The Role of Aloe vera L. Extract in Modulating Oxidative Stress and Inflammation in Wistar Rats on a High-Fat Diet: Insights from In Vivo and In Silico Studies
Research Article
The Role of Aloe vera L. Extract in Modulating Oxidative Stress and Inflammation in Wistar Rats on a High-Fat Diet: Insights from In Vivo and In Silico Studies
Roy Sukbir Singh1, Jekson Martiar Siahaan2,3*, Endy Juli Anto4, Syafruddin Ilyas5, Putri Eyanoer6, Hendrika Andriani7,8
1Master Program in Biomedical Sciences, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia; 2Department of Physiology, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia; 3Department of Molecular Biology, Master Program Biomedical Sciences, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia; 4Department Parasitology and Immunology, Faculty of medicine, Universitas Methodist Indonesia, Medan, Indonesia; 5Study Program of Biology, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia; 6Department of Community and Preventine Medicine, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia; 7Department of Histology, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia; 8Department of Research Methodology, Master Program Biomedical Sciences, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia.
Abstract | Hyperlipidemia is a major health problem that is connected to cardiovascular events. It is characterized by disruptions in lipid profiles and is frequently accompanied by endothelial dysfunction, oxidative stress, and inflammation. Aloe vera, renowned for its secondary metabolites, is suggested as a potential treatment because of its hypolipidemic, antioxidant, and anti-inflammatory qualities. This study employed both in-silico and in-vivo approaches to explore these effects. The in-silico analysis involved molecular docking simulations to evaluate the interactions of Aloe vera compounds with inflammatory mediators such as TNF-α and COX-2. Furthermore, this study also employed a laboratory design with a post-test randomized controlled group. The study included 30 male white rats divided into six groups. Prior to the study, the rats were induced with a high-fat diet (HFD) for 14 days. The groups consisted of a normal group receiving distilled water, a negative control group following a high-fat diet, a positive control group treated with cholestyramine (200 mg/kgBW/day), and three experimental groups receiving Aloe vera at doses of 200, 300, and 400 mg/kgBW/day. At the end of the study, lipid profiles, MDA (malondialdehyde), and Hs-CRP (high-sensitivity C-reactive protein) levels were assessed. The in silico results indicated strong binding affinities for aloin in TNF-α, and Aloinoside in COX-2, this suggesting its potential inhibition of these targets. Moreover, at doses of 400 mg/KgBW Aloe vera extract had a significant effect improving their lipid profiles, and reducing MDA activity and inflammation (P < 0.05) with significance measured using ANOVA followed by Tukey’s Multiple Comparison Test. Hence, the ethanolic extract of Aloe vera has demonstrated efficacy as a hypolipidemic, and anti-inflammatory agent.
Keywords | Aloe vera, Anti-inflammatory, Lipid, In Vivo, In Silico
Received | May 29, 2024; Accepted | June 28, 2024; Published | August 23, 2024
*Correspondence | Jekson Martiar Siahaan, 2Department of Physiology, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia; 3Department of Molecular Biology, Master Program Biomedical Sciences, Faculty of Medicine, Universitas Methodist Indonesia, Medan, Indonesia; Email: [email protected]
Citation | Singh RS, Siahaan JM, Anto EJ, Ilyas S, Eyanoer P, Andriani H (2024). The role of Aloe vera L. extract in modulating oxidative stress and inflammation in wistar rats on a high-fat diet: insights from in vivo and in silico studies. Adv. Anim. Vet. Sci. 12(10): 1884-1895.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.10.1884.1895
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The growing number of diseases associated with lifestyle factors, such as obesity, diabetes, and cardiovascular diseases, has contributed to the search for improved therapeutic strategies. Hyperlipidemia, characterised by abnormally elevated levels of lipids in the blood, is a common risk factor associated with the progression of various medical diseases (Dubey et al., 2023). An increasing correlation has been observed between it and systemic reactions, such as oxidative stress and inflammation. These reactions are essential in the progression of atherosclerosis and other metabolic disorders (Masenga et al., 2023). Approximately 53% of the adult population in the United States, which amounts to over 100 million individuals, have elevated levels of LDL cholesterol. In addition, over 31 million adults had total cholesterol levels exceeding 240 mg/dl (Beheshti et al., 2020). The World Health Organization (WHO) reported that the occurrence of hyperlipidemia in Southeast Asia was approximately 30.3%, which is comparatively lower than the rates in Europe (53.7%) and America (47.7%) (Lin et al., 2018). The prevalence of hyperlipidemia in persons aged 25 years in Indonesia is approximately 36%, with a rate of 33.1% in men and 38.2% in women. In Indonesia, the occurrence of cardiovascular problems linked to hyperlipidemia is approximately 37% (Lin et al., 2018).
The observed differences in hyperlipidemia prevalence among regions can be attributed to various factors including dietary habits, genetic predisposition, healthcare infrastructure, and socioeconomic status. For instance, dietary patterns rich in saturated fats and low in fiber, which are more prevalent in Western countries, contribute significantly to higher cholesterol levels. Treating hyperlipidemia not only helps to regulate lipid levels but also diminishes these interrelated systemic responses. Currently, there are pharmaceutical options available for managing cholesterol levels, such as statins and fibrates (Ferraro et al, 2022). However, it is important to note that these medications often have negative side effects that can affect patient compliance and general health. As a result, there has been a growing interest in studying natural compounds that may offer comparable therapeutic benefits while reducing negative side effects. Aloe vera, a widely used plant in traditional medicine across several cultures, has been scientifically proven to possess several health-promoting properties, including anti-inflammatory, antioxidant, and antihyperlipidemic effects (Kumar et al., 2024; Lanka, 2018; Hęś et al., 2019).
Aloe vera comprises a diverse range of phytochemical elements, such as vitamins, enzymes, minerals, carbohydrates, lignin, saponins, salicylic acids, and amino acids (Bista et al., 2020). The plant’s therapeutic properties are mainly due to its abundant polyphenolic content, which has the ability to eliminate harmful free radicals and reduce oxidative stress (Marianne et al., 2021). Moreover, it is believed that Aloe vera’s anti-inflammatory effects are achieved by regulating the synthesis of cytokines and suppressing the activation of proinflammatory pathways.
The justification for utilizing ethanol extract of Aloe vera in experimental investigations stems from its superior ability of ethanol to extract these bioactive polyphenols in comparison to alternative solvents. Ethanol extracts are recognized for their heightened capacity to dissolve a broad spectrum of phytochemicals, hence optimizing the medicinal efficacy of the resultant extracts.
Despite the established benefits of Aloe vera, there remains a need for a comprehensive evaluation of its effects on lipid metabolism and inflammation, particularly in the context of hyperlipidemia. Previous studies have demonstrated the plant’s potential in modulating various physiological processes, but the precise mechanisms and the efficacy of different dosages require further exploration. This study aims to bridge this gap by employing a dual approach that combines in-silico molecular docking simulations and in-vivo experiments. The in-silico component provides insights into the potential interactions between Aloe vera compounds and key molecular targets involved in oxidative stress and inflammation, while the in-vivo experiments validate these findings in a hyperlipidemic Wistar rat model.
This study employs a comprehensive approach that integrates computer-based simulations (in silico) and experiments on living organisms (in vivo) to examine the effects of the ethanol extract of Aloe vera on the regulation of oxidative stress and inflammation. Specifically, it focuses on how this extract can mitigate the impact of lipid-induced stress (hyperlipidemia) in Wistar rats. In silico studies provide an initial understanding of the possible links between the bioactive compounds present in Aloe vera and the important molecular targets involved in oxidative stress and inflammation pathways. This is corroborated by in vivo studies that showcase the physiological importance and concrete impact of these extracts on hyperlipidemic Wistar rats.
This study aims to bridge this gap by employing a dual approach that combines in-silico molecular docking simulations and in-vivo experiments. The in-silico component provides insights into the potential interactions between Aloe vera compounds and key molecular targets involved in oxidative stress and inflammation, while the in-vivo experiments validate these findings in a hyperlipidemic Wistar rat model. The specific hypothesis being tested is that ethanol extract of Aloe vera L. can significantly improve lipid profiles, reduce oxidative stress, and mitigate inflammation in hyperlipidemic Wistar rats.
Materials and Methods
Materials
30 male rats, Aloe vera ethanol extract, Rice Husk, Feed (Commercial Pellets), Heparin Sodium, Nacl 0.9%, Cell Lysis Buffer, TBA Reagent, Aquabidest, Alcohol 70%, 80%, 96%, Acid Alcohol 1% Solution, Absolute Alcohol, Xylol, Liquid Paraffin, Glyserin, Haemotoxylin Eosin Dye 1% Solution, Xylene, Cholesterol assay kit, MDA assay kit and Hs-CRP assay kit.
Animal
Experimental animals used in this study were male Wistar rats (Rattus norvegicus) sourced from the Animal Research Center at Universitas Sumatera Utara, Medan, Indonesia. The rats were 8 weeks old and weighed between 200-250 grams at the start of the experiment. The selection of Wistar rats as experimental animals was based on the consideration that genetically, rats have similarities with humans and have the ability to adapt to the laboratory environment.
Extraction Process
Aloe vera extract was made by maceration using 80% ethanol. For the extraction, 500 grams of dried Aloe vera powder was placed into a dark container, and 2000 mL of 80% ethanol was poured over it. The container was covered and left for 5 days, protected from light, and stirred frequently. After 5 days, the mixture was filtered, and the residue was washed with an additional 500 mL of 80% ethanol. The combined filtrate was then transferred to a closed vessel and left in a cool place, protected from light, for 2 days to allow for sedimentation. The clear liquid was carefully decanted to avoid wasting the sediment. The filtrate was evaporated using a rotary evaporator at a temperature of ± 40°C until a thick extract was obtained (Nabila and Putra, 2020).
Phytochemical Assessment
Qualitative methodologies were used to determine the presence of various phytochemicals. Flavonoids were identified using 2 mL of concentrated hydrochloric acid, 0.5 grams of magnesium powder, and 5 mL of amyl alcohol, with quercetin (1 mg/mL) as the positive control. Alkaloids were identified using Mayer’s (1.36%), Bouchardat’s (1.35%), and Dragendorff’s (1.35%) reagents, with atropine sulfate (1 mg/mL) as the positive control. Saponins were detected using the foam test, with saponin (1 mg/mL) as the positive control. Tannins were detected by adding 2 mL of 5% ferric chloride solution to the extract, with tannic acid (1 mg/mL) as the positive control. Steroids and terpenoids were assessed using the Liebermann-Burchard reagent (2 mL of acetic anhydride and 1 mL of concentrated sulfuric acid) (Agidew, 2022).
Experimental Design
The research used 30 male rat that divided into 6 group. These samples were then separated into:
- Group 1 (G1), the control group, did not receive any therapy and were provided with normal food and water in their cage.
- Group 2 (G2), the negative control, received no treatment but was administered a HFD.
- Group 3 (G3) consisted of subjects who were administered a HFD together with cholestyramine at a dosage of 200 mg per kgBW per day. This group served as the positive control.
- Group 4 (G4) received a treatment of HFD combined with Aloe vera extract at a dosage of 200 mg per kg/BW per day.
- Group 5 (G5) received a treatment of HFD + Aloe vera extract at a dosage of 300 mg per KgBW perday.
- Group 6 (G6) received treatment with a high-fat diet (HFD) combined with Aloe vera extract at a dosage of 400 mg per KgBW per day.
The study begins by exposing male albino rats to a two-week phase of acclimatisation. After the test animals were acclimated, they were randomized to 6 groups using a block randomization method to ensure unbiased allocation (Berger et al., 2021). The strip test cholesterol method was used to test the total cholesterol level, specifically through the tail, as the initial data before HFD induction. After a two-week period of consuming a diet high in cholesterol, all rats had a second evaluation to measure their overall cholesterol levels. Next, the test animals were treated according to the pre-determined groups for a period of 14 days. Subsequently, a comprehensive assessment was carried out by a final examination to analyse the lipid profile (including total cholesterol, LDL, HDL, and triglycerides), MDA levels, and Hs-CRP levels by collecting blood from the rats’ hearts.
Ethical Clearance
The use and handling of experimental animals in research laboratories is carried out in accordance with the rules of ethics for animal research as regulated in the Declaration of Helsinki and obtained Ethical clearance from the ethics committee of the Faculty of Medicine UMI Medan, with approval number: 0174/T/FKUMI/2023 on March 17, 2023.
In silico tools
The in-silico analysis was conducted using several computational tools. The 3D structures of TNF-α (Tumor Necrosis Factor-alpha) and COX-2 (Cyclooxygenase-2) were obtained from the Protein Data Bank (PDB). The ligands, including Aloinoside, Aloin, Hydroxyaloin, and Aloesin, were retrieved from the PubChem database. UCSF Chimera 1.16 was used for molecular visualization and preparation of protein and ligand structures. Molecular docking simulations were performed using SwissDock, which predicts the binding affinity and possible interactions between the ligands and target proteins. The binding interactions were analyzed and visualized using Chimera, providing insights into the potential inhibitory effects of Aloe vera compounds on inflammatory mediators.
Preparation of ligands and proteins
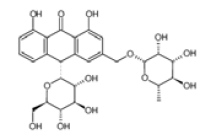
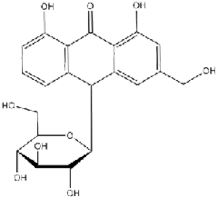
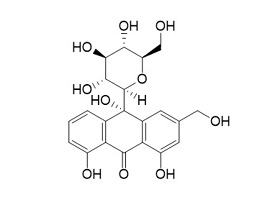
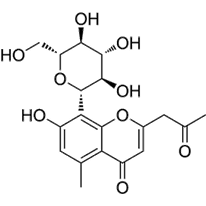
TNF- (Tumor Necrosis Factor-alpha) and COX-2 (Cyclooxygenase-2) are critical proteins involved in the inflammatory response, making them significant targets in the development of anti-inflammatory drugs (Ferrer et al., 2019). Their roles in inflammation and the utility of targeting them in in silico docking studies for anti-inflammatory activity. The TNF-α, AND COX-2 was obtained from the Protein Data Bank website (*). PDB file format. Subsequently, the UCSF Chimera 1.16 tool was used to prepare the sample by eliminating residues. The test compounds were generated using UCSF Chimera 1.16. This was achieved by inputting the PubChem CID of the ligand, which was acquired earlier using the PubChem online service and stored in the mol2 format. Molecular docking involves interactions between proteins and either test chemicals or natural ligands, and the SwissDock platform was used to execute the docking procedure. Docking data were quantified using the Gibbs free energy (∆G) value (Martins da Silva et al., 2023). Table 1 lists the precise attributes of these ligands.
|
Name |
Formula |
Chemical Structure |
|
ALOINOSIDE |
C27H32O13 |
|
|
ALOIN |
C21H22O9 |
|
|
HYDROXYALOIN |
C21H22O1 |
|
|
ALOESIN |
C19H22O9 |
|
Rendering of docking outcomes
The visualisation procedure was carried out through the USCF Chimaera 1.16 software. The protein data and docking results were converted into the *.pdb file format. Visualisation demonstrates the particular sort of bond interaction formed with the amino acid that acts as the binding site. The visualisation results are displayed in the *.png file format (Pettersen et al., 2004).
Statistical analysis
The in vivo results were analyzed using one-way analysis of variance (ANOVA) to determine the overall significance among the groups. A priori comparisons were planned to evaluate the effects of Aloe vera extract doses (200, 300, and 400 mg/kgBW) compared to the negative control group (high-fat diet without treatment) and the positive control group (cholestyramine treatment). Post hoc comparisons were conducted using Tukey’s Multiple Comparison Test to identify specific differences between the treatment groups, including pairwise comparisons among all groups to determine which specific groups differed significantly from each other. P-values for significance were set at P < 0.05. Values for all measurements are expressed as mean ± standard deviation (SD). The histogram data were constructed using GraphPad Prism Software 9.0.
RESULTS AND DISCUSSION
Phytochemical Screening
Phytochemical screening is a qualitative test that is used as an initial step for Aloe vera extract. The purpose of phytochemical screening is to determine the secondary metabolites contained in Aloe vera extract. The results of phytochemical screening on Aloe vera can be seen in Table 2.
Tabel 2. Phytochemical Screening.
|
No. |
Phytochemical Content |
Reagent |
Result |
|
1. |
Flavonoid |
MgHCl |
- |
|
Pb (CH3COO)2 1 – 5% |
+ |
||
|
NaOH |
+ |
||
|
2. |
Alkaloid |
Mayer |
- |
|
Dragendroof |
- |
||
|
3. |
Saponin |
HCl |
+ |
|
4. |
Tannin |
FeCl3 |
+ |
|
5. |
Terpenoid |
Libermann – Burchard’s |
+ |
The Table 2 shows test results for various phytochemicals with specific reagents. Flavonoids did not react with MgHCl but tested positive with Pb(CH3COO)2 1 – 5% and NaOH, indicating their presence. Alkaloids showed no reaction with Mayer’s or Dragendroff’s reagents, confirming their absence. Saponins reacted positively with HCl, tannins with FeCl3, and finally terpenoids with Liebermann–Burchard’s reagent, all suggesting their presence. The results were in line with another studies that also revealing the presence of various bioactive compounds such as tannins, saponins, flavonoids, terpenoids, alkaloids, glycosides, and proteins, with the absence of cardiac glycosides and steroids (Kumar et al., 2020; Bista et al., 2020).
Effects of Aloe vera Extract in Lipid Profile
The study examined the effect of Aloe vera extract on the reduction of blood lipid profile levels in male white rats of the Wistar strain (Rattus novergius sp.) induced by a high fat diet (HFD). The levels of triglycerides, LDL, HDL, and total cholesterol were assessed. Figure 1 displays the test results illustrating the impact of Aloe vera ethanol extract on the improvement of lipid profile.
This study examines the impact of Aloe vera ethanol extract on lipid profiles in male Wistar rats with hyperlipidemia. Total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol were among the lipid parameters that were evaluated. Aloe vera ethanol extract was administered at three different dosage levels: 200 mg/kg BW, 300 mg/kg BW, and 400 mg/kgBW. In comparison to the control group, both concentrations of Aloe vera extract significantly decreased triglyceride levels; the 400 mg/kg dose demonstrated the most pronounced effect (P< 0.05). In the same way, the groups that received Aloe vera extract demonstrated a substantial reduction in LDL cholesterol levels, with the highest dose group experiencing the most substantial decline (P< 0.05), comaper to negative control group. According to these results, the effectiveness of Aloe vera extract in decreasing triglyceride and LDL cholesterol levels showed dose dependent manner.
On the contrary, there were no substantial alterations observed in HDL cholesterol levels following treatment with Aloe vera, only dose of 400 mg/kgBW that showed improvement of HDL compare to negative control group (P<0.05). Furthermore, In the treatment groups, total cholesterol levels decreased substantially, with the greatest reduction observed in the rodents administered the 400 mg/kg dose (P<0.05). This finding emphasizes the potential of Aloe vera extract in reducing overall cholesterol levels. Additionally, the study also indicated the effect sizes (Cohen’s d) of 400 mg/kg ranged from 2.0 to 3.2, with 95% confidence intervals confirming the significance of these changes.
The effectiveness of Aloe vera in improving lipid profiles can be attributed to its rich phytochemical contents, which have capabilities that change lipids. Polysaccharides, phytosterols, and flavonoids are the main phytochemicals involved in crucial metabolic processes that regulate lipid balance (Amri et al., 2022; Egbuna et al., 2020). These substances have demonstrated efficacy in reducing levels of blood lipoproteins and triglycerides, therefore alleviating hyperlipidemia. Therefore, Aloe vera shows potential as a beneficial plant-based treatment for dyslipidemia, which can improve cardiovascular health by regulating lipid metabolism. A study conducted by Hosseini et al. (2020) revealed that Aloe vera has a substantial impact on lowerin serum cholesterol and triglyceride levels in individuals with
hyperlipidemia (Hosseini et al., 2020). Furthermore, Aloe vera may affect lipid profiles through its bioactive com- pounds, such as aloin, aloesin, and flavonoids, which inhibit key enzymes like HMG-CoA reductase and pancreatic lipase. This inhibition reduces cholesterol synthesis in the liver and decreases dietary fat absorption, leading to lower levels of LDL cholesterol and triglycerides. The observed reductions in triglycerides, LDL, and total cholesterol suggest Aloe vera’s potential in modulating lipid metabolism (Misawa et al., 2012).
Additionally, the current study demonstrates a direct correlation between the dosage of Aloe vera extract and the extent of reduction in cholesterol levels. The research conducted by Sánchez et al. (2020), supports the notion that the effectiveness of herbal extracts, such as Aloe vera, is typically dependent on the dosage. This highlights the significance of dosage in the therapeutic application of natural products.
Extensive research highlights the safety and effectiveness of Aloe vera extract as a natural therapy. However, it is important to exercise caution because the content of the extract might vary depending on the production processes and the specific portions of the plant that are employed. The ethanol extract used in our study contains distinct bioactive constituents that differ from the commonly studied water extracts. These unique components may account for the discrepancies in effectiveness, specifically in relation to HDL cholesterol. Additional study is essential to identify the precise molecules accountable for the lipid-lowering effects and to confirm these discoveries in different biological systems and metabolic circumstances by conducting comparative studies in various animal models or human clinical trials.
Aloe vera Extract in Reducing MDA and HsCRP Activity
The study assessed the effectiveness of Aloe vera ethanol extract in managing oxidative stress and inflammatory response in male Wistar rats exposed to hyperlipidemia caused by a high-fat diet. This study quantified the concentrations of malondialdehyde (MDA), a biomarker for lipid peroxidation (Alvarez-Mon et al., 2022), and high-sensitivity C-reactive protein (Hs-CRP), an indicator of inflammation (Beatty et al., 2024). The impact of Aloe vera extract on lowering MDA and HsCRP activity in male white rats of the Wistar strain (Rattus novergius sp.) with hyperlipidemia produced by a high fat diet is illustrated in Figure 2.
Administration of the ethanol extract of Aloe vera led to a notable decrease in MDA levels among the groups that received treatment. Significantly, the group that received a dose of 400 mg/kgBW showed a more prominent reduction in MDA levels compared to both the control group and the group that received a lower dose of 200 mg/kg. The efficacy of Aloe vera in decreasing MDA levels was highly significant in comparison to the negative control group (P<0.05). In the same way, the concentrations of Hs-CRP were notably reduced in the groups that received 400 mg/kgBW of Aloe vera extract. The decrease in high-sensitivity C-reactive protein (Hs-CRP) indicates a significant anti-inflammatory impact of the extract. Although the lower dose led to a reduction in Hs-CRP levels, one of the treatment groups did not exhibit a statistically significant difference compared to the control group, denoted as ‘ns’ for not significant (P>0.05). The Aloe vera extract at a dose of 400 mg/kgBW significantly decreased MDA and Hs-CRP levels. The effect sizes (Cohen’s d) ranged from 3.3 to 3.5, with 95% confidence intervals supporting the significance of the reductions.
The decrease in MDA levels seen in the groups treated with Aloe vera indicates that the extract possesses strong antioxidant characteristics that can effectively reduce lipid peroxidation. This is especially significant because MDA is a widely recognized indicator of oxidative stress and harm to cells (Mayasari et al 2024). The varying impact of Aloe vera extract based on dosage emphasizes its potential usefulness in therapeutic environments, where the adjustment of oxidative stress is needed. Aloe vera extract has shown potential in therapeutic environments, particularly in the management of oxidative stress in hyperlipidemia. Studies have demonstrated its significant reduction of malondialdehyde and luminol levels in diabetic rats (Şeker et al, 2023). Furthermore, Aloe vera gel has been associated with potential protective effects on cardiovascular diseases, including hyperlipidemia (Sabbaghzadegan et al., 2021).
Moreover, the substantial decrease in Hs-CRP levels observed in rats administered with Aloe vera provides evidence for its efficacy in regulating inflammatory processes. Inflammation has a crucial role in numerous chronic illnesses, such as cardiovascular disorders, which are frequently associated with hyperlipidemia (Banait et al., 2022) Aloe vera extract may provide preventive advantages against the inflammatory elements of hyperlipidemia by decreasing Hs-CRP levels. The results align with previous research that highlights the positive impact of Aloe vera on health, particularly its ability to reduce inflammation and act as an antioxidant (Manye et al, 2023; Ramírez et al., 2024). Furthermore, the significant reduction in Hs-CRP levels suggests a direct anti-inflammatory effect, likely through inhibiting pro-inflammatory cytokines like TNF-α and IL-6. In silico results support this, showing strong binding affinities of Aloe vera compounds to COX-2 and TNF-α, key inflammation mediators (Sánchez et al., 2020). Although our findings show promise, they are currently restricted to an animal model. Further research is required to investigate the specific mechanisms through which Aloe vera extract affects oxidative and inflammatory pathways, and to verify if these effects can be consistently observed in human participants. Finally, the improvements in lipid profiles, oxidative stress, and inflammation in our study suggest Aloe vera extract could help manage hyperlipidemia and related inflammatory conditions. However, translating these findings to clinical practice requires careful consideration of dosage and side effects. Our study found the highest dose of 400 mg/kgBW most effective. Future clinical studies should determine the optimal human dosage and monitor for side effects like gastrointestinal discomfort or allergic reactions.
To address potential biases, we used SYRCLE’s Risk of Bias tool. Selection bias was minimized through block randomization, ensuring unbiased group allocation. Performance bias was reduced by blinding caregivers and researchers and by using random housing to prevent environmental influences. Detection bias was mitigated by conducting random outcome assessments and blinding outcome assessors. Attrition bias was addressed by accounting for all animals and explaining any exclusions. Reporting bias was minimized by reporting all pre-specified outcomes. No other sources of bias were identified.
Insilico Study
The in-silico analysis was conducted to determine the interaction of the principal compounds in Aloe vera with key inflammation markers, namely TNF-α (Tumor Necrosis Factor-alpha) and COX-2 (Cyclooxygenase-2). The results, which include binding affinity data and structural visualizations, are presented in Tables 3 and 4, and Figures 3 and 4 respectively.
The in silico analysis was conducted to determine the interaction of the principal compounds in Aloe vera with key
Table 3. Docking Affinity Score in TNF-α.
|
Ligand |
Docking Affinity Score in TNF-α |
|
|
ΔG (kkal/ mol) |
Amino acid residue |
|
|
Aloinoside |
-6.9 |
Chain A: SER9 ASP10 LYS11 ALA156 LEU157; Chain B: ASP10 LYS11 PRO12 SER52 GLU53 GLY54 LEU55 TYR56 GLN125 ALA156 LEU157; Chain C: SER9 ASP10 LYS11 PRO12 ASN39 SER52 GLU53 GLY54 LEU55 GLN125 ILE155 ALA156 LEU157 |
|
Aloin |
-8.2 |
Chain A: CYS69 HIS73 SER99 PRO100 CYS101 GLN102 ARG103 GLU104 THR105 PRO106 GLU107 PRO113 TRP114 TYR115 GLU116; Chain B: CYS69 HIS73 LYS98 SER99 PRO100 CYS101 GLN102 ARG103 GLU104 THR105 PRO106 GLU107 GLU110 LYS112 PRO113 TRP114 TYR115 GLU116; Chain C: CYS69 HIS73 LYS98 SER99 PRO100 CYS101 GLN102 ARG103 GLU104 PRO113 TRP114 TYR115 GLU116 |
|
Hydroxyaloin |
-7.6 |
Chain B: THR77 HIS78 THR79 SER81 ARG82 LYS90 VAL91 ASN92 LEU93 LEU94 SER95 ALA96 ILE97 VAL123 PHE124 GLN125 LEU126 GLU135; Chain C: HIS15 VAL16 VAL17 ALA18 PRO20 LEU29 ARG31 LEU36 ALA145 GLU146 SER147 GLY148 GLN149 TYR151 |
|
Aloesin |
-7.5 |
Chain A: CYS69 LYS98 SER99 PRO100 CYS101 GLN102 ARG103 TRP114 TYR115 GLU116; Chain B: CYS69 HIS73 LYS98 SER99 PRO100 CYS101 GLN102 ARG103 PRO113 TRP114 TYR115 GLU116; Chain C: CYS69 LYS98 SER99 PRO100 CYS101 GLN102 ARG103 PRO113 TRP114 TYR115 GLU116 |
Table 4. Docking Affinity Score in COX 2.
|
Ligand |
Docking Affinity Score in COX 2 |
|
|
ΔG (kkal/ mol) |
Amino acid residue |
|
|
Aloinoside |
-10.3 |
Chain C: ALA33 ASN34 PRO35 CYS36 CYS37 ASN39 PRO40 GLU46 CYS47 MET48 SER49 PHE52 ASN131 VAL132 HIS133 TYR134 GLY135 TYR136 PRO153 PRO154 VAL155 ALA156 ASP157 ASP158 CYS159 LYS459 GLN461; Chain D: PRO321 GLU322 TRP323 GLY324 ASP325 GLU326 GLN327 LEU328 |
|
Aloin |
-9.6 |
Chain A: PRO127 TRP139 GLU140 PHE142 SER143 ASN144 LEU145 SER146 LYS211 ARG216 ARG222 GLY223 LEU224 GLY225 HIS226 GLY227 VAL228 ASP229 ASN231 GLY235 GLU236 THR237 LEU238 ASP239 GLN241 LYS333 TYR373 GLN374 ASN375 ARG376 GLY533 GLY536 ASN537 PRO538; Chain B: PRO127 PRO128 TRP139 GLU140 PHE142 SER143 ASN144 LEU145 SER146 ARG222 GLY223 LEU224 GLY225 HIS226 GLY227 VAL228 ASP229 ASN231 TYR234 GLY235 GLU236 THR237 LEU238 GLN241 GLN330 LYS333 TYR373 GLN374 ASN375 ARG376 GLY536 ASN537 PRO538 |
|
Hydroxyaloin |
-9.8 |
Chain C: ALA33 ASN34 PRO35 CYS36 CYS47 MET48 SER49 GLY51 PHE52 TYR130 VAL132 HIS133 TYR134 GLY135 TYR136 PRO153 PRO154 VAL155 ALA156 ASP157 ASP158 |
|
Aloesin |
-8.1 |
Chain A: PRO128 TRP139 GLU140 PHE142 SER143 ASN144 LEU145 SER146 LYS211 ARG222 GLY223 LEU224 GLY225 HIS226 GLY227 ASP229 ASN231 TYR234 GLY235 GLU236 THR237 LEU238 ASP239 GLN241 LYS333 TYR373 GLN374 ASN375 ARG376 GLY536 PRO538 |
inflammation markers, namely TNF-α (Tumor Necrosis Factor-alpha) and COX-2 (Cyclooxygenase-2). The docking affinity score for Aloinoside with COX-2 was -10.3 kcal/mol, indicating a strong binding affinity, with significant interactions involving residues ALA33, ASN34, and GLY135. Aloin showed a docking affinity score of -8.2 kcal/mol with TNF-α, suggesting a strong inhibitory potential, with binding involving residues CYS69, HIS73, and GLU104. Hydroxyaloin had a docking affinity score of -9.8 kcal/mol with COX-2, demonstrating a high binding affinity with significant interactions at residues ALA33 and TYR134. Aloesin showed a moderate binding affinity with a docking score of -7.5 kcal/mol with TNF-α, with key interactions occurring at residues CYS69 and PRO113. These docking scores reflect the Gibbs free energy (ΔG) values, where more negative scores indicate stronger binding affinities. The interaction patterns suggest that Aloe vera compounds have the potential to inhibit these inflammation markers effectively. These results align with previous studies highlighting the anti-inflammatory properties of Aloe vera compounds.
Comparing these results with existing research, such as the study by Park et al. (2009), which highlights the anti-inflammatory properties of Aloe vera, we find consistency in the potential routes through which these compounds exert their effects. The interactions involving multiple amino acid chains and diverse residues suggest complex interaction dynamics that could potentially increase the effectiveness of Aloe vera compounds in treating inflammation. Other In silico studies have identified potential anti-inflammatory compounds in Aloe vera, including aloinoside, aloin, hydroxyaloin, and aloesin, which have shown binding affinity to key inflammation markers TNF-α and COX-2 (Jerine et al., 2020). These compounds have also been found to inhibit COX enzymes, with specific interactions identified at amino acid residues Leu93, Val116, Leu352, and Ala527 (Liang et al., 2020). Furthermore, the current investigation underscores the similar binding type sites found in both and several compounds of Aloe vera highlighting a substantial correlation between their biochemical structures and functions. Therefore, comprehensive studies using in vitro and in vivo assays should be conducted to confirm the therapeutic potential of Aloe vera extracts and the biological relevance of our in-silico findings.
CONCLUSION AND RECOMMENDATIONS
Aloe vera L. has hypolipidemic and anti-inflammatory properties in hyperlipidemic male white rats (Rattus novergius sp.) who were fed a high-fat diet. The dose of 400 mg/kgBW demonstrated the greatest efficacy. Furthermore, It is important to carefully assess potential adverse effects and establish appropriate, efficient dosages for clinical application. Therefore, it is important to view these discoveries as a first stage in the direction of more extensive investigation, rather than as definitive evidence of their clinical significance.
NOVELTY STATEMENT
The novelty of this study is the evaluation of the effect of Aloe vera extract on the treatment of rat induced high fat died.
AUTHOR’S CONTRIBUTION
Roy Sukbir Singh conceptualized the study, conducted formal analysis, and drafted the manuscript. Jekson Martiar Siahaan supervised, validated the study, and edited the manuscript as the corresponding author. Endy Juli Anto managed investigation and data curation. Syafruddin Ilyas provided resources and oversaw project administration. Putri Eyanoer contributed to analysis, visualization, and manuscript review. Hendrika Andriani supported investigation and data curation. All authors approved the final manuscript and take responsibility for its content.
Conflict of Interest
The authors assert that they do not have any conflicts of interest, whether financial, personal, authorship-related, or otherwise, that could potentially impact the research and the findings described in this paper.
REFERENCES
Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Lahera G, Monserrat J, Gomez-Lahoz AM, Alvarez-Mon M (2022). Differential malondialdehyde (MDA) detection in plasma samples of patients with major depressive disorder (MDD): A potential biomarker. J. Int. Med. Res., 50(5): 03000605221094995. https://doi.org/10.1177/03000605221094995
Agidew MG (2022). Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Nat. Res. Centre., 46(1): 87. https://doi.org/10.1186/s42269-022-00770-8
Amri A, Bouraoui Z, Balbuena-Pecino S, Capilla E, Gharred T, Haouas Z, Guerbej H, Hosni K, Navarro I, Jebali, J (2022). Dietary supplementation with Aloe vera induces hepatic steatosis and oxidative stress together with a disruption of cellular signaling pathways and lipid metabolism related genes’ expression in gilthead sea bream (Sparus aurata). Aquaculture, 559: 738433. https://doi.org/10.1016/j.aquaculture.2022.738433
Beheshti SO, Madsen CM, Varbo A and Nordestgaard BG (2020). Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J. Am. Coll. Cardiol., 75(20): 2553-2566. https://doi.org/10.1016/j.jacc.2020.03.057
Banait T, Wanjari A, Danade V, Banait S, Jain J (2022). Role of high-sensitivity C-reactive protein (Hs-CRP) in non-communicable diseases: a review. Cureus, 14(10): e30225 https://doi.org/10.7759/cureus.30225
Beatty C, Richardson KP, Tran PM, Satter KB, Hopkins D, Gardiner M, Sharma A, Purohit S (2024). Multiplex analysis of inflammatory proteins associated with risk of coronary artery disease in type-1 diabetes patients. Clin. Cardiol., 47(1): e24143. https://doi.org/10.1002/clc.24143
Berger VW, Bour LJ, Carter K, Chipman JJ, Everett CC, Heussen N and Randomization Innovative Design Scientific Working Group Robert A Beckman (2021). A roadmap to using randomization in clinical trials. BMC medical research methodology, 21: 1-24.
Bista R, Ghimire A, Subedi S (2020). Phytochemicals and antioxidant activities of Aloe vera (Aloe barbadensis). J. Nut. Sci. Heal. Diet., 1(1): 25-36. https://doi.org/10.47890/jnshd/2020/rbista/10243803
Dubey A, Dash SL, Kumari M, Patel S, Singh S, Agarwal S (2023). A Comprehensive Review on Recent Progress in Invivo and Invitro Models for Hyperlipidemia Studies. Pak. Heart J., 56(1): 286-297.
Egbuna C, Gupta E, Ezzat, SM, Jeevanandam J, Mishra N, Akram M, Onyekere PF (2020). Aloe species as valuable sources of functional bioactives. Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations, 337-387. https://doi.org/10.1007/978-3-030-42319-3_18
Ferrer MD, Busquets-Cortés C, Capó X, Tejada S, Tur JA, Pons A, Sureda A (2019). Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem., 26(18): 3225-3241. https://doi.org/10.2174/0929867325666180514112124
Ferraro RA, Leucker T, Martin SS, Banach M, Jones SR and Toth PP (2022). Contemporary management of dyslipidemia. Drugs, 82(5): 559-576. https://doi.org/10.1007/s40265-022-01691-6
Hęś M, Dziedzic K, Górecka D, Jędrusek-Golińska A, Gujska E (2019). Aloe vera (L.) Webb.: natural sources of antioxidants–a review. Plant Foods Hum. Nutr., 74: 255-265. https://doi.org/10.1007/s11130-019-00747-5
Hosseini A, Khoshsovt F, Ahmadi M, Azarbayjani MA, Salehi O, Farkhaie F (2020). Effects of Aloe vera and swimming training on lipid profile of streptozotocin induced diabetic rats. Nutrition and food sciences research, 7(1): 9-16. https://doi.org/10.29252/nfsr.7.1.9
Jerine PS, Kannath JJ, John P, Abraham R, Evan PS (2020). A Study on the pharmacological activity of the seeds of Aloe vera: in-silico and GC-MS approach. International Research Journal of Pharmacy. 11(6): 20-24. https://doi.org/10.7897/2230-8407.110660
Kumar A, Mahajan A, Begum Z (2020). Phytochemical screening and in vitro study of free radical scavenging activity of flavonoids of Aloe vera. Res. J Pharm. Technol., 13(2): 593-598. https://doi.org/10.5958/0974-360x.2020.00112.2
Kumar S, Kalita S, Basumatary IB, Kumar S, Ray S, Mukherjee A (2024). Recent advances in therapeutic and biological activities of Aloe vera. Biocatal. Agric. Biotechnol., 57: 103084. https://doi.org/10.1016/j.bcab.2024.103084
Lanka S (2018). A review on Aloe vera-The wonder medicinal plant. J. Drug. Deliv. Sci. Ther., 8(5-s): 94-99. https://doi.org/10.22270/jddt.v8i5-s.1962
Liang JJ, Bonvino NP, Hung A, Karagiannis TC (2020). In silico characterisation of olive phenolic compounds as potential cyclooxygenase modulators. Part 1. J. Mol. Graph. Model, 101: 107719 . https://doi.org/10.1016/j.jmgm.2020.107719
Lin CF, Chang YH, Chien SC, Lin YH, Yeh HY (2018). Epidemiology of dyslipidemia in the Asia Pacific region. Int. J. Gerontol, 12(1): 2-6. https://doi.org/10.1016/j.ijge.2018.02.010
Manye SJ, Saleh JS, Ishaya HB, Chiroma SM, Attah MOO, Dibal NI (2023). Phytochemical screening and in-vitro antioxidant activities of aqueous and methanol extracts of Aloe vera. Pharmacol. Res.-Mod.Chin. Med., 8: 100291. https://doi.org/10.1016/j.prmcm.2023.100291
Marianne M, Mariadi M, Nugraha SE, Nasution R, Syuhada PN, Pandiangan S (2021). Characteristics and hepatoprotective activity of the Curcuma heyneana rhizome extract toward wistar rats induced by ethanol. Jundishapur J. Nat. Pharm. Prod., 16(4): e112653. https://doi.org/10.5812/jjnpp.112653
Martins da Silva AY, Arouche T da S, Siqueira MRS, Ramalho TC, de Faria LJG, Gester R do M, Carvalho Junior RN de, Santana de Oliveira M, Neto AM de JC (2023). SARSCoV-2 external structures interacting with nanospheres using docking and molecular dynamics. J. Biomol. Struct. Dyn., pp. 1–16. https://doi.org/10.1080/07391102.2023.2252930
Masenga SK, Kabwe LS, Chakulya M, Kirabo A (2023). Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci., 24(9): 7898. https://doi.org/10.3390/ijms24097898
Mayasari CK, Gunarti DR, Octavia LI (2024). Potential Role of Propolis Flavonoid on Malondialdehyde and Superoxide Dismutase Levels on Endometriosis. J. La Medihealtico, 5(2): 323-339. https://doi.org/10.37899/journallamedihealtico.v5i2.1202
Misawa E, Tanaka M, Nomaguchi K, Nabeshima K, Yamada, M, Toida T, and Orally H (2012). Oral ingestion of Aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in Zucker diabetic fatty rats. J. Agric. Food Chem., 60(11): 2799-2806. https://doi.org/10.1021/jf3001173
Nabila VK, Putra IB. (2020). The effect of Aloe vera ethanol extract on the growth inhibition of Candida albicans. Med Glas, 17(2): 485-9. https://doi.org/10.17392/1098-20
Park MY, Kwon HJ, Sung MK (2009). Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotechnol. Biochem., 73(4): 828-832. https://doi.org/10.1271/bbb.80714
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004). UCSF Chimera. A visualization system for exploratory research and analysis. J. Comput. Chem., 25(13): 1605–1612. https://doi.org/10.1002/jcc.20084
Ramírez O, Pomareda F, Olivares B, Huang YL, Zavala G, Carrasco-Rojas J, Alvarez S, Leiva Sabadini C, Hidalgo V, Romo P, Sanchez M, Vargas A, Martinez J, Aguayo S, Schuh, C. M. (2024). Aloe vera peel-derived nanovesicles display anti-inflammatory properties and prevent myofibroblast differentiation. Phytomedicine, 122: 155108. https://doi.org/10.1016/j.phymed.2023.155108
Singh A, Uzun G, Bakchoul T (2021). Primary immune thrombocytopenia: novel insights into pathophysiology and disease management. J. Clin. Med., 10(4): 789. https://doi.org/10.3390/jcm10040789
Sabbaghzadegan S, Golsorkhi H, Soltani MH, Kamalinejad M, Bahrami M, Kabir A, Dadmehr M. (2021). Potential protective effects of Aloe vera gel on cardiovascular diseases: A mini-review. Phytotherapy Res., 35(11): 6101-6113. https://doi.org/10.1002/ptr.7219
Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP (2020). Pharmacological update properties of Aloe vera and its major active constituents. Molecules, 25(6): 1324. https://doi.org/10.3390/molecules25061324
Şeker U, Güzel BC, Şener D, Baygeldi SB, Yüksel M, Demirel ÖU, Soker S (2023). Nephroprotective Effect of Aloe vera extract with regulation of oxidative stress, apoptosis and aquaporin 3 expression levels in streptozotocin induced diabetic rats. J. Fac. Pharm. Ankara., 47(2): 12-12. https://doi.org/10.33483/jfpau.1225760
To share on other social networks, click on any share button. What are these?