Spermicidal Activity of Gold Nanoparticles on Male Mice in Vitro
Research Article
Spermicidal Activity of Gold Nanoparticles on Male Mice in Vitro
Heba Ali Salih*, Mohanad A. Al-Bayati
Department Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | The effect of Gold Nanoparticles (GNPs) on sperm concentration and motility was studied in the acute toxicity of Gold nanoparticles on semen suspensions using MTT, revealing an increase in sperm death markers with rising Gold nanoparticles concentrations. The lethal concentrations of Gold nanoparticles were determined, with LC50 and LC95 found to be 176.48 and 349.63 respectively. The study examined sperm motility, showing a significant variation at the onset of recording motile sperm, suggesting that sperm concentration was dependent on turbidity. There was a positive correlation between the delay at the start of absorbance recording and Gold nanoparticle exposure concentrations. However, the overall population of motile sperm showed a negative correlation with increased Gold nanoparticle concentration. The Gold nanoparticle concentrations effectively hindered the progressive motility of sperm. Lag time results indicated decreased time values with increased Gold nanoparticle concentrations, suggesting a negative prognosis. The velocity of sperm within a semen suspension exhibited a marked decrease as the concentration of gold nanoparticles increased. Concurrently, The Fraction of Rapidly Moving Sperm was reduced with increasing Gold nanoparticle concentrations, indicating a negative correlation.The motility index of sperm was notably influenced by the presence of gold nanoparticles, exhibiting a pronounced decline as the concentration of these nanoparticles escalated. The study investigated the impact of Gold nanoparticles on DNA abnormalities. The concentration of Gold nanoparticles increased; there was an increase in histone residues and abnormal DNA chromatin, indicating a concentration-dependent relationship. These findings highlight the potential deleterious effects of Gold Nanoparticles on sperm motility and DNA integrity. The study concluded that Gold nanoparticles affect sperm concentration and motility, with increased Gold nanoparticle concentrations leading to higher sperm death markers and reduced motility. An observed correlation exists between the concentrations of gold nanoparticles and the incidence of DNA abnormalities; elevated levels of gold nanoparticles are associated with an increase in histone residues and anomalies in DNA chromatin structure.
Keywords | Gold nanoparticles, Sperm, MTT, LD50, Acute toxicity, Lag time, Motility index, DNA, Acridine, X-ray diffraction, Alanine blue
Received | May 13, 2024; Accepted | June 10, 2024; Published | December 31, 2024
*Correspondence | Heba Ali Salih, Mohanad A. Al-Bayati, Department Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Baghdad, Iraq; Email: hiba.ali2206m@covm.uobaghdad.edu.iq, aumnmumu@covm.uobaghdad.edu.iq
Citation | Salih HA, Al-Bayati MA (2025). Spermicidal activity of gold nanoparticles on male mice in vitro. Adv. Anim. Vet. Sci. 13(1): 176-188.
DOI | https://dx.doi.org/10.17582/journal.aavs/2025/13.176.188
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
A spermicide is a chemical substance revealed to destroy or immobilize sperm in the vagina and act as a contraceptive effect on the female genital tract. Thus, the spermicide mode of action ranged between disruption and distorting sperm physiology activity in topical application, as well as causing sperm mortality in the vaginal route, which prevents fertilizing the ovum (Hifnawy et al., 2021).
The problematic issue with conventional contraceptive methods lies in their reliance on hormonal manipulation, which disrupts physiological hormonal equilibrium and may signal various systemic conditions. These methods can interfere with normal ovum production and induce substantial alterations in the endometrial lining. all the pharmaceutics surveyed to be used as plane infertility inducers in male gametes or as spermicides into the vagina-cervical canal were not sufficiently efficient, in addition to not having complete safety due to low selective toxicity or low efficiency as a barrier with their adverse effect outcomes in female (Hifnawy et al., 2021). Moreover, a reduction in testosterone production (Liu et al., 2020) was observed. Regrettably, the reversibility of GNP-induced reproductive toxicity was not explored in those particular studies. For this reason, Gold nanoparticles were chosen and challenged due to their capability of penetrating inside the cells and combining with DNA molecules due to their extremely small sizes and unique physical and chemical properties sperm quality and quantity may interfere. Several Notable Research on the ability of GNPs with different sizes in penetration into the DNA molecule and positioning in DNA large grooves has been shown (Appasamy et al., 2007). This evidence that certain nanoparticles exhibit spermiotoxic properties, potentially inhibiting sperm motility and hindering sperm chromatin decondensation (Wiwanitkit et al., 2009). This research paves the way for the development of effective spermicides in animals and the exploration of targeted actions as concentrations change. The study aimed to modify GNP activity on semen suspension and reset the efficiency concentrations of prepared heparinized Gold nanoparticles as a spermicidal agent.
MATERIALS AND METHODS
The design, technique, and protocols were based on the usage and care of laboratory animals in the teaching guide and research (AUCAC) (Al-Bayati and Khamas., 2015) and were documented by the Graduated approval system of Iraq’s College of Veterinary Medicine and approved by document report No. 2336 and dated 14/12/2023: University of Baghdad - College of Veterinary Medicine.
Preparation and Standardization of Heparinized Gold Nanoparticles
Several steps were provided as a sequence step for the achievement capping initiator for synthesis GNPs and stabilizing the preparative methodology was run as flow steps (Vidya and Saji, 2018). The stock solution of AuCl4 was processed by dissolving 0.67g of Auric chloride in 100 ml deionized water to achieve 0.02M expressed as 0.67%. Auric chloride 1ml was transferred to a conical flask (50ml) and mixed with 4.5ml of deionized water 0.148 %. The working diluted AuCI4 5.5 ml was boiled at 95C° for 45 minutes with stirring at 500 rpm/ min on the magnetic stirred-hot plate. Heparin 10ml (5000 IU) was dropped on boiled Auric chloride gradually; to prevent clumping, with stirrings until the yellow solution became a red-brown Nanoparticles solution (Vidya and Saji, 2018). Forming Gold nanoparticles were stabilized by adding Hydrochloride acid 1ml, 1n for stabilizing and increasing the reducing rate (King et al., 2015).
Standardization of GNP
The physicochemical properties of the Nano-gold particles were characterized using various techniques. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) were employed at the Alkhora company’s Nano research lab. Dynamic light scattering (DLS) and zeta potential measurements were performed at the Ministry of Science and Technology laboratory, while atomic force microscopy (AFM) analysis was conducted at the Chemistry Analysis Centre.
Experimental Design and Protocol
The thirty mice semen suspension was grouped first as the control group, and second Gold Nanoparticles (GNP) group: The treated GNP groups were divided into five subgroups according to exposure concentrations of Heparinized-Gold Nanoparticles in semen suspension and set as follows: 100, 150, 200, 250, and 300 µg/ml modified from (Batool et al., 2022), for achieved Log concentrations Response curve.
Animal
The mice were obtained from the Industrial Development Al-Razi Center for Research and Medical Diagnostic Kits productions animal house: Corporation for Research and Industry Development, Ministry of Industry and Minerals, the mice were left for one week for adaptation, and supplied free tap water and ad libidum concentrated meal, the room was controlled temperature (25±2 Cº), humidity and ventilation.
Sperm Suspension Preparation
The male mouse was euthanized via inhalation of diethyl ether. It was necropsied for the excision of the epididymis (Martinez, 2022). The cauda of the epididymis was separated and The caudal of the epididymis was weighed. each cauda epididymis was placed in 0.5 ml of Dulbecco’s Modified Eagle Medium (DMEM) at 37 C° in a pre-warmed glass watch. and then minced 200 times with microsurgical scissors (Abd-alhussein and Al-Bayati, 2021). Before proceeding with the experimental protocols, the individual motility of the stock semen suspension for each mouse was checked to confirm the healthy functioning of the sperm (Hadi, 2013a). Sperm concentration and viability were assessed using a standard curve generated by serial dilutions of sperm suspensions in Dulbecco’s Modified Eagle Medium (DMEM). Aliquots containing 20, 30, 40, 50, and 60% of the calculated sperm count (determined using a hemocytometer) were prepared. Absorbance was then measured at a wavelength of 450 nm using a spectrophotometer. then blotted best-fitted line versus concentrations (Brito et al., 2016). As well as The determination of viable sperm percentages at dilutions of semen suspension 2x102, 2x103, 2x104, 2x105 and 2x106 per ml. A 50 trypan blue was added to 0.1 ml of each concentration of semen and examined the slide under a light microscope at 100 X magnification. Then semen suspension was checked MTT, the MTT method was placing 0.1 ml of semen of each concentration on the microliter plate well, and then 0.1 of MTT was left mixture for 15 minutes at room temperature and the color-forming was estimated at wavelength 450 nm in spectrophotometers. The viable sperm was plotted with absorbance values for each concentration of semen suspension as a calibrated curve of viability (Strober, 2015).
Acute Toxicity (Lcs) on Mice Semen Suspension
According to (Finney, 1971) the acute toxicity of Gold Nanoparticles in semen suspension 2×106 sperm/ml was determined by the calculation of LCs (LC50 and LC95) by determining via MTT on 0.1 ml GNPs concentrations 0, 100, 150, 200, 250, and 300 ug/ml. The semen reaction was diluted with 1.7ml normal saline and transferred to a cuvette for determination of the absorbance at wavelength 450 nm.
Turbidimetric Analysis of Sperms Motility
According to (Sakoloski et al., 1977; Al-Bayaty, 1999), the spectroscopic Turbidiametric method of sperm motility analysis was used to describe the motility indices; this approach was assessed as follows: a masked cuvette narrow window containing 1 ml of normal saline was filled with a 50 µl of diluted semen suspensions. Sperm motility was assessed using spectrophotometry. The motility of sperm was recorded as sperm kinetic and traced curve absorbance versus time for each GNP-treated semen suspension and control. The kinetic parameters of motility were determined as follows (Figure 1). Log-transformed dose concentrations were plotted against motility indices. Half-maximal effective concentration (EC₅₀) and the maximal effect (Emax) were calculated using a fitted curve, following the method described by (Al-Bayaty, 1999). The parameters of sperm motility analysis were:
Lag Time (Sec.): The lag time refers to the time between injecting diluted semen suspension and the first rise in absorbance, indicating the active injection port. The quickest fraction of sperm to exit the sample is 4 mm in our equipment, with a bottom window of 1 cm (Hadi, 2013b).
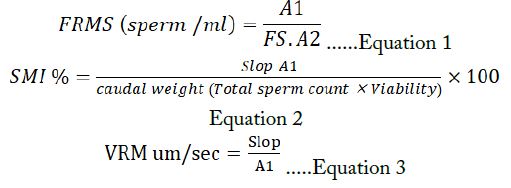
Fraction of Rabidly Moving Sperm (FRMS): The percentage of fast-traveling sperm was discovered by Equation 1.
Sperm Motility Index (SMI) %: (Ibrahim and Al-Bayati, 2014) the total motile of all types of wave’s motility in semen samples of whole total viable sperm percent Equation 2.
The velocity of Fraction Rapidly Moving Sperm (FRMS): According to (Al-sabaawy et al., 2020) the speed of sperm per unite time of elite rapidly moving sperm was determined via Equation 3.
DNA Abnormality: The DNA abnormality test for assessment of chromatin quality via different vital staining methods according to (Talebi et al., 2007; Talebi et al., 2011) and explored 1. DNA Integrity and Condensation: Toluidine Blue Dye (TB) 2. Abnormal Sperm DNA: Acridine Orange (AO) 3. Histone Residue: Aniline Blue (AB).
Statical Analysis
Data were analyzed for mean and standard error. One-way and two-way analysis of variance (ANOVA) with F-test was used to identify statistically significant differences. The least significant difference (LSD) post-hoc test was employed to determine specific group differences. Dose-response curves were generated using Microsoft Excel 2010. The best-fit line was calculated for linear and quadratic equations. Lethal concentration (LC) values were determined using the Probit method within Microsoft Excel 2010, as described by (Steel and Torrie, 1981).
RESULTS AND DISCUSSIONS
Standardizations OF Heparinized Gold Nanoparticles
Gold nanoparticles synthesized the color change from yellow to red-brown after 45 minutes at boiled degree 95 C° depicted in Figure 2.
Scanning Electron Microscope (SEM): The morphology and size of Gold nanoparticles in Figure 3, showed the heparinized GNP shape as a cubic cage box.
X-Ray Diffraction (XRD): The X-ray diffraction was performed to establish conformation of the GNP composition with the presence of other elements as well as the crystalline nature of heparin-GNPs the pattern of analyzed GNP showed diffraction peaks were recorded from 15° to 75° at 2 theta angles. the diffraction peaks 23.31°, 27°, 31.30°, 53.12°, 55.89° and 74.30° as a reflection of metallic Gold The diffraction peaks of all peaks corresponded to the (177), (445), (405), (145), (298) and (331) lattice planes in Figure 4.
Zeta Potential: The colloidal stability of GNPs was assessed using zeta potential to evaluate their surface charge. The analysis revealed a surface charge of -17.09 mV on the GNPs, indicating sufficient electrostatic repulsion for stability. A decrease in zeta potential is observed in Figure 5.
Atomic Force Microscopy (AFM): Analysis was used to determine the surface morphology of Gold nanoparticles. GNPs showed heterogeneity of shape GNPs with a thickness range from 5.809 nm to 67.62 nm had a surface cubic to triangular Nanoparticles shape had a surface distance of 11.61 nm and vertical distance of 9 nm as well as a fraction number of size (Figure 6).
Gold Nanoparticles Lethal Concentrations of Sperm
The acute toxicity of Gold nanoparticle concentrations (GNPs) challenged by serial concentrations on semen suspensions (2×106) with serial concentrations of GNPs were tested by MTT, the graduation of GNPs increment concentrations 100, 150, 200, 250, 300 µg/ml exhibited increased the percentage of MTT absorbance value indicative to increase sperms death markers 1.4654, 1.4854, 1.5568, 1.5754 and 1.582 respectively. The dose-response relationship between GNP concentrations and Probit lethal responses exhibited a sigmoidal curve with a lower plateau, characteristic of threshold responses (Figure 7). The median lethal concentration (LC₅₀) and lethal concentration for 95% mortality (LC₉₅) of GNPs were 176.48 and 349.63, respectively.
Sperm Motility Analysis
Figure 8 presents a schematic representation of the Turbidiametric analysis of sperm motility. The experiment involved groups treated with varying concentrations of (GNP) - Control, 100, 150, 200, 250, and 300 µg/ml. Changes in sperm suspension absorbance over time exhibited a positive correlation with motile sperm density, which was recorded over time, showed an upward trend corresponding to the density of motile spermatozoa. The overall sperm curve indicated a significant variation (p≤0.05) at the onset of recording motile sperm, suggesting that sperm concentration was dependent on turbidity. There was a significant positive correlation (p≤0.05) between the delay at the start of absorbance recording and GNP exposure concentrations. Moreover, the motility slopes deviated and shifted to the left, depending on the GNP concentrations. This shift was more pronounced in certain treatment groups, showing a negative correlation between the overall population of motile sperm and increased GNP concentration. The final analysis of sperm motility focused on two specific concentrations of treated semen: 250 µg/mL and 300 µg/mL. These concentrations effectively hindered the progressive motility of sperm during the screening process.
LAG Time
The quantitative linear movement of ascending upward of progressive sperm achieved through Lag time results referred to decreased time values with increased concentration of GNPs as a negative prognosis significant (p≤0.05) as compared with control and between GNP treated semen suspension groups followed increased to GNP concentrations attributed to dependent on initiation of fraction rapidly moving sperm progressive motility waves in (Figure 9) and displayed Lag time 0.9±0.005, 2.5±0.03, 4.5±0.06, 5.1±0.34, 0±0, 0±0 minutes for control and exposure concentration,100, 150, 200, 250, and 300 µg/ml respectively, which significant (p≤0.05) increase as increase concentration of GNPs (dose-dependent). The GNP ECs of Lag time ECmax = 277.9.
The Velocity of the Fraction of Rapidly Moving Sperm µm\sec
Sperm velocity in the semen suspension exhibited a typical decline with increasing concentrations of GNPs (Figure 10). This figure illustrates the relationship between sperm velocity of mice semen suspension and exposure to increasing concentrations of GNPs (0, 100, 150, 200, 250, and 300 µg/mL). The percentage of the velocity of the fraction of rapidly moving sperm for each GNP concentration is presented (0.231±0.021, 0.155±0.006, 0.133±0.002, 0.128±0.008, 0±0 and 0±0 µm/sec) significant (p≤0.05) reduction as compared with control significant and EC50 =15.84 µg/ml EC max = 245.47 µg/ml.
Fraction of Rapidly Moving Sperm (FRMS)
The FRMS ×106 per ml in the figure represented the elite sperm having a high-velocity fraction of sperm. (Figure 11) showed and displayed the negative correlation between FRMS and Gold Nanoparticles significant (p≤0.05) reduction of FRMS at concentrations 100, 150, 200, 250, and 300 µg/ml as compared with control semen suspension. The FRMS for each concentration of GNPs (0.017±0.0001, 0.003±0.0005, 0.0004±0.00006, 0.0001±0.000009, 0±0 and 0±0 sperm/ml) and ECmax = 194.98.
Motility Index
The percentages of sperm motility index in (Figure 12) were intensively affected by GNPs and sensitive concentration changes, the control group sperm motility index and GNP-treated semen suspension were (55.46±5.82, 21.05±2.04, 8.11±1.21, 1.16±0.19, 0±0, and 0±0). The motility index was steeply decreased with an increase in GNPs at a concentration of 100 µg/ml, then fast regress with increased concentrations at 150 and 200 µg/ml significant (p ≤0.05) and EC50 =19.49, EC max = 345.8
DNA Abnormality: Histone Residues (Alanine Blue Stain)
Figure 13 investigates the concentration-dependent effects of GNPs on histone residue formation, as assessed by staining with Alanine Blue. The results demonstrate a positive correlation between GNP concentration and the observed defects. Higher GNP concentrations corresponded to a greater degree of disruption, as evidenced by the non-linear fitted line relationship. This effect was described as concentration-dependent and exhibited an aggressive deleterious impact at high concentrations. The experimental concentrations of GNPs tested were 0, 100, 150, 200, 250, and 300 µg/ml, corresponding to histone residue percentages of 1.5±0.02, 16.3±2.75, 22.33±1.42, 30.33±2.22, 34.66±4.05, and 47.33±3.81, respectively (p>0.05). These findings highlight the concentration-dependent and deleterious effects of GNPs on histone residues, Figure 14. The EC50= 152.4 ±0.185 µg/ml and EC max= 331.89 ± 9.026 µg/ml.
The Integrity of Chromatin and DNA Condensation (Toluidine Blue Stain)
Figure 13 the observed result of abnormal DNA chromatin with an increased concentration of GNP, the concentrations tested of GNP were 0, 100, 150, 200, 250, and 300 µg/ml, and the result of abnormal DNA chromatin was 1.01±0.003, 29.22±0.04, 34.33±3.58, 69.32±5.21, 65.66±5.92 and 69.54±4.92 ), the two end concentrations displayed no significant in DNA abnormality values (p>0.05), Figure 15 depicts the relationship between increasing GNP concentration (logarithmic scale) and abnormal DNA chromatin formation. The data reveal a sigmoidal dose-response curve, with a half-maximal effective concentration (EC₅₀) of 119.6 ± 0.025 µg/mL and a max imum effect (ECmax) of 264.8 ± 12.542 µg/ml.
Abnormal Sperm DNA (Acridine Orange)
Acridine orange staining, as illustrated in Figure 13, revealed a significant increase in abnormal sperm DNA with increasing concentrations of GNPs. The experiment employed five GNP concentrations ranging from 0 µg/mL to 300 µg/ml, while the corresponding abnormal sperm DNA levels were measured as percentages 4.59±0.05, 59.10±6.83, 58.15±6.82, 61.04±5.92, 62.72±4.60, and 60.54±3.97, respectively, the third end concentrations displayed no significant in DNA abnormality values (p>0.05). The results indicate a notable relationship between the increase in abnormal DNA and the logarithmic increase in GNP concentrations Figure 16 The EC50= 13 ±0.025 µg/ml and EC max= 188.36 ± 10.638 µg/ml.
The red-brown (wine color) Gold Nanoparticle’s suspension appearance was attributed to size-dependent color reported the size of Gold Nanoparticles (Gadge et al., 2020; Sangwan and Seth, 2022) at less than 100 nm was given dark reddish or brown-reddish color agreed with (Vidya and Saji, 2018). The Scan EM GNP result agreed with (Oliveira et al., 2023), The Scan EM exerted cubic GNPs from that was synthesized due to the method depending on polyol reduction for synthesized Cub Nano metal, as well as capping of as acidifier HCl media, which occurs the replacement of galvanic may be between heparin and AuCl4 was matching this synthesized to three steps morphological structure changes cub Au first initial Au dissolution at sharp corner second bulk dissolution of Au interior through the initial site, third forming of Nano for sharp cub (Xia et al., 2011). The result of XRD The outcome is attributed to the phenomenon where X-rays are specifically targeted at the atoms within the specimen, resulting in their dispersion and the creation of a diffraction pattern, which can be utilized to ascertain the crystal structure and organizational phase of the substance. The presence of peaks in an X-ray diffraction (XRD) pattern suggests the existence of a structured crystalline form in the specimen that agrees with (Roddu et al., 2020). The emergence of six distinct peaks in the XRD pattern related to Gold Nanoparticles (Anbu et al., 2020).
The quantified surface charge zeta potential provides net charges of GNP surface area which facilitates surface absorption of GNPs in an electric double layer of diffusion that develops the fact of absorption of the negative charge of zeta potential that prevents neutralization with the membrane and gives non-stability with membrane negatively charged ions. That was attributed to the accumulation of GNPs on the outer layer of the membrane without penetration in higher concentrations, which considered immobilized ions and allowed diffused according to influence opposite electrostatic force, which considered defined of electrostatic potential with a biological agent and reliable suggestion of a pinocytosis route than other routes (Wang et al., 2016). The AFM showed the heterogeneity of shape GNPs with thickness range from 5.809 nm to 67.62 nm had a surface cubic to triangular Nanoparticles shape had a surface distance of 11.61 nm and vertical distance of 9 nm as well as the fraction number of size, all these were give displayed small size was highly fraction portion GNPs by heparinized methods it useful to uniform their function and that facilitated the binding of membrane and conjugated with membrane protein (Kumar et al., 2008). The median lethal concentration (LC₅₀) determined by the MTT assay, which reflects sperm viability, was used to assess the lethality of GNPs in semen, the toxic mechanisms of GNP on cellular levels presumably had been elicited to adhere to cell membranes (Ghitescu and Fixman, 1984) and be endocytosis gulped by cells (Parak et al., 2002). The breaching of the sperm cell membrane with the intracellular compartment storage may presumably have a dual mode negative charge and passive consequence on the sperm cells regardless of the toxic-cellularity of the GNPs and their succeeding functionality (Vijayakumar and Ganesan, 2012).
The dominance of acute toxicity escalated in the semen suspension viability exposed to treated in vitro with GNP concentrations, this was particularly noticeable at the LC95 level, where the highest toxicity was observed. As the concentration of Nanoparticles increased, which was a corresponding gradual increase in sperm, mortality (sperm viability) was marked via MTT. This result agrees with in vitro toxicity was agreed also with MTT assay in a healthy human cell line (HaCat) and human cancer cell lines (A375, MCF-7, Bx-PC3), GNP concentrations (100, 300, and 600 µM) resulted in a loss of cell viability (Echeverry-Rendón et al., 2022; Rosłon et al., 2019), may be due to the acute toxicity of GNPs on semen suspensions, which may be due to attributed to the impairment of various metabolic pathways and DNA damage (Gutiérrez et al., 2017). The outcomes of the LC95 for sperm exposed to GNPs were attributed to the LC95 of GNP effect on the increase in DNA abnormalities. This is followed by defective motility, which may provide a rational explanation for the observed increase in LC95 concentration Figure17, whether it be cumulative or singular causes. Furthermore, the effects presumably stem from various factors, suggesting different toxic modulated mechanisms of GNP action on semen. This initiates and progressively increases the percentage of dead sperm (Nazari et al., 2016; Zakhidov et al., 2010; Xiao et al., 2020).
The internalization of GNPs within cells can potentially alter their stability and permeability due to interactions with the cellular environment, including the cell membrane. Cationic nanoparticles, with their positive charge, tend to exhibit a higher affinity for negatively charged cell membranes, facilitating their uptake even by non-targeted cells (Ferreira-Gonçalves et al., 2021; Singh et al., 2019). The Cage rectangular, hexagonal, and prism forms of GNPs result in a differentiated distribution of Gold atoms on to the cellular surface, with more edged and angular Nanoparticles endorsing greater reactivity, but it differs the spherical form being more efficiently internalized by cells (Kus-Liśkiewicz et al., 2021; Jia et al., 2017; Behzadi et al., 2017; Didamson et al., 2022). The concept of the surface area of GNPs is an essential element as Pro-oxidants in their tense toxicity due to their interaction with biological systems (Choi and Hu, 2008; Ringsted and Todeschini, 2012). ROS formation causes oxidative stress, inflammation, and consequent damage to the vital proteins, cell membrane, and DNA as well as advanced effects involving mitochondrial respiration and inactivation of NADPH-dependent enzyme systems (Daoud, 2007; Regoli and Giuliani, 2014; Chen et al., 2011; Taha and MJ Ali, 2015). The Lag time term indicates to selection time of moving sperm to reach the light-estimated peak turbidity reflected light referring in biology to the time required from sperm deposit to migration to the fertilizing area (Sokoloski et al., 1977). The period depends on the velocity of sperm rapidly moving sperm and a detectable number of motile speedy sperm. The delay of Lag time as concentration-dependent was presumably attributed to the reduction of sperm velocity (Sokoloski et al., 1977).
The velocity of the fraction of rapidly moving sperm depends on several vital processes due to the direct effect GNPs on sperm structure or metabolic direct or indirect by deprived ATP demined may be through decrease eventually impaired metabolic pathway (Li et al., 2023). The reduction in VRMS presumably directly causes Loss of fluidity and elasticity of the sperm’s outer structure, disposal of the tail axial fiber structure of kinetic motion of sperm, and loss of partial regular vigorous forward movement. As well as the GNP reduced activity of GNP on the sperm tail limiting mitochondria directly through translocation transport and Plasmon biocompatibility. That presumably led to reduced metabolic activity reduction of ATP demined (Xiao et al., 2020).
The fact of indirect defective mechanisms affected by GNP including free radical dominancy may be due to i. Oxidant-generating properties of particles themselves as well as their ability to stimulate the generation of ROS as a part of cellular response to Nanoparticles ii. Transition metal-based Nanoparticles or transition metal contaminants are used as catalysts during the production of non-metal Nanoparticles. iii. Relatively stable free radical intermediates are present on reactive surfaces of particles. iv. Redox-active groups result from the functionalization of nanoparticles (Alani et al., 2011; Fard et al., 2015). The FRMS may be affected due to the direct effect of GNP on spermatozoa membrane and their structures suggesting the importance of structural membranous ionic regulatory action potential and their control of Ca++channels, which is essential for sperm motility. The Ca2+ permeation pathway in sperm motility is essential for mammalian fertilization, their report attributed a candidate sperm cation channel, Cat Sper, that has an amino-acid sequence similar to a single. Sperm motility is significantly reduced in CatSper blocker in mice. Moreover, the cAMP-induced Ca2+ influx is eliminated in the sperm of blocked this channel. Therefore, Cat Sper is crucial for cAMP-mediated Ca2+ influx in sperm, and sperm motility (Ren et al., 2001).
The findings of SMI results were consistent with those of (Wiwanitkit et al., 2009) observed that Gold Nanoparticles exerted dose-dependent effects on sperm motility in their study on donor sperm. Similarly, found that both silver and Gold Nanoparticles influenced sperm motility in a dose-dependent manner (Moretti et al., 2013). It is agreed to highlight that research focusing on the impact of gold nanoparticles on the male reproductive system is scarce. The result of sperm motility index at exposure to GNP concentrations decreased sperm motility this result agrees with (Nazari et al., 2016). While reaching the end two concentrations of Gold Nanoparticles (250, 300 µg/ml) presented absent sperm motility and attributed the effect of GNPs on the motility of sperm to numerous causes, including defect of cell membrane integrity. That led to decreased fluidity and elasticity, as well as the presumably causal of GNP exerted via the GNP have high affinity to free thiol on sperm surface aggregation that causes a massive loss of motility. The thiol is a component of membrane-bound Na+/k+-ATPase and Ca+-ATPase, which were major responsible for the initiation and demined of sperm motility (Hanon et al., 2022; Taylor et al., 2014).
An increased concentration of Gold Nanoparticles led to a significantly increased (p≤0.05) abnormal percentage of Histone residues as compared with the group of control semen suspension. The GNP-treated semen suspension groups increased the histone defect as concentrations dependent on these results agreed with (Nazari et al., 2016). The protamine-like proteins that under normal conditions played an important role in the condensation and stabilization of gamete’s nuclear chromatin. The results of toluidine blue were denoted to abnormal chromatin and referred to the decondensation pattern of the nucleosome, which means a reduced number of protamines, resulting in an effect on sperm genetic sources (Zakhidov et al., 2010). In addition, the rate of abnormal chromatin condensation was increased when exposure sperm was to high concentrations of Gold Nanoparticles significantly (p≤0.05) at the two-end concentration of Gold Nanoparticles (250 and 300 µg/ml) displayed no significance in DNA abnormality value (p>0.05) (Zakhidov et al., 2010). The results noticed in this outcome of stain were a positive correlation with an increased Gold nanoparticle concentrations significantly (p≤0.05) reflex to the increased rate of abnormal DNA. That includes DNA fragmentation, and chromatin decondensation due to maybe decreased protamine confirmation and turnover and increased rate of histone residue, these results of presumably the DNA, GNPs can directly interact with DNA and cause DNA fragmentation. That is the breaking of DNA strands into smaller pieces GNPs can induce chromatin decondensation, it is loose of the tightly packed DNA-protein complex that forms the chromosomes. This can result from the alteration of protamine and histone proteins, which are responsible for maintaining the stability and compaction of chromatin (Nazari et al., 2016; Wang et al., 2017).
CONCLUSIONS AND RECOMMENDATIONS
The conclusion The study successfully prepared heparinized Gold nanoparticles (GNP) and tested them in both in vitro and in vivo experiments. These GNPs, synthesized particularly in an acidic environment with heparin, formed a cubic–cubic cage shape and were less than 100 nm in size. They exhibited stability, crystalline properties, and homogeneous surface roughness. In the in vitro experiment, the GNPs demonstrated spermicidal efficacy of more than 300 µg/ml. They affected sperm motility and limited the proportion of rapidly moving sperm at concentrations less than LC values. The GNPs also induced DNA abnormalities with higher efficacy than other vital functional motivations. These findings suggest that the GNPs, when used in Gel GNP vaginal spermicides, could minimize vaginal pathological deterioration, making them a potential candidate for contraception.
ACKNOWLEDGEMENTS
The authors are thankful to the College of Veterinary Medicine /University of Baghdad for their assistance in conducting this research.
NOVELTY STATEMENT
The novelty of the research is focused on the toxicity of gold nanoparticles leading to induced the toxicity effects on sperm mice in vitro.
AUTHOR’S CONTRIBUTION
These researchers each contributed equally to this research.
Conflict of Interest
There is no conflict of interest.
REFERENCES
Abd-alhussein FM, Al-Bayati MA (2021). THE REPRODUCTIVE EFFECT OF LIPOSOMAL CIMETIDINE ON MALE MICE. Plant Arch., 21(1): 757–769. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.114
Alani G, Khonda S, El Yaseen H (2011). Analysis of DNA damage and oxidative stress in human spermatozoa and some biochemical changes in seminal plasma and their correlation with semen quality of infertile men.The Iraqi Postgrad. Med. J.,10(1): 81– 88.
Al-Bayati MA, Khamas W, Al America B (2015). The Importance to Implement and Enforce of Standardized Guidelines For The Care And Use Of Laboratory Animals in Research And Teaching In Iraqi Scientific Institutions. TOFIQ J. Med. Sci.., 2(1): 34 – 43.
Al Bayaty MA, (1999). The Reproductive Effects of Cottonseeds Extract on male mice. PHD Thesis in Pharmacology. College of Veterinary Medicine, university of Baghdad.
Al-sabaawy HB, Al-kaisie BI, Diseases P (2020). Effects of Sub Lethal Concentrations of Sodium Fluoride on Sperm Activity and on the Level of Sex Hormones of Adult Male Albino Rats.The Iraqi J. Vet. Med., 44(2): 92– 98. https://doi.org/10.30539/ijvm.v44i2.980
Anbu P, Gopinath SCB, Jayanthi S (2020). Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomaterials Nanotechnol.,10: 1847980420961697. https://doi.org/10.1177/1847980420961697
Appasamy M, Muttukrishna S, Pizzey AR, Ozturk O, Groome NP, Serhal P, Jauniaux E (2007). Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reproductive Biomedicine Online, 14(2): 159 –165. https://doi.org/10.1016/S1472-6483(10)60783-3
Batool Z, Muhammad G, Iqbal MM, Aslam MS, Raza MA, Sajjad N, Abdullah M, Akhtar N, Syed A, Elgorban AM (2022). Hydrogel assisted synthesis of gold nanoparticles with enhanced microbicidal and in vivo wound healing potential. Sci. Rep., 12(1): 6575. https://doi.org/10.1038/s41598-022-10495-3
Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad, OC, Mahmoudi M (2017). Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev., 46(14): 4218–4244. https://doi.org/10.1039/C6CS00636A
Brito LFC, Althouse GC, Aurich C, Chenoweth PJ, Eilts BE, Love CC, Luvoni GC, Mitchell JR, Peter AT, Pugh DG (2016). Andrology laboratory review: Evaluation of sperm concentration. Theriogenology, 85(9): 1507–1527. https://doi.org/10.1016/j.theriogenology.2016.01.002
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011). Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants and Redox Signaling, 14(8): 1505–1517. https://doi.org/10.1089/ars.2010.3576 https://doi.org/10.1089/ars.2010.3576
Choi O, Hu Z (2008). Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol., 42(12): 4583–4588. https://doi.org/10.1021/es703238h
Daoud MS (2007). DNA Content of Human Spermatozoa with Respect To Sperm Morphology. Iraqi Postgrad. Med. J., 6(2):136-140
Didamson OC, Chandran R, Abrahamse H (2022). A Gold Nanoparticle Bioconjugate Delivery System for Active Targeted Photodynamic Therapy of Cancer and Cancer Stem Cells. Cancers,14(19): 4558. https://doi.org/10.3390/cancers14194558
Echeverry-Rendón M, Stančič B, Muizer K, Duque V, Calderon DJ, Echeverria F, Harmsen MC (2022). Cytotoxicity Assessment of Surface-Modified Magnesium Hydroxide Nanoparticles. ACS Omega., 7(21): 17528–17537. https://doi.org/10.1021/acsomega.1c06515
Fard JK, Jafari S, Eghbal MA (2015). A review of molecular mechanisms involved in toxicity of nanoparticles. Adv. Pharm. Bull., 5(4): 447– 454. https://doi.org/10.15171/apb.2015.061
Ferreira-Gonçalves T, Ferreira D, Ferreira HA, Reis CP (2021). Nanogold-based materials in medicine: From their origins to their future. Nanomedicine, 16(30): 2695–2723. https://doi.org/10.2217/nnm-2021-0265
Finney DJ (1971). Statistical logic in the monitoring of reactions to therapeutic drugs. Methods Inf. Med., 10(04): 237–245. https://doi.org/10.1055/s-0038-1636052
Gadge AN, Wadher SJ, Landge AD (2020). Gold Nanoparticle. Int. J. Sci. Healthc. Res., 5(2): 21–35.
Ghitescu L, Fixman A (1984). Surface charge distribution on the endothelial cell of liver sinusoids. J. Cell Biol., 99(2): 639–647. https://doi.org/10.1083/jcb.99.2.639
Gutiérrez L, Stepien G, Gutiérrez L, Pérez-Hernández M, Pardo J, Pardo J, Grazú V, De La Fuente J M (2017). Nanotechnology in drug discovery and development. InElsevier eBooks, pp.264–295. https://doi.org/10.1016/B978-0-12-409547-2.12292-9
Hanon MS, Mazhir SN, Al-Ahmed HI, Haddad RA (2022). Influence of Non-Thermal Plasma (DBD) on Infertility Male Semen with Low Sperm Motility and Dna Damage. Iraqi J. Sci., 63(4): 1491–1497. https://doi.org/10.24996/ijs.2022.63.4.9
Hadi IH (2013a). Effect of Cryopreservation on Sperms Function Parameters and In vitro Fertilization Rate in Mice. The Iraqi J. Vet. Med., 37(2): 218–225. https://doi.org/10.30539/ijvm.v37i2.1381
Hadi S (2013b). Effect of Sperm Selection by ‘’Swim-Up” Technique on the Sex Ratio of In Vitro Produced Ovine Embryos. The Iraqi J. Vet. Med., 37(2): 175–179. https://doi.org/10.30539/iraqijvm.v37i2.279
Hifnawy MS, Aboseada MA, Hassan HM, Tohamy AF, EI Naggar EMB, Abdelmohsen UR (2021). Nature-inspired male contraceptive and spermicidal products. Phytochemistry Reviews, 20(4):797–843. https://doi.org/10.1007/s11101-020-09721-5
Ibrahim Mohammed A, Al-Bayati MA (2014). Comparative Study Among Watermelon Crud Extract, Citrulline and Lycopene on Some Reproductive Indices in Male Mice. Int. J. Res. Med. Health Sci., 3(6): 7-14.
Jia Y-P, Ma B-Y, Wei X-W, Qian Z-Y (2017). The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett., 28(4): 691–702. https://doi.org/10.1016/j.cclet.2017.01.021
King SR, Massicot J, McDonagh AM (2015). A straightforward route to tetra chloroauric acid from gold metal and molecular chlorine for nanoparticle synthesis. Metals, 5(3): 1454–1461 .https://doi.org/10.3390/met5031454
Kumar SA, Peter YA, Nadeau JL (2008). Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology, 19(49): https://doi.org/10.1088/0957-4484/19/49/495101
Kus-Liśkiewicz M, Fickers P, Ben Tahar I (2021). Biocompatibility and cytotoxicity of gold nanoparticles: recent advances in methodologies and regulations. Int. J. Mol. Sci., 22(20): 10952. https://doi.org/10.3390/ijms222010952
Li Y, Zhang G, Wen F, Xian M, Guo S, Zhang X, Feng X, Hu Z, Hu J (2023). Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm. Animals, 13(9): 1442. https://doi.org/10.3390/ani13091442
Liu Y, Li X, Xiao S, Liu X, Chen X, Xia Q, Lei S, Li H, Zhong Z, Xiao K (2020). The effects of gold nanoparticles on Leydig cells and male reproductive function in mice. Int. J. Nanomed., 15(11):9499–9514. https://doi.org/10.2147/IJN.S276606
Martinez G (2022). First-line Evaluation of Sperm Parameters in Mice (Mus musculus). Bio-Protocol, 12(20): 4529 – 4529. https://doi.org/10.21769/BioProtoc.4529
Moretti E, Terzuoli G, Renieri T, Iacoponi F, Castellini C, Giordano C, Collodel G (2013). In vitro effect of gold and silver nanoparticles on human spermatozoa. Andrologia, 45(6): 392–396. https://doi.org/10.1111/and.12028
Nazari M, Talebi AR, Hosseini-Sharifabad M, Abbasi A, Khoradmehr A, Danafar AH (2016). Acute and chronic effects of gold nanoparticles on sperm parameters and chromatin structure in Mice. Int. J. Reprod. Biomed., 14(10): 637–642. https://doi.org/10.29252/ijrm.14.10.637
Taha M, MJ Ali A (2015). Changes in the weights of the testes and epididymes as well as sperm characteristic of male albino mice treated with silver nanoparticles. Ibn AL-Haitham J. Pure and Appl. Sci., 28(3): 309–316.
Oliveira AEF, Pereira AC, Resende MAC, Ferreira LF. (2023). Gold Nanoparticles: A Didactic Step-by-Step of the Synthesis Using the Turkevich Method, Mechanisms, and Characterizations. Analytica, 4(2): 250–263. https://doi.org/10.1007/s11051-023-05768-5
https://doi.org/10.4236/anp.2023.121004
Parak WJ, Boudreau R, Le Gros M, Gerion D, Zanchet D, Micheel CM, Williams SC, Alivisatos AP, Larabell C (2002). Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Adv. Mater., 14(12): 882–885. https://doi.org/10.1002/1521-4095(20020618)14:12<882::AID-ADMA882>3.0.CO;2-Y
Regoli F, Giuliani ME (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res., 93: 106–117. https://doi.org/10.1016/j.marenvres.2013.07.006
Ren D, Navarro B, Perez GI, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE (2001). A sperm ion channel required for sperm motility and male fertility. Nature, 413(6856): 603–609. https://doi.org/10.1038/35098027
Ringsted T,Todeschini R (2012). Marie Curie Initial Training Network Environmental Chemoinformatics (ECO).
Roddu AK, Wahab AW, Ahmad A, Taba P, Sutapa IW (2020). Theoretical Analysis Properties of Gold Nanoparticles Resulted by Bioreduction Process. J. Phys. Conf. Ser.,1463(1): 1-8. https://doi.org/10.1088/1742-6596/1463/1/012008
Rosłon M, Jastrzębska A, Sitarz K, Książek I, Koronkiewicz M, Anuszewska E, Jaworska M, Dudkiewicz-Wilczyńska J, Ziemkowska W, Basiak D (2019). The toxicity in vitro of titanium dioxide nanoparticles modified with noble metals on mammalian cells. International J. Appl. Ceram. Technol., 16(2): 481–493. https://doi.org/10.1111/ijac.13128
Sangwan S, Seth R (2022). Synthesis Characterization and Stability of Gold Nanoparticles (AuNPs) in Different Buffer Systems. J. Cluster Sci., 33(2): 749–764. https://doi.org/10.1007/s10876-020-01956-8
Singh AV, Laux P, Luch A, Sudrik C, Wiehr S, Wild AM, Santomauro G, Bill J, Sitti M (2019). Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design.Toxicol. Mech. Methods, 29 (5): 378–387. https://doi.org/10.1080/15376516.2019.1566425
Sokoloski JE, Blasco L, Storey BT, Wolf DP (1977). Turbidimetric analysis of human sperm motility. Fertil. Steril. 28(12): 1337–1341. https://doi.org/10.1016/S0015-0282(16)42980-8
Steel RGD,Torrie JH (1980). Principles and Procedures of Statistics: A Biometrical approach.
Strober W (2015). Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol.,111(1): 111(1): 1-3. https://doi.org/10.1002/0471142735.ima03bs111
Talebi AR, Khalili MA, Hossaini A (2007). Assessment of nuclear DNA integrity of epididymal spermatozoa following experimental chronic spinal cord injury in the rat. Int. J. Androl., 30(3): 163–169. https://doi.org/10.1111/j.1365-2605.2006.00736.x
Talebi AR, Sarcheshmeh AA, Khalili MA, Tabibnejad N (2011). Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol., 45(4): 403 – 409. https://doi.org/10.1016/j.alcohol.2010.10.005
Taylor U, Barchanski A, Petersen S, Kues WA, Baulain U, Gamrad L, Sajti L, Barcikowski S, Rath D (2014). Gold nanoparticles interfere with sperm functionality by membrane adsorption without penetration. Nanotoxicology, 8(sup1)., 118–127. https://doi.org/10.3109/17435390.2013.859321
Vidya R, Saji A (2018). Naked eye detection of infertility based on sperm protamine-induced aggregation of heparin gold nanoparticles. Anal. Bioanal. Chem., 410: 3053–3058. https://doi.org/10.1007/s00216-018-1026-6
Vijayakumar S, Ganesan S (2012). In VitroCytotoxicity Assay on Gold Nanoparticles with Different Stabilizing Agents. J. Nanomaterials, 14: 1–9. https://doi.org/10.1155/2012/734398
Wang E, Huang Y, Du Q, Sun Y (2017). Silver nanoparticle induced toxicity to human sperm by increasing ROS(reactive oxygen species) production and DNA damage. Environ. Toxicol. Pharmacol., 52: 193–199. https://doi.org/10.1016/j.etap.2017.04.010
Wang W, Ding X, Xu Q, Wang J, Wang L, Lou X (2016). Zeta-potential data reliability of gold nanoparticle biomolecular conjugates and its application in sensitive quantification of surface absorbed protein. Colloids and Surfaces B: Biointerfaces, 148: 541–548. https://doi.org/10.1016/j.colsurfb.2016.09.021
Wiwanitkit V, Sereemaspun A, Rojanathanes R (2009). Effect of gold nanoparticles on spermatozoa: the first world report. Fertil. Steril., 91(1): 7– 8. https://doi.org/10.1016/j.fertnstert.2007.08.021
Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK (2011). Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res., 44(10): 914–924. https://doi.org/10.1021/ar200061q
Xiao Y, Xu W, Komohara Y, Fujiwara Y, Hirose H, Futaki S, NiidomeT (2020). Effect of Surface Modifications on Cellular Uptake of Gold Nanorods in Human Primary Cells and Established Cell Lines. ACS Omega., 5(50): 32744–32752. https://doi.org/10.1021/acsomega.0c05162
Zakhidov ST, Marshak TL, Malolina EA, Kulibin AY, Zelenina IA, Pavluchenkova SM, Rudoi VM, Dement’eva OV, Skuridin SG, Evdokimov YM (2010). Gold nanoparticles disturb nuclear chromatin decondensation in mouse sperm in vitro. Biochemistry (Moscow) Supplement Series A: Membr. Cell Biol., 4(3): 293–296. https://doi.org/10.1134/S1990747810030074
To share on other social networks, click on any share button. What are these?