Impact of Adding Different Sweet Potato Flour Types (Ipomoea Batatas L.) at Various Percentages on the Antioxidant Activity and Microbiological Quality of Probiotic Fermented Milk (Pediococcus acidilactici BK01)
Research Article
Impact of Adding Different Sweet Potato Flour Types (Ipomoea Batatas L.) at Various Percentages on the Antioxidant Activity and Microbiological Quality of Probiotic Fermented Milk (Pediococcus acidilactici BK01)
Doni Supadil1, Sri Melia2*, Indri Juliyarsi2
1Magister Program of Animal Science, Universitas Andalas, Padang, West Sumatra, Indonesia. 25163; 2Faculty of Animal Science Universitas Andalas, Padang, West Sumatra, Indonesia, 25163.
Abstract | Probiotics are live microorganisms that, when consumed in sufficient amounts, benefit the body’s health. One such probiotic is Pediococcus acidilactici BK01. This study evaluates the impact of different types and amounts of sweet potato flour on the prebiotic and antioxidant properties of fermented milk. The objective is to determine the effects of different types and percentages of sweet potato flour on prebiotic and antioxidant properties. An experimental design was employed, preparing three types of sweet potato flour: white (A1). purple( A2), yellow (A3), and These flours were added to fermented milk in varying proportions: 0% (B1), 3% (B2), and 6% (B3), with three repetitions for each treatment. The results showed a significant effect (P<0.05) of sweet potato type and flour percentage on increasing total lactic acid bacteria, decreasing pH value, increasing acidity, and antioxidant activity. The optimal treatment was fermented milk with 6% purple sweet potato flour, yielding 117.67 x 109 CFU/ml of lactic acid bacteria, a pH value of 3.75, titratable acidity of 1.40%, and antioxidant activity of 78.90%. This study suggests that fermented milk with added sweet potato flour can serve as a functional food, offering health benefits through its prebiotic and antioxidant properties.
Keywords | Antioxidant activity, Lactic acid bacteria, Acidity, Prebiotic, Probiotic, Functional food
Received | June 10, 2024; Accepted | August 03, 2024; Published | August 23, 2024
*Correspondence | Sri Melia, Faculty of Animal Science Universitas Andalas, Padang, West Sumatra, Indonesia, 25163; Email: srimelia75@ansci.unand.ac.id
Citation | Supadil D, Melia S, Juliyarsi I (2024). Impact of adding different sweet potato flour types (ipomoea batatas l.) at various percentages on the antioxidant activity and microbiological quality of probiotic fermented milk (pediococcus acidilactici bk01). Adv. Anim. Vet. Sci. 12(10): 1903-1910.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.10.1903.1910
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The development of science and technology is growing more and more. One of them is the development of food products. In addition, high public awareness of eating healthy food encourages people to consume functional foods. Milk is one of the food products from livestock favored by various groups of people and functional. Milk has a high nutritional content such as lactose, protein, fat, and minerals, making it susceptible to irreversible damage due to its nutrient-rich composition that supports microbial growth (Sujata et al., 2022). Such microbial growth can alter milk’s taste, color, aroma, and texture, leading to milk spoilage. Various processing and preservation techniques can be carried out to improve milk quality so it is not easily damaged. One type of processing and preservation of milk is to process it into fermented milk products as a functional food.
Fermented milk is a dairy product produced from the fermentation process by lactic acid bacteria using processed milk raw materials or without the addition and modification of milk by breaking down lactose using lactase so that it decreases pH and without coagulation (Horiuchi et al., 2022; Chairunnisa, 2006). The fermentation process in milk can inhibit pathogenic microorganisms to extend milk’s shelf life (Nadirova et al., 2023). Milk fermentation requires probiotic microorganisms to play a role. Probiotics are microorganisms that, when consumed in sufficient quantities, cities can have advantages such as increasing nutritional value and affecting body health by improving the balance of gut microbiota (Prem and Savitri, 2023). Probiotics will affect the gut microflora and provide health effects such as lowering the risk of colon cancer, anti-hypertension, enhancing immunity, and anti-carcinogenic (Zaib et al., 2024). One type of probiotic used in fermented milk is Pediococcus acidilactici BK01.
Pediococcus acidilactici BK01 is a probiotic isolated from spontaneously fermented Bekasam or swamp sepat fish (Tricopodus trichopterus) from Banyuasin, South Sumatra. This bacterium can survive in stomach acid and bile salt conditions and has antimicrobial activity against Escherichia coli 0157:H7, Staphylococcus aureus ATTCC25923, and Listeria monocytogenes CFSAN0044330 (Melia et al., 2019). These bacteria can be used as probiotics in fermented milk, where in previous studies, research has been carried out on Pediococcus acidilactici BK01 fermented milk with the addition of red ginger with 30 days of storage has an antioxidant content of 48.39%, pH 4.3%, titratable acidity 1.716%, moisture content 80%, protein 3%, fat 3%, syneresis 28%, and water holding capacity (WHC) 63% (Melia et al., 2022). In another study, Melia et al. (2020) examined the quality of Pediococcus acidilactici BK01 fermented goat milk at refrigerator temperature for 28 days which had a probiotic count of 9,106 log CFU/ml, titratable acidity 1.73%, pH 4.28, total plate count (TPC) 4,012 log CFU/ml, protein 3.57%, fat 3.49%, and moisture content 85.51%. Synbiotic foods are an essential strategy in functional foods (Avila-Reyesa et al., 2014). Synbiotics are a combination of probiotics and prebiotics that affect body health (Lyon et al., 2023). One type of prebiotic that can be added to fermented milk is sweet potato flour.
For years, traditional foods in numerous cultures have included fermented dairy products like kefir and yogurt. An increasing number of people are interested in enhancing these goods’ nutritional profile and health benefits by adding different functional ingredients. The addition of sweet potato is one such change. The global demand for healthier functional food encourages this trend, which is observed in several regions.
Sweet potatoes are high in dietary fiber, minerals, and vitamins, particularly vitamin A. Sweet potatoes are a source of essential minerals and antioxidants that can be added to fermented dairy products to boost their nutritional value. Inulin, a prebiotic fiber found in sweet potatoes, can encourage the development of beneficial gut microbes. This suggests that sweet potato-based fermented milk products may improve intestinal health. Sweet potatoes’ inherent sweetness can enhance the flavor of fermented dairy products, negating the need for additional sugar. Its fiber content could also affect the texture’s creaminess and thickness. Several types of sweet potato that are quite familiar among the public include purple sweet potato (Ipomoea batatas var Ayumurasaki), yellow sweet potato (Ipomoea batatas var Lam), and white sweet potato (Ipomoea batatas linneaus). Purple sweet potato has 0.72% fructooligosaccharide and 2.73% inulin (Long et al., 2014). In addition, purple sweet potatoes contain anthocyanins 61.85 mg/100 g (Husna et al., 2013). Yellow sweet potato has oligosaccharide compounds of 2.165% and inulin of 4.6% (Afriani et al., 2016). In addition, yellow sweet potatoes are rich in beta-carotene (pro-vitamin A), ranging from 1.3-3.9 mg/100g, and vitamin C (Bungan, 2016; Kammona et al., 2015). White sweet potato contains 0.07% raffinose, 2.46% sucrose, 2.53% oligosaccharide, and 5.5% inulin (Lesmanawati et al., 2013; Afriani et al., 2016).
In a previous study, fermented goat milk Levilactobacillus brevis DSM02 with the addition of porang flour (Amorphophallus oncophyllus) as much as 0.25% had a total lactic acid bacteria of 19 x 109 CFU/mL, titratable acidity of 1.21%, pH value of 4.10%, dietary fibre of 0.18%, moisture content of 84.33%, protein content of 2.77% and viscosity of 6,114 cP (Mayasari et al., 2024). Research by Rizki et al. (2019) showed that yogurt with the addition of 8% purple sweet potato flour had a total lactic acid bacteria content of 13.19 log CFU/ml, pH 3.73, total acid titration of 1.23%, and antioxidant activity of 90.33%.
By investigating the impact of adding various kinds and percentages of sweet potato flour (Ipomoea batatas L.) on the antioxidant activity and microbiological quality of probiotic fermented milk (P. acidilactici BK01), this study attempts to fill this gap. This study investigates the impact of different types and percentages of sweet potato flour on the antioxidant activity and microbiological quality of probiotic fermented milk.
MATERIALS AND METHODS
The materials used in this study were Etawa crossbreed goat milk (Capra aegagrus circus) for making starters obtained from Dairy Goat Farm in Korong Gadang, Kuranji District, Padang City, West Sumatra, Indonesia. Diamond® Ultra High Temperature (UHT) milk as much as 2700 ml. The compotion of UHT milk (per 200ml): fat 7 g, protein 6 g, Carbohydrat 12 g, glukose 7 g. Probiotic microencapsulated powder Pediococcus acidilactici BK01 (The P. acidilactici starter was microencapsulated with a mixture of maltodextrin and whey protein isolate, and encapsulated microbes can thrive in fresh milk media in the fermentation process) from the Animal Product Technology Laboratory, Faculty of Animal Husbandry, Andalas University, Padang. White, purple, and yellow sweet potatoes were obtained from Bandar Buat traditional market in Padang City. In addition, the materials used were MRS Agar (Neogen Culture Media®), MRS Broth (Neogen Culture Media®), distilled water, NaOH 0.1N, Phenolphthalein Indicator, buffers 4.01 and 6.86, Folin Ciocalteu Reagent, Na2CO3, DPPH (Diphenylpicryl-Hydrazy), and Methanol.
This research was conducted with an experimental design This research was conducted with experimental design. The method was a 3 x 3 factorial complete randomized design (CRD) with 3 replicates. The treatments were sweet potato flour type, namely white sweet potato (A1), purple sweet potato (A2), yellow sweet potato (A3), and the percentage of sweet potato flour addition to fermented milk, namely 0% (B1), 3% (B2), and 6% (B3).
Preparation of Sweet Potato Flour (Ipomoea batatas L.)
White, purple, and yellow sweet potatoes were peeled, washed, and sliced thinly, then dried using an Electric Food Dehydrator DHY-06X at 90o for 3 hours. Pureed the dried sweet potato with a blender, then sifted with a 60 mesh sieve to produce fine sweet potato flour (Santi et al., 2022; Hardoko et al., 2010). Table 1 shows the composition of sweet potatoes fluor.
Table 1: Compotition of Sweet Potatoes Flour.
|
Component |
Sweet Potatoes Flour |
||
|
White |
Purple |
Yellow |
|
|
Moisture |
6,35 % |
5,53% |
7,00% |
|
Protein |
2,07% |
1,39% |
1,52% |
|
Fat |
0,26% |
0,25% |
0,30% |
|
Total Phenol |
7,39 mg GEA/g |
124,78 mg GEA/g |
35,65 mg GEA/g |
|
Antioxidant Activity |
44,94% |
87,11% |
85,18% |
Preparation of Fermented Milk Starter
100 ml of goat’s milk was pasteurized at 85oC for 15 minutes and reduced to room temperature. Microencapsulated probiotic powder Pediococcus acidilactici BK01 was put into goat milk as much as 1%. Milk was incubated at 37oC for 18 hours (Wakhidah et al., 2017).
Preparation of Fermented Milk
UHT milk was put into each milk bottle. Sweet potato flour was added according to the treatment, and pasteurized milk was pasteurized at 85oC for 15 minutes. The milk temperature was lowered to room temperature. Pediococcus acidilactici BK01 starter was inoculated as much as 5% and homogenized. Incubation occurred at 37oC for 18 hours (Andriani et al., 2021).
Test Parameters
Total Colony of Lactic Acid Bacteria: After preparing a 1.0 mL sample, it is diluted in 9 mL of de Man Rogosa Sharpe Broth (Neogen Culture Media) and homogenised by vortexing. Following this, the fluid is diluted to 108. A hockey stick is used to level the dilution results after they have been planted using the spread method on a petri dish filled with de Man Rogosa Sharpe Agar (Neogen Culture Media). For 48 hours, the samples are incubated at 37°C. Colony Forming Units (CFU) are used to count colonies (Sivakumar and Kalairasu, 2010).
Testing pH and Total Acid Titration: The pH value was tested using a digital pH meter (HANNA Instrument) that was calibrated using buffers pH 4.01 and pH 6.86 (AOAC, 2012). Total acid titration was measured by mixing yogurt samples with 10 mL sterile distilled water and titrated using 0.1 N NaOH using phenolphthalein indicator (AOAC, 2005).
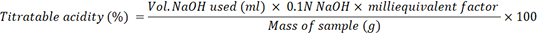
Antioxidant activity: 1 ml sample was mixed with 1 ml methanol solution containing 80 ppm DPPH. Then homogenized and allowed to stand for 30 minutes in a dark room. Measurements were made with a spectrophotometer (Shimadzu UV-1800®) with an absorbance reading of 517 nm (Huang et al., 2005).
Statistical Analysis
All data obtained in this study were analyzed using SPSS 26 statistical analysis. Data were analyzed using SPSS version 26 statistics, and if there was a significant difference from the treatment, it was continued with the Duncan Multiple Range Test (DMRT).
RESULTS AND DISCUSSION
Total Colony of Lactic Acid Bacteria
The results show that the significant difference (P<0.05) was due to the type of sweet potato used and the percentage of sweet potato flour addition that could increase lactic acid bacteria in Pediococcus acidilactici BK01 fermented milk. The average colonies of lactic acid bacteria of each treatment are presented in Table 2.
In the A3B3, A2B3, and A1B3 treatments, the addition of yellow, purple, and white sweet potato flour as much as 6% has the highest total lactic acid bacteria in Pediococcus acidilactici BK01 fermented milk. This is due to the prebiotic content contained in sweet potato flour, such as dietary fiber, oligosaccharides, fructooligosaccharides, inulin, raffinose, and resistant starch, which can stimulate the growth of Pediococcus acidilactici BK 01 bacteria in fermented milk. The addition of sweet potato flour, both yellow, purple, and white yam, as much as 6% in fermented milk, experienced an average LAB increase of 40% when compared to fermented milk without the addition of yam flour.
Table 2: Average total lactic acid bacteria (109 CFU/mL) of fermented milk.
|
Type of Sweet Potato Flour |
Persentages of Sweet Potato Flour Addition |
Average |
||
|
0% (B1) |
3% (B2) |
6% (B3) |
||
|
White (A1) |
70.00a |
88.00ab |
117.00bc |
91.67 |
|
Purple (A2) |
73.00a |
113.33b |
117.67bc |
101.33 |
|
Yellow (A3) |
82.67ab |
113.67b |
149.33c |
115.22 |
|
Average |
75.22 |
105.00 |
128.00 |
|
Note: abc Different superscripts in the columns indicate significant differences (P<0.05).
Adding sweet potato flour can increase LAB growth because sweet potatoes have prebiotic sources found in sweet potatoes, such as dietary fiber, oligosaccharides, fructooligosaccharides, inulin, raffinose, and resistant starch. Prebiotic components such as fructooligosaccharides (FOS), inulin, raffinose, fiber, oligosaccharides, and resistant starch can selectively stimulate probiotic growth (de Albuquerque et al., 2020; Muchiri et al., 2017; Lestari et al., 2013). This follows the opinion of (Celestin et al., 2017), which states that fructooligosaccharides, oligosaccharides, and inulin play an essential role in fermented dairy products by encouraging the growth of probiotics. Gouveia et al. (2020) and Alam (2021), added that apart from being rich in carbohydrates, sweet potato (Ipomoea batatas L.) also contains other bioactive components such as protein, minerals, rubberenoids (yellow sweet potato), flavonoids, anthocyanins (purple sweet potato), phenolic acids, and ascorbic acid. Sancho et al. (2017) added that fructooligosaccharides in sweet potatoes have a 29.08 mg/100g concentration. According to Yuliansar (2020), the starch content of sweet potatoes in white, orange, and purple is 85.15%, 78.14%, and 46.98%, respectively. Furthermore, according to Nindyariani et al. (2011), Saman et al. (2019) and Correa et al. (2018), the fiber content of purple, white, and yellow sweet potatoes is 2.40%, 0.62%, and 1.92%, respectively.
In the A1B1, A2B1, and A3B1 treatments, the treatment with the addition of 0% white, purple, and yellow sweet potato flour (control) had the lowest value because there was no addition of sweet potato flour, which could be used as a source of prebiotics to increase lactic acid bacteria in fermented milk. This is the opinion of Perez et al. (2022), which states that adding tuber flour to fermented milk increases total lactic acid bacteria. In contrast, the addition of sweet potato flour (Ullucus tuberosus) to low-fat yogurt can increase total LAB so that it can produce acid production during the fermentation process. Pramono et al. (2020) added ganyong flour (Dioscorea esculenta L) as much as 2% in yogurt produced a total LAB of 9.2 x 109 CFU / ml. Added research by Rizki et al. (2019) fermented milk with 8% purple sweet potato flour produced a total LAB of log 13.19 CFU/ml. In this study, the total LAB of fermented milk with the addition of sweet potato flour total LAB of 70-149.33 x 109 CFU/ml, which has met the probiotic drink standard and the WHO (2001) standard of 109 CFU/ml.
Table 3: Average pH value of fermented milk.
|
Type of Sweet Potato Flour |
Persentages of Sweet Potato Flour Addition |
Average |
||
|
0% (B1) |
3% (B2) |
6% (B3) |
||
|
White (A1) |
4.49e |
3.96bcd |
3.80ab |
4.08 |
|
Purple (A2) |
4.52e |
3.97cd |
3.75a |
4.08 |
|
Yellow (A3) |
4.54e |
4.07d |
3.89abc |
4.17 |
|
Average |
4.52 |
4.00 |
3.81 |
|
Note: abcd Different column superscripts indicate significant differences (P<0.05).
Table 3 shows that fermented milk with several types of sweet potatoes with dufferent persentages of sweet potatoes have a significant effect (P<0.05) on the pH value of Pediococcus acidilactici BK01 fermented milk. The average pH value of Pediococcus acidilactici BK01 fermented milk was 3.75-4.54. The average pH value of each treatment is presented in Table 3.
Fermented milk containing 6% purple, white, and yellow sweet potato flour exhibited the lowest pH value in the A2B3, A1B3, and A3B3 treatments. Compared to fermented milk without adding sweet potato flour, the average pH decreased by 15%. This is because lactic acid bacteria use prebiotic sources higher than lower percentages of sweet potato flour to make lactic acid, which can lower the pH of fermented milk.
The more lactic acid is a metabolite produced by LAB, so with more lactic acid metabolites the lactic acid can dissociate into H+ ions so that the pH becomes lower. This follows the opinion of Purwati et al. (2018), which states that the more acid produced, the more H+ ions are formed, so the pH meter electrode’s pH measurement shows a decreasing value. Abouloifa et al. (2020) stated that the addition of prebiotics such as fructooligosaccharides (FOS) and oligosaccharides can cause a decrease in pH during fermentation.
This follows the opinion of Jenimat et al. (2023), which states that sweet potatoes contain polyphenol compounds, chlorogenic acid, caffeoylquinic acid, and oxalic acid 77.66-197.90 mg/100g. Added opinion Aboyeji et al. (2020) state that sweet potatoes, as a carbon source, can produce acids through simultaneous saccharification and fermentation processes. In addition, according to Li et al. (2019), the presence of inulin in milk causes the growth of Lactococcus lactis bacteria, thus reducing the pH value. In the research of Melia et al. (2022) on fermented goat milk, Pedioccoccus acidilactici BK01, with red ginger extract, produced a pH value of 4.07-4.43.
Titratable Acidity
Table 4 shows that fermented milk with several types of sweet potatoes with different persentages has a significant effect (P<0.05) on the titratable acidity of Pediococcus acidilactici BK01 fermented milk. The average titratable acidity of Pediococcus acidilactici BK01 fermented milk is 1.03-1.56. This shows that the increase in titratable acidity in fermented milk is in line with the decrease in the pH value of fermented milk, along with the addition of the percentage of several types of sweet potato flour.
Compared to fermented milk without the addition of sweet potato flour, there was a 33% increase in titratable acidity in the fermented milk of P. acidilactici BK01 in the A1B3, A2B3, and A3B3 treatments. This rise was caused by the addition of numerous forms of sweet potato fluor, up to 6%. The titratable acidity of the fermented milk of P. acidilactici BK01 in the A1B3, A2B3, and A3B3 treatments increased by 33% compared to fermented milk without adding sweet potato fluoride. The addition of several types of sweet potato flour, up to 6%, was the cause of this surge.
This follows the research findings of Imelda et al. (2020), who claim that the higher the sweet potato content, the lower the pH value. Lactic acid bacteria use lactose and prebiotic sources as a substrate for their metabolism, converting them into organic acids and lactic acid, the main acid component. Novelina et al. (2012) conducted more research on fermented beverage products, including red sweet potato extract, which has a pH of 3.55-4.47, titratable acidity of 1.56-1.97%, and total LAB of 6.1-8.3 x 108 CFU/ml.
Phahlane et al. (2022) state that sweet potatoes primarily contain chlorogenic acid (CGA). However, they also include other types of acids. Where the many forms of chlorogenic acid, including chlorogenic acid and 3,5-, 3,4-, and 4,5-dicaffeoylquinic acids, are found. According to Lebot et al. (2021), yellow sweet potatoes had an average of 0.95 µg/mg of chlorogenic acid (CGA), 0.86 µg/mg of 3,5-caffeoylquinic, 0.09 µg/mg of 3,4-caffeoylquinic, and 0.16 µg/mg of 4,5-caffeoylquinic. This study found that the average titratable acidity of yellow sweet potato (A3), purple sweet potato (A2), and white sweet potato (A1) was 1.29%, 1.23%, and 1.31%, respectively. According to Codex Alimentarius (2003), the standard for titratable acidity is a minimum of 0.3%.
Table 4: Average titratable acidity (%) of fermented milk.
|
Type of Sweet Potato Flour |
Persentages of Sweet Potato Flour Addition |
Average |
||
|
0% (B1) |
3% (B2) |
6% (B3) |
||
|
White (A1) |
1.08a |
1.30b |
1.56d |
1.31 |
|
Purple (A2) |
1.03a |
1.26b |
1.40bc |
1.23 |
|
Yellow (A3) |
1.10a |
1.30b |
1.48cd |
1.29 |
|
Average |
1.07 |
1.29 |
1.48 |
|
Note: abcd Different superscripts in the columns indicate significant differences (P<0.05).
Antioxidant Activity
The statistical analysis results showed a significant effect (P<0.05) between several types of sweet potato and the percentage of sweet potato flour addition on the antioxidant activity of Pediococcus acidilactici BK01 fermented milk. The average antioxidant activity of each treatment is presented in Table 5.
Table 5: Average antioxidant activity (%) of fermented.
|
Type of Sweet Potato Flour |
Persentages of Sweet Potato Flour Addition |
Average |
||
|
0% (B1) |
3% (B2) |
6% (B3) |
||
|
White (A1) |
20.06ab |
22.09abc |
25.26bc |
22.47 |
|
Purple (A2) |
19.42ab |
58.85d |
78.90e |
52.39 |
|
Yellow (A3) |
18.75a |
23.23abc |
27.06c |
23.01 |
|
Average |
19.41 |
34.72 |
43.74 |
|
Note: abcde Different superscripts in the columns indicate significant differences (P<0.05).
Compared to fermented milk without flour addition, the highest antioxidant activity was found in fermented milk with 6% purple sweet potato flour addition, as revealed by a 75.38% increase in antioxidant activity. This may be because, as compared to other flours, purple sweet potato flour had higher total phenol content (124.78 mg GEA/g) and antioxidant activity (87.11%) than the different flours.
According to Winardi et al. (2020), purple sweet potato flour has an anthocyanin content of 20.196–62.138 mg/100g. Lee et al. (2021) add that purple sweet potatoes have been shown to boost the antioxidant content of fermented milk products like yogurt. Similarly, fermented milk containing Changsey purple sweet potato added by Lactobacillus acidophilus, Lactobacillus delbrueckii subsp Lactis, and Lactobacillus gasseri bacteria has a high concentration of butyric amino acids (GABA), organic acids, and antioxidant content, as indicated by the additional opinion of Wu et al. (2012). On the other hand, the abundance of antioxidants in Chingshey Purple Sweet Potatoes may increase the antioxidant activity of functional meals.
The high antioxidant activity in the A2B2 and A2B3 treatments is due to flavonoids such as anthocyanins in the purple sweet potato flour added to the fermented milk of Pediococcus acidilactici BK01. This is following Lee et al. (2021), which states that purple sweet potatoes are proven to increase antioxidants in fermented products such as yogurt. Winardi et al. (2020) stated that the anthocyanin content in purple sweet potato flour ranged from 20,196 mg/100g to 62,138 mg/100g. Pediococcus acidilactici BK01 fermented milk with white sweet potato did not differ significantly (P>0.05) from fermented milk with yellow sweet potato. This is due to the relatively low beta carotene content. This follows the opinion of Nogueira et al. (2018) that the beta-carotene content in sweet bread with yellow sweet potato flour ranges from 0.1656-0.4715 µg/g. Ishiguro et al. (2010) added that yellow sweet potato flesh contains beta carotene 1.3-3.9 mg/100g. In addition, the beta-carotene content is prone to degradation when exposed to heat, so although there is an increase in antioxidant activity, it is not significant. This follows the statement of Ospina et al. (2023), where exposure to high temperatures causes a decrease in carotenoid compounds in sweet potatoes with a significantly decreased retention rate at cooking temperatures of 75oC, 85oC, and 95oC.
CONCLUSIONs AND RECOMMENDATIONS
The optimal sweet potato type addition to the fermented milk of P. acidilactici BK01 was purple sweet potato flour (Ipomoea batatas var Ayumurasaki), added at a percentage of sweet potato flour addition of 6%. The fermented milk had a pH value of 3.75, a total acid content of 1.40%, and a 78.90% antioxidant activity. P. acidilactici BK01 fermented milk can be made with several types of sweet potato flour (Ipomoea batatas L.), a source of prebiotics and antioxidants that is functional foods.
ACKNOWLEDGEMENTS
We are grateful to the Dean of Faculty of Animal Science, Universitas Andalas for supporting this research.
NOVELTY STATEMENT
The addition of several types of sweet potato flour (Ipomoea batatas L.) to the fermented milk of P. acidilactici BK01 still meets the criteria of probiotics that have the potential to be functional food and are beneficial for health.
AUTHOR’S CONTRIBUTIONS
Sri Melia and Doni Supadil: Data conceptualization and curation.
Doni Supadil: Data Curation, formal analysis, and writing of the original manuscript.
Sri Melia and Indri Juliyarsi: Validation and writing editor.
Conflict of Interest
The authors have declared no conflict of interest.
REFERENCES
Abouloifa H, Khodaei N, Rokni Y, Karboune S, Brasca M, D’Hallewin G, Asehraou A (2020). The prebiotics (Fructo-oligosaccharides and Xylo-oligosaccharides) modulate the probiotic properties of Lactiplantibacillus and Levilactobacillus strains isolated from traditional fermented olive. World J. Microbiol. Biotechnol., 36: 1-12. https://doi.org/10.1007/s11274-020-02961-9
Aboyeji OO, Oloke JK, Arinkoola AO, Oke MA, Ishola, MM (2020). Optimization of media components and fermentation conditions for citric acid production from sweet potato peel starch hydrolysate by Aspergillus niger. Sci. Afr., 10: e00554. https://doi.org/10.1016/j.sciaf.2020.e00554
Afriani, Yana, Farida (2016). Uji kadar inulin pada beberapa varietas ubi jalar (Ipomoea batatas) dikabupaten ngawi jawa timur. Skripsi. Universitas Islam Sunan Kalijaga Yogyakarta.
Alam MK (2021). A comprehensive review of sweet potato (Ipomoea batatas L. Lam): Revisiting the associated health benefits. Trends in Food Sci. Technol., 115: 512-529. https://doi.org/10.1016/j.tifs.2021.07.001
Andriani RD, Rahayu PP, Apriliyani MW, Manab A, Sawitri ME (2021). Characterization of Fermented Milk with the Addition of Gembili (Dioscorea esculenta) Flour. Asian Food Sci. J., 20(2): 56-65. https://doi.org/10.97 34/afsj/2021/v20i230267
AOAC (2005). Official Methods of Analysis. Association of Official Analytical Chemists. Benjamin Franklin Station, Washington DC.
AOAC (2012). Official Method of Analysis: Association of Analytical Chemists. 19th Edition. AOAC, Washington DC.
Avila-Reyes SV, Garcia-Suarez FJ, Jiménez MT, San Martín-Gonzalez MF, Bello-Perez LA (2014). Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym., 102: 423-430.
Bungan AS (2016). Kajian Sifat Fisik, Organoleptik, Dan Kadar Beta Karoten Kroket Dengan Variasi Campuran Ubi Jalar Kuning. Doctoral dissertation, Poltekkes Kemenkes Yogykarta.
Celestin S, Thorat SS, Desale RJ, Chavan UD (2015). Effect of milk supplementation with fructooligosaccharides and inulin on viable counts of probiotic bacteria in goat and cow milk yoghurts. IOSR J. Environ. Sci. Toxicol. Food Technol., 9(7): 6-12.
Chairunnisa H (2006). Utilization of lactic acid bacteria in fermented milk product lifihome. J. Ilmu Ternak Universitas Padjadjaran, 6(2).
Codex Alimentarius (2003). Standard for Fermented Milks. Codex. STAN 243. FAO/WHO Food Standards.
Correa D, Castillo PMM, Martelo RJ (2018). Physicochemical characterization of sweet potato flour from the Colombian Caribbean. Contemp. Eng. Sci., 11(37). Pp: 1845-1851. https://doi.org/10.12988/ces.2018.84167
de Albuquerque TMR, Borges CWP, Cavalcanti MT, dos Santos Lima M, Magnani M, de Souza EL (2020). Potential prebiotic properties of flours from different varieties of sweet potato (Ipomoea batatas L.) roots cultivated in Northeastern Brazil. Food Biosci., 36: 100614. https://doi.org/10.1016/j.fbio.2020.100614
Gouveia CS, Ganança JF, Lebot V, Pinheiro de Carvalho MA (2020). Changes in oxalate composition and other nutritive traits in root tubers and shoots of sweet potato (Ipomoea batatas L. Lam.) under water stress. J. Sci. Food and Agric., 100(4): 1702-1710. https://doi.org/10.1002/jsfa.10185
Hardoko L, Hendarto dan Tagor Marsillam S (2010). Pemanfaatan Ubi Jalar Ungu Sebagai Pengganti Tepung Terigu Dan Sumber Antioksidan Pada Roti Tawar. J. Teknologi dan Industri Pangan, 21 (1): 25-31.
Horiuchi H, Ichimura T, Inoue N, Takagi N (2022). U.S. Patent No. 11,344,040. Washington, DC: U.S. Patent and Trademark Office.
Huang YC, Chang YH, and Shao YY (2005). Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem., 98(3): 529-538. https://doi.org/10.1016/j.foodchem.2005.05.083
Husna NE, Novita M, Rohaya S (2013). Anthocyanins content and antioxidant activity of fresh purple-fleshed sweet potato and selected products. Agritech, 33(3), 296-302.
Imelda F, Purwandani L, Saniah S (2020). Total Bakteri Asam Laktat, Total Asam Tertitrasi dan Tingkat Kesukaan pada Yoghurt Drink dengan Ubi Jalar Ungu sebagai Sumber Prebiotik. Vokasi: Jurnal Publikasi Ilmiah, 15(1): 1-7.
Ishiguro K, Yoshinaga M, Kai Y, Maoka T, Yoshimoto M (2010). Composition, content and antioxidative activity of the carotenoids in yellow-fleshed sweetpotato (Ipomoea batatas L.). Breeding Sci., 60(4): 324-329.
Jenimat AD, Lawung YD, Baunsele AB, Boelan EG, Wariani T, Leba MAU (2023). Phytochemical Content Of Fresh Purple Sweet Potato (Ipomea batatas L.) Extract as Acid-Base Titration Indicator. Jurnal Sains Natural, 13(2): 57-66.
Kammona S, Othman R, Jaswir I, Jamal P (2015). Characterisation of carotenoid content in diverse local sweet potato (Ipomoea batatas) flesh tubers. Int. J. Pharm. Pharm. Sci., 2: 347-351.
Lebot V, Leo P, Legendre L (2021). Phenotyping chlorogenic acids and coumarins in sweet potato (Ipomoea batatas L. Lam.) breeding lines for enhanced tolerance to periderm pathogens. Euphytica, 217(4): 59. https://doi.org/10.1007/s10681-021-02808-w
Lee SG, Chae J, Kim DS, Lee JB, Kwon GS, Kwon TK, Nam JO (2021). Enhancement of the antiobesity and antioxidant effect of purple sweet potato extracts and enhancement of the effects by fermentation. Antioxidants, 10(6): 888. https://doi.org/10.3390/antiox10060888
Lesmanawati W, Widanarni W, Sukenda S, Purbiantoro W (2013). The Potential of Sweet Potato Oligosaccharide Extract as Aquaculture Probiotic Bacteria Prebiotic. Jurnal Sains Terapan: Wahana Informasi dan Alih Teknologi Pertanian, 3(1), 16-20.
Lestari LA, Soesatyo MHNE, Iravati S, Harmayani E (2013). Characterization of Bestak sweet potato (Ipomoea batatas) variety from Indonesian origin as prebiotic. International Food Research Journal, 20(5).
Li R, Ding Q, Zhao XH (2019). Impact of milk fortification on the microbiological and physicochemical properties of set-type skimmed yogurt using three commercial soluble prebiotics. Foods, 8(6), 181. https://doi.org/10.3390/foods 8060181
Long E, Billard C, Adenet S (2014). Comparison of Physicochemical, Organoleptic and Nutritional Abilities of Eight Sweet Potato (Ipomoea batatas) Varieties. Food and Nutr. Sci., 5.
Lyon IIIJ, Connell M, Chandrasekaran K, Srivastava S (2023). Effect of synbiotics on weight loss and metabolic health in adults with overweight and obesity: A randomized controlled trial. Obesity, 31(8): 2009-2020. https://doi.org/10.1002/o by.23801
Mayasari J, Oktavia V, Juliyarsi I, Sukma A, Melia S (2024). Quality of Levilactobacillus brevis DSM02 fermented goat milk with the addition of porang flour (Amorphophallus oncophyllus). Adv. Anim. Vet. Sci, 12(3):566-572. https://dx.doi.org/10.17582/journal.aavs/2024/12.3.566.572
Melia S, Juliyarsi I, Kurnia YF (2022). Physicochemical properties, sensory characteristics, and antioxidant activity of the goat milk yogurt probiotic Pediococcus acidilactici BK01 on the addition of red ginger (Zingiber officinale var. rubrum rhizoma). Vet. World, 15(3): 757. https://doi.org/10.14202%2Fvet world.2022. 757-764
Melia S, Juliyarsi I, Kurnia YF, Pratama YE, Pratama DR (2020). The quality of fermented goat milk produced by Pediococcus acidilactici BK01 on refrigerator temperature. Biodiversitas J. Biol. Divers., 21(10). https://d oi.org/10.13057/biodiv/d211017
Melia S, Purwati E, Kurnia YF, Pratama DR (2019). Animal potential of Pediococcus acidilactici from bekasam fermentation of sepat rawa fish (Tricopodus trichopterus) from Banyuasin, South Sumatra, Indonesia, 20: 12. https://doi.org/10.13057/biodiv/d201210
Muchiri MN, McCartney AL (2017). In vitro investigation of orange-fleshed sweet potato prebiotic potential and its implication on human gut health. 7: 10. https://doi.org/10.31989/ffhd.v7i10.361
Nadirova S, Sinyavskiy Y (2023). Justification of the shelf life of fermented dairy products based on goat’s milk. Eurasian Journal of Applied Biotechnology, (2): 29-37. https://doi.org/10.11134/btp.2.2023.4
Nindyarani AK, Sutardi S, dan Suparmo S (2011). Karakteristik kimia, fisik dan inderawi tepung ubi jalar ungu (Ipomoea batatas Poiret) dan produk olahannya. Agritech., 31 (4). https://doi.org/10.22146/agritech.9634
Nogueira AC, Sehn GA, Rebellato AP, Coutinho JP, Godoy HT, Chang YK, Clerici MTP (2018). Yellow sweet potato flour: use in sweet bread processing to increase β-carotene content and improve quality. An. Acad. Bras. ciências, 90: 283-293. https://doi.org/10.1590/0001-3765201820150804
Novelina N, Eliyasmi R, Ariani S, Firdausni F (2012). Pengaruh Penambahan Susu Bubuk Fullcream Terhadap Mutu Produk Minuman Fermentasi dari Ekstrak Ubi Jalar Merah (Ipomoea batatas L). Indones. J. Ind. Res., 2(2), 93-102. http://dx.doi.org/10.24960/jli.v2i2.605.93-102
Ospina MA, Moreno JL, Tran T, Jaramillo AM, Gallego‐Castillo S, Ospina B, Dufour D (2023). Kinetics of thermal degradation of carotenoids related to potential of mixture of wheat, cassava and sweet potato flours in baking products. J. Sci. Food Agric.. https://doi.org/10.1002/jsfa.12831
Pérez LV, Sánchez HJ, Cando VM, Sánchez ÁE, Salazar DM (2022). Fortification of low-fat yogurt with melloco flour (Ullucus tuberosus): Physicochemical and rheological effects. Afr. J. Food Agric. Nutr. Dev.,. 22(10): 22041-22058. https://doi.org/10.18697/ajfa nd.115.20870
Phahlane CJ, Laurie SM, Shoko T, Manhivi VE, Sivakumar D (2022). Comparison of caffeoylquinic acids and functional properties of domestic sweet potato (Ipomoea batatas (L.) Lam.) storage roots with established overseas varieties. Foods, 11(9), 1329. https://doi.org/10.3390/foods11091329
Pramono YB, Dwiloka NB, Mulyani S, Setiani BE, Rochmayani M, Bahtiar DE (2020). Utilization of lesser yam (Dioscorea esculenta L.) flour as prebiotic in yogurt to total lactic acid bacteria (LAB), sugar reduction, and organoleptic properties. Digit. Press Life Sci., 2, 00011. https://doi.org/10.29037/digitalpress.22325
Prem, Lata, Savitri (2023). Probiotics and human health. Res. J. Biotechnol., 18(7):173-180. Doi: 10.25303/1807rjbt1730180
Primurdia EG, Kusnadi J (2014). Aktivitas Antioksidan Minuman Probiotik Sari Kurma (Phoenix dactilyfera L.) dengan isolat L. Plantarum dan L. casei. J. Pangan dan Agroindustri, 2(3), 98-109.
Purwati E, Hallyward J, Juliyarsi I, Melia S, Purwanto H, Hartini P (2018). Effect of addition cinnamon bark extract (Cinnamomum burmannii) of water content, total lactic acid bacteria colonies, antioxidant activity and cholesterol levels from goat’s milk yoghurt. J. Adv. Res. Dyn. Control Syst., 10(4). 272-278.
Rizki GCKA. Nocianitri dan Sugitha IM (2019). Pengaruh Penambahan Tepung Ubi Jalar Ungu (Ipomea batatas L. var. ayamurasaki) terhadap Karakteristik Health-Promoting Yogurt. Jurnal Ilmu Dan Teknologi Pangan (Itepa), 8(4), 341.
Saman WR, Yuliasih I, Sugiarto M (2019). Physicochemical characteristics and functional properties of white sweet potato starch. Int. J. Eng. Manage. Res. e-ISSN, 2250-0758. https://doi.org/10.31033/ijemr.9.3.7
Sancho RAS, Souza DJR, de Lima FA, Pastore GM (2017). Evaluation of oligosaccharide profiles in selected cooked tubers and roots subjected to in vitro digestion. LWT-Food Sci. Technol., 76, 270-277. https://doi.org/10.1016 /j.lwt.2016.07.046
Santi EN, Murdianto W, Ahmadi NR, Sulistyaningrum A (2022). Physicochemical Characteristics of Three Local Sweet Potato Flour from East Kalimantan. In IOP Conference Series: Earth and Environ. Sci., 1024: 012037. IOP Publishing.
Sivakumar N, Kalaiarasu S (2010). Microbiological approach of curd samples collected from different locations of Tamilnadu India. Int. J. Curr. Res, 2: 27-30.
Sujata, Dubey KK, Raj T, Kumar P (2022). Pathogenic microorganisms in milk: their source, hazardous role and identification. In Advances in Dairy Microbial Products (pp. 145-161). Woodhead Publishing. https://doi.org/10.1016/B978-0-323-85793-2.00005-9
Tomovska J, Gjorgievski N, Makarijoski B (2016). Examination of pH, titratable acidity and antioxidant activity in fermented milk, journal of materials science and engineering. J. Mater. Sci. Eng., 6(11): 326-333. https://doi.org10.17265/2161-6213/2016.11-12.006
Wakhidah N, Jati G, dan M, Utami R (2017). Yoghurt susu sapi segar dengan penambahan ekstrak ampas jahe dari detilasi minyak atsiri. Proceeding Biol. 12 Education Conference, 14: 278-284.
Wang T, Ye Z, Liu S, Yang Y, Dong J, Wang K, Liu D (2021). Effects of crude Sphallerocarpus gracilis polysaccharides as potential prebiotics on acidifying activity and growth of probiotics in fermented milk. LWT, 149: 111882. https://doi.o rg/10.1016/j.lwt.2021.111882
WHO/FHO (2001). Health and Nutritional Properties of Probiotics in Food Including Power of Milk with Live Lactic Acid Bacteria. Procedings of the FAO/WHO Expert Consultation On Evalution of Health and Nutritional Properties of Probiotic in Food Including Power of Milk With Live Lactid Acid Bacteria. Cordoba, Argentina
Winardi RR, Prasetyo HA (2020). Perubahan komposisi kimia dan aktivitas antioksidan pada pembuatan tepung dan cake ubi jalar ungu (Ipomoea batatas L.). Agrica Ekstensia, 14(1).
Wu TY, Tsai CC, Hwang YT, Chiu TH (2012). Effect of antioxidant activity and functional properties of chingshey purple sweet potato fermented milk by Lactobacillus acidophilus, L. delbrueckii subsp. lactis, and L. gasseri Strains. J. Food Sci., 77(1): M2-M8. https://doi.org/10.1111/j.1750-3841.2011.02507.x
Yuliansar Y, Ridwan R, Hermawati H (2020). Karakterisasi pati ubi jalar putih, orange, dan ungu. Jurnal Saintis, 1(2): 1-13.
Zaib S, Hayat A, Khan I (2024). Probiotics and their beneficial health effects. Mini Rev. Med. Chem., 24(1): 110-125. http://dx.doi.org/10.2174/138955 7523666230608163823
To share on other social networks, click on any share button. What are these?






