The Content of the Secondary Metabolite and Anti-Oxidant of Various Onion and its Effects on the Growth Performance and Blood Profile of Broiler Chickens
Research Article
The Content of the Secondary Metabolite and Anti-Oxidant of Various Onion and its Effects on the Growth Performance and Blood Profile of Broiler Chickens
Saadatin Nurul Jannah1, Edhy Sudjarwo1, Osfar Sjofjan2*
1Department of Animal Production, Faculty of Animal Science, Universitas Brawijaya, Malang 65145, East Java, Indonesia; 2Department of Animal Nutrition and Feed Science, Faculty of Animal Science, Universitas Brawijaya, Malang, East Java, Indonesia.
Abstract | This study aimed to determine the contents of food substances, total flavonoid active compounds, and antioxidant activity as a substitute for synthetic antibiotic feed additives given to broiler chickens. This study was conducted using a laboratory experiment using garlic peel powder as a material, an active substance content test using a quantitative flavonoid test, and antioxidant activity. Additionally, onion peels are used as a feed additive for broiler chickens to evaluate production performance and blood profile. The material used was 189 one-day-old commercial broiler chickens (unsexing). This study consisted of 9 treatments and 3 tests. The treatment used was T0(-): basal diet, T0(+): basal diet + antibiotic (zinc bacitracin 0.10%), T1: basal diet + 0.50 % garlic peel, T2: basal diet + 0.50 % shallot peel, T3: basal diet + 0.50 % onion peel, T4: basal diet + 0.25 % garlic peel and 0.25% shallot peel, T5: basal diet + 0.25 % garlic peel + 0,25% onion peel, T6: basal diet + 0.25% shallot peel + 0.25% onion peel, T7: basal diet + 0.17% garlic peel + 0.17% shallot peel + 0.17% onion peel. The variables studied were production appearance (feed consumption, body weight gain, feed conversion ratio, production index) and blood profile (haemoglobin, haematocrit, erythrocytes, leukocytes). The experimental design used a Complete Randomized Design, and the research data were analysed using Analysis of Variance (ANOVA), if a significantly different result is obtained, it is continued with Duncan’s Multiple Range Test. The results showed no significant effect of treatment (P>0.05) on production appearance (feed consumption, body weight gain, feed conversion ratio, production index) and blood profile (haemoglobin, haematocrit, erythrocytes, leukocytes. Although the addition of garlic peel powder, shallot peel, and onion peel powder can be used as a feed additive, these three ingredients can improve the immune system in broiler chickens.
Keywords | Antioxidant, Blood profile, Flavonoid, Onion peel, Production performance
Received | February 28, 2024; Accepted | March 10, 2024; Published | April 16, 2024
*Correspondence | Osfar Sjofjan, Department of Animal Nutrition and Feed Science, Faculty of Animal Science, Universitas Brawijaya, Malang, East Java, Indonesia; Email: osfar@ub.ac.id
Citation | Jannah SN, Sudjarwo E, Sjofjan O (2024). The content of the secondary metabolite and anti-oxidant of various onion and its effects on the growth performance and blood profile of broiler chickens. Adv. Anim. Vet. Sci., 12(6):1055-1060.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.6.1055.1060
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Demand for broiler chickens in Indonesia increases yearly in line with the increase in population and public awareness of the importance of meeting protein needs. The development of purebred chicken meat production in Indonesia over the last five years (2018-2022) has experienced fluctuations with a tendency to increase by 2.94% per year or an average meat production of 3.42 million tons per year (Directorate General of Animal Husbandry and Animal Health, 2022). Several factors influencing broiler chicken production are feed factors, environmental conditions, rearing management, and health and biosecurity management. Feed is the most critical factor in broiler farming to achieve optimal productivity. Therefore, the quantity and quality of feed need to be considered because feed costs reach 65-75% of total production costs (Sjofjan et al., 2021). One of the efforts made by breeders to increase broiler chicken productivity is the addition of growth promoters in synthetic antibiotics. Providing antibiotics in feed must take into account several aspects, including high prices, the working system of antibiotics, which can damage the balance of intestinal microflora, and resistance to microorganisms and can cause adverse effects in the form of residues on broiler carcasses when used continuously (Abd-El-Hack et al., 2022).
The development of science and technology in various fields, including animal husbandry and animal health, supports technological developments that efficiently use synthetic antibiotic substitutes. The use of this technology is in line with the law which regulates the cessation of the use of antibiotics, namely the Republic of Indonesia Minister of Agriculture Regulation No.14/PERMENTAN/PK.350/5/2017 concerning the classification of veterinary drugs, which explains the prohibition on the use of antibiotics as feed additives. Efforts that can be made to optimize feed use and broiler chicken production are by adding feed additives. Potential local food additives can use underutilized market waste and still have relatively good nutritional content, including garlic peel, shallot peel, and onion peel.
Garlic peels (Allium sativum L.), shallot peels (Allium ascalonicum L.), and onion peels (Allium cepa L.) are all viable options for utilization. The maximum utilization of onion peels has yet to occur. It is common to encounter onion shells in restaurants and markets. Active constituents of garlic peels (Allium sativum L.) include flavonoids, alkaloids, saponins, polyphenols, and essential oils. These active compounds function synergistically as antibacterials by causing bacterial cell wall damage and lysis. Active shallot peel extract (Allium ascalonicum L.), constituents include flavonoids, triterpenoids, saponins, steroids, and polyphenols. As antioxidants, flavonoid compounds can repair damaged body cells and prevent the formation of free radicals (Bordin et al., 2023). Saponins, glycosides, steroids, flavonoids, and glycosides are all present in the onion peels (Allium cepa L.). Onions comprise quercetin, a flavonoid compound classified as a polyphenol group that inhibits lipid peroxidation and thus can protect against free radicals and heavy metals (Gawlik-Dziki et al., 2013). When added to animal feed, the active compounds found in garlic peel, shallot peel, and onion peel can function as antibacterials, restoring bacterial balance to the digestive tract and enhancing livestock production. This study aims to assess the impact of incorporating onion peel powder into broiler chickens feed on their production performance and blood profile.
MATERIALS AND METHODS
Experimental design
A total of 189 days old chick (DOC) chickens from the Lohmann strain (unsexed). Each treatment was randomly assigned within a completely randomized design, with nine treatments and three replications with total in 27 pens. Each cage housed 7 chicks and they were raised for a period of 35 days. All broiler chickens were kept in environmentally controlled rooms within pens measuring 50 x 50 x 50 square meters, filled with rice hull litter. Broiler chickens were provided unrestricted access to water via adjustable nipple drinkers. The treatments included Shallot peel (Allium ascalonicum L.), Garlic peel (Allum sativum L.), and Onion peel (Allium cepa L.). The treatment feeds were as follows: T0(-) (basic feed), T0(+) (basic feed + antibiotic zinc bacitracin 0.1%), T1 (basic feed + 0.5% garlic peel), T2 (basic feed + 0.5% shallot peel), T3 (basic feed + 0.5% onion peel), T4 (basic feed + 0.25% garlic peel + 0.25% shallot peel), T5 (basic feed + 0.25% garlic peel + 0.25% onion peel), T6 (basic feed + 0.25% shallot peel + 0.25% onion peel), and T7 (basic feed + 0.17% garlic peel + 0.17% shallot peel + 0.17% onion peel).
Garlic peel, shallot peel, and onion peel powder preparation
The garlic peels, shallot peels, and onion peels were obtained from traditional markets in Malang, East Java, Indonesia. The procedure for producing onion peel powder involved the following steps: The sorted onion peels were cleaned using flowing water, after that, the onion peels were dried for 24 hours at 50oC in the oven, reducing the amount of water to less than 10% then proceed with grinding using a grinder until it had a powder texture. The onion peel powder was filtered using a 100-micro mesh sieve to form a powder and then the total flavonoid content and antioxidant activity were analysed.
Total flavonoid compound
The total flavonoid test of onion peel powder was carried out at the Plant Pest and Disease Laboratory, Faculty of Agriculture, Universitas Brawijaya. The quantity of flavonoids in onion peel powder will be determined using the UV-Vis Spectrophotometry methods and the subsequent processes. A 2000 µg/ml concentration of onion peel powder was dissolved using methanol. The test sample, weighing 0.5 grams, was put into 1.5 millilitres of methanol. Add 0.1 ml of sodium acetate (C2H3NO3) 1M, 2.8 ml of Aqueduct, and 0.1 ml of AlCl310%. UV-Vis spectrophotometry determined the mixture’s absorbance at 510 nm after 30 minutes of incubation. The determination of the total flavonoid compound in the sample was conducted by quantifying it in milligrams of equivalents per gram of sample, utilizing the dry weigh.
Antioxidant activity index
The antioxidant activity of onion peel powder was carried out at the Plant Pest and Disease Laboratory, Faculty of Agriculture, Universitas Brawijaya. The DPPH (2,2-diphenyl-1-picrylhydrazyl) method in UV-Vis spectrophotometry was used to measure antioxidant activity, and the following procedure was followed: Materials and tools were ready. 50 µg/ml of sample concentration was created by combining 0.5 grams of onion peel with 9.5 millilitres of distilled water. 50 mg of DPPH powder was combined with 100 ml of methanol to create a 50 ppm DPPH solution. To produce a comparison solution, 2 millilitres of methanol and 1 millilitre of 50 parts per million DPPH were combined. To find the IC50 value, use two millilitres of the sample and two millilitres of the DPPH solution. After 30 minutes of incubation at 27°C, the mixture turned yellow instead of purple, and the absorbance value was determined using a UV-Vis spectrophotometer set to 517 nm. After that, the percentage of DPPH absorption inhibition was used to calculate the antioxidant activity of the sample based on the degree of DPPH radical absorption inhibition.

The linear regression equation, y = a(x) + b, is displayed with the sample concentration and extract inhibition values on the x and y axes, respectively. The IC50 value (inhibitor concentration 50%) for each sample was determined using the following equation by setting the dependent variable (y) as 50 and solving for the corresponding independent variable (x), which represents the IC50 value. The IC50 value is the concentration of the sample solution necessary to achieve a 50% reduction in DPPH (Scherer and Godoy, 2009). The Antioxidant Activity Index (AAI) value may be calculated utilizing the formula provided below:
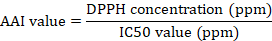
Growth performance
The chickens were initially weighed at the start of the trial, and then weighed weekly until the experiment concluded after 35 days by following (Sjofjan and Adli, 2021). The feed intake was determined by calculating the difference between the feed offered to broiler chickens and the remaining feed each week (Sjofjan et al., 2021). Additionally, the feed intake was adjusted for the mortality of broilers that occurred during the experiment. Feed efficiency, measured as feed/gain, was calculated by dividing the feed intake by the body weight gain of the broiler chickens throughout the experiment. Mortalities were recorded per pen as a percentage from the start to the end of the experiment (Sjofjan et al., 2021).
Blood profile
Analysis of blood profile hematology observations was carried out at the Healthy Animal Laboratory Indonesia, Malang. Blood samples for haematological examination were obtained from 35-days old chickens. The birds are fasted for eight hours before blood collection. Using a 3 ml disposable syringe (BD syringe), blood is collected through the brachial vein and transferred directly into a sterile tube containing the anticoagulant ethylene diamine tetra acetic acid (EDTA) (Adeyemi et al., 2021). Blood samples were taken to the laboratory in a refrigerated tray containing ice gel in less than two hours from collection. Haematological parameters were analysed, such as hemoglobin, erythrocytes, and leukocytes. The haematological analysis is performed automatically with the ABX Micro 60 reagents and the Vet Auto Haematology Analyzer M-HEMA100.
Statistical analysis
An analysis of variance (ANOVA) was performed using the general linear model (GLM) in GraphPad Prism 5.2.1. The findings were reported as standard error mean (SEM), and probability values were determined using least significant difference testing. The model utilized for analysis is outlined below adapted from (Adli et al., 2023).
Yij = μ + Ti + eij
Where Yij was parameters observed, μ was the overall mean, Tithe effect level of various onion meal, and eij the amount of error number.
RESULTS AND DISCUSSION
Total flavonoid and antioxidant activity index (AAI)
Flavonoids are a secondary bioactive compound found in plants, including onion peels. This compound has potential antioxidant and anti-inflammatory activity. The total average of flavonoids contained in onion peel powder can be seen in Table 1. The laboratory analysis results show differences in the flavonoid content of the three samples analysed. Garlic peel powder 311,256 ppm of flavonoids, 348,213 ppm of shallot peel, and 351,223 ppm of onion peel (Table 1). Numerically, the highest total flavonoid content is onion peel powder. This is supported by research by Albishi et al. (2013), which states that the flavonoid content in onion peel is higher than in garlic peel, onion peel contains a total of 10.69 mg QE/g of flavonoids and 0.08 mg QE/g in garlic peel. The high total flavonoid content in onions is caused by the main components of flavonoids in quercetin, kaempferol, myricetin, and catechin (Kumar et al., 2022). Providing onion peel powder is expected to inhibit the growth of pathogenic bacteria and improve the balance of intestinal microflora, thereby increasing the digestibility and absorption of feed, which improves the performance of broiler chicken production.
Table 1: Total flavonoid and antioxidant of garlic peel, shallot peel and onion peel.
|
Parameters |
Items |
||
|
Garlic peel |
Shallot peel |
Onion peel |
|
|
Flavonoid (ppm) |
311.256 |
348.213 |
351.223 |
|
AAI |
0.762 |
0.773 |
0.723 |
AAI: antioxidant activity index; ppm: part per millions.
The average antioxidant activity contained in onion peel powder can be seen in Table 1. The laboratory analysis results show that garlic peel has an antioxidant activity index value of 0.762, shallot peel 0.773, and onion peel 0.723 Figure 1. The medium antioxidant category includes the antioxidant activity index value of garlic peel, shallot peel, and onion peel flour. This refers to Scherer and Godoy (2009) that the value of AAI ˂ 0.5: weak antioxidant, AAI ˃ 0.5-1: moderate antioxidant, AAI ˃ 1-2: potent antioxidant, and AAI ˃ 2: powerful antioxidant.
Numerically, the highest antioxidant activity index (AAI) value is shallot peel powder 0.773. Supported by research by Yang et al. (2019) shows that onion peel extract using ethanol as a solvent has an IC50 antioxidant activity of 10.60 µg/mL, higher than the IC50 antioxidant activity content of onion peel extract IC50 4.50 µg/mL. The high value of antioxidant activity in shallot peel is due to its dominant flavonoid content (quercetin, isorhamnetin, and kaempferol). Quercetin is the main component in the flavonoid group contained in onion peels. The potent antioxidant properties of quercetin play a role in protecting body cells from damage caused by free radicals (Gawlik-Dziki et al., 2013). Other research conducted by Albishi et al. (2013) showed that the onion peels content of four varieties (shallot, white, onion, and pearl peels) had higher antioxidant activity compared to the onion bulb due to the higher phenolic and flavonoid content in the onion peel. Based on this, garlic peel powder, shallot peel, and onion peel have good antioxidant activity. Antioxidants play a role in protecting body cells from damage caused by free radicals. The application of onion peels as a feed additive is expected to help reduce the impact of oxidative stress in chickens and support the immune system by protecting cells from oxidative damage (Bouhenni et al., 2021).
Effect of onion peel powder on the growth performance of the broiler chickens
Table 2 illustrates the impact of onion peels as a feed additive on the production performance of broiler chickens. Additionally, the result showed that treatment did not significantly impact (p>0.05) on feed intake, feed conversion ratio, body weight, and Production Index broiler chickens. The inability of the active ingredients in garlic powder to function at their best is the reason for the insignificant impact on feed intake. In this study, the phytobiotic was treated with a concentration of 1% in 1 kg of feed, so this caused the nutritional content of the feed to be similar between treatments. However, flavonoids have antibacterial capabilities that can effectively impede the proliferation of harmful microorganisms in the gastrointestinal tract. Flavonoid chemicals serve as antioxidants by protecting body cells from damage by free radicals, reducing the effects of oxidative stress on hens, and boosting the immune system of broiler chickens (Bouhenni et al., 2021). The improved feed utilization efficiency can be achieved by incorporating onion peel powder alone or combined with three different types. The result is attributed to the well-balanced antibacterial and antioxidant properties present in all three varieties of onion peel. According to (Ao et al., 2011) garlic supplementation (5-20g/kg) positively affected the histological structure of the small intestine in broiler chickens, which may change nutrient digestion and absorption to increase growth performance.
Effect of onion peel powder on the blood profile of the broiler chickens
Blood profile observations are parameters used to indicate the health status of livestock. They serve as transport media and nutrient absorption indicators for the treatment given. Research was conducted on the effect of giving onion peel flour on blood profiles, including haemoglobin, haematocrit, erythrocytes, and leukocytes. The results of this study are shown in Table 3. Additionally, the result showed that treatment did not significantly impact (p>0.05) on blood profile such as haemoglobin, haematocrit, erythrocytes, and leukocytes broiler chickens. Haematocrit and haemoglobin levels increase or decrease together based on the number of erythrocytes. Changes in the haematocrit level can have an impact on the viscosity of blood (Reisinger et al., 2012). The amount of haemoglobin in an animal’s body is influenced by the protein and mineral (Fe) content of the feed they consume. This is important because haemoglobin is essential for the process of hemopoiesis, which is the formation of blood cells that takes place in the spinal marrow. Adding a small percentage of onion peel powder to the animal feed does not affect its nutritional value, including its protein and Fe content (Adeyemi et al., 2021). Haemoglobin requires minerals and protein as precursors for succinyl Co-A to form Heme molecules.The protein needed in the hemopoiesis process is acidic. The amino acids glycine, methionine, and Fe are necessary to form haemoglobin in red blood cells (Garcia-Santos et al., 2017).
Table 2: Effect of onion peel powder on the growth performance of the broiler chickens.
|
Treatments |
Variables |
||||
|
Feed intake (g/bird) |
Body weight (g/bird) |
FCR |
Production index |
||
|
T0 (-) |
2947.18 ± 48.60 |
1890.08 ± 81.24 |
1.56 ± 0.05 |
320.54 ± 40.32 |
|
|
T0 (+) |
3050.33 ± 74.41 |
1978.00 ± 31.60 |
1.54 ± 0.02 |
285.08 ± 30.14 |
|
|
T1 |
3082.00 ± 66.67 |
1887.18 ± 59.99 |
1.64 ± 0.08 |
306.76 ± 45.36 |
|
|
T2 |
2982.85 ± 115.50 |
1912.62 ± 64.11 |
1.56 ± 0.03 |
341.98 ± 8.10 |
|
|
T3 |
2965.40 ± 27.82 |
1968.33 ± 104.63 |
1.51 ± 0.07 |
367.19 ± 37.49 |
|
|
T4 |
3060.52 ± 51.68 |
1892.86 ± 65.95 |
1.62 ± 0.05 |
294.45 ± 46.40 |
|
|
T5 |
3106.55 ± 30.82 |
1940.00 ± 114.69 |
1.61 ± 0.11 |
339.98 ± 41.23 |
|
|
T6 |
3008.59 ± 76.78 |
1907.38 ± 86.77 |
1.58 ± 0.06 |
323.55 ± 52.35 |
|
|
T7 |
3067.71 ± 37.37 |
1989.52 ± 83.91 |
1.54 ± 0.08 |
358.59 ± 26.69 |
|
|
SEM |
33.97 |
44.44 |
0.03 |
18.91 |
|
|
p |
0.07 |
0.65 |
0.41 |
0.34 |
|
FCR: feed conversion ratio; g: gram; T0(-): basic feed + free antibiotic; T0(+): basic feed + antibiotic (zinc bacitracin 0.1%); T1: basic feed + 0.5 % garlic peel; T2: basic feed + 0.5 % shallot peel; T3: basic feed + 0.5 % onion peel; T4: basic feed + 0.25 % garlic peel + 0.25% shallot peel; T5: basic feed + 0.25 %. garlic peel + 0,25% onion peel; T6: basic feed + 0.25% shallot peel + 0.25% onion peel; T7: basic feed + 0.167% garlic peel + 0.17% shallot peel + 0.17% onion peel.
Table 3: Effect of onion peel powder on the blood profile of the broiler chickens.
|
Variables |
||||
|
T |
Hemoglobin (g/dl) |
Hematocrit (%) |
Erythrocytes (x 106/mm3) |
Leukocytes (x 106/mm3) |
|
T0(-) |
13.47 ± 1.15 |
54.33 ± 4.03 |
10.53 ± 0.95 |
19.77± 4.76 |
|
T0(+) |
22.70 ± 3.21 |
54.67 ± 5.31 |
10.92 ± 0.50 |
22.53 ± 4.51 |
|
T1 |
18.03 ± 7.52 |
57.70 ± 0.00 |
10.65 ± 0.57 |
22.75 ± 9.26 |
|
T2 |
18.50 ± 4.50 |
55.93 ± 2.49 |
10.76 ± 0.19 |
18.63 ± 2.40 |
|
T3 |
18.27 ± 6.48 |
55.37 ± 0.57 |
10.31 ± 0.31 |
18.40 ± 8.17 |
|
T4 |
16.17 ± 1.69 |
58.00 ± 2.95 |
10.96 ± 0.75 |
15.27 ± 2.64 |
|
T5 |
19.43 ± 6.23 |
58.20 ± 5.07 |
10.45 ± 0.42 |
28.70 ± 2.42 |
|
T6 |
15.23 ± 1.66 |
56.17 ± 2.87 |
10.56 ± 0.34 |
23.33 ± 5.17 |
|
T7 |
15.00 ± 1.40 |
59.57 ± 2.25 |
10.63 ± 0.32 |
15.16 ± 4.18 |
|
SEM |
2.17 |
1.64 |
0.28 |
2.79 |
|
p |
0.36 |
0.56 |
0.85 |
0.12 |
dl: decilitre; g: gram; T0(-): basic feed + free antibiotic; T0(+): basic feed + antibiotic (zinc bacitracin 0.1%); T1: basic feed + 0.5 % garlic peel; T2: basic feed + 0.5 % shallot peel; T3: basic feed + 0.5 % onion peel; T4: basic feed + 0.25 % garlic peel + 0.25% shallot peel; T5: basic feed + 0.25 %. garlic peel + 0,25% onion peel; T6: basic feed + 0.25% shallot peel + 0.25% onion peel; T7: basic feed + 0.167% garlic peel + 0.17% shallot peel + 0.17% onion peel.
Leukocyte values within the normal range indicate that feeding broiler chickens with garlic peel powder, shallot peel powder, and onion peel powder does not lead to any inflammatory response or decrease in immune function. Flavonoids have antioxidant properties that can protect leukocytes and other cells in the chicken’s body against free radicals. Using photobiotic containing flavonoids can improve the immune system by maintaining the number of leukocytes, thus protecting the body from disease-causing microbes (Geevarghese et al., 2023). Flavonoids are beneficial compounds that help protect the endothelial cells lining blood vessels, as well as cardiovascular cells and tissue, from oxidative damage caused by free radicals (Reisinger et al., 2012).
CONCLUSIONS and Recommendations
This study concludes that using garlic peel powder, shallot peel powder, and onion peel powder can be used as a feed additive. This choice yields positive effects on production performance and blood profile, particularly in terms of Feed Conversion Ratio (FCR) and the Performance Index (IP).
ACKNOWLEDGeMENTS
The authors thanks to Universitas Brawijaya and LPDP (Lembaga Pengelola Dana Pendidikan) for funding this research.
NOVELTY STATEMENT
The novelty of this research is the use of secondary various garlic (garlic peel powder, shallot peel powder, and onion peel powder) as an alternative feed additive.
AUTHOR’S CONTRIBUTION
SNJ contributed to data collection, nutritional analysis, data analysis and manuscript preparation. ES and OS contributed to research design, supervision and revision of the manuscript. All authors read and approved the final version of the manuscript in the journal at this time.
Ethical approval
The Animal Research Ethics Committee, Universitas Brawijaya approved in vivo experiments with approval number 170-KEP-UB-2023.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abd-El-Hack ME, El-saadony M, Salem H, El-Tahan A, Soliman MM, Youssef GB, Taha AE, Soliman SM, Ahmed AE, Rl-kott A, Al syaad KM, Swelum AA (2022). Alternatives to antibiotics for organic poultry production: Tipes, modes of action and impacts on bird’s health and production. J. Poult. Sci., pp. 1–20. https://doi.org/10.1016/j.psj.2022.101696
Adeyemi KD, Oseni AI, Asogwa TN (2021). Onionskin waste versus synthetic additives in broiler diet: Influence on production indices, oxidative status, caecal bacteria, immune indices, blood chemistry and meat quality. Ital. J. Anim. Sci., 20(1): 587–599. https://doi.org/10.1080/1828051X.2021.1892545
Adli DN, Sadarman S, Irawan A, Jayanegara A, Wardiny TM, Prihambodo TR, Nayohan S, Permata D, Sholikin MM, Yekti AP (2023). Effects of oligosaccharides on performance, egg quality, nutrient digestibility, antioxidant status, and immunity of laying hens: A meta-analysis. Ital. J. Anim. Sci., 22: 594-604. https://doi.org/10.1080/1828051X.2023.2223211
Albishi T, John JA, Al-Khalifa AS, Shahidi F (2013). Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods, 5(3): 1191–1203. https://doi.org/10.1016/j.jff.2013.04.002
Ao X, Yoo JS, Zhou TX, Wang JP, Meng QW, Yan L, Cho JH, Kim IH (2011). Effect of fermented garlic powder supplementation on growth performance, blood profiles and breast meat quality in broilers. Livest. Sci., 141: 85–89. https://doi.org/10.1016/j.livsci.2011.05.002
Bordin V, Piovesan N, Mello RDO, Barin JS, Fogaca ADO, Bizzi CA, De Moraes Flores ÉM, Dos Santos Costa AC, Pereira DE, Soares JKB, Hashime KE (2023). Ultrasonic assisted extraction of phenolic compounds with evaluation of red onion skin (Allium cepa L.) antioxidant capacity. J. Culinary Sci. Technol., 21(1): 156–172. https://doi.org/10.1080/15428052.2021.1910095
Bouhenni H, Doukani K, Hanganu D, Olah N, Şekeroğlu N, Gezici S (2021). Analysis of bioactive compounds and antioxidant activities of cultivated garlic (Allium sativum L.) and red onion (Allium cepa L.) in algeria. Int. J. Agric. Environ. Food Sci., 5(4): 550–560. https://doi.org/10.31015/jaefs.2021.4.15
Directorate General of Animal Husbandry and Animal Health. (2022). Livestock and Animal Health Statictics 2022. Jakarta
Garcia-Santos D, Schranzhofer M, Bergeron R, Sheftel AD, Ponka P (2017). Extracellular glycine is necessary for optimal hemoglobinization of erythroid cells. Haematologica, 102(8): 1314–1323. https://doi.org/10.3324/haematol.2016.155671
Gawlik-Dziki U, Świeca M, Dziki D, Baraniak B, Tomiło J, Czyz J (2013). Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem., 138(3): 1621–1628. https://doi.org/10.1016/j.foodchem.2012.09.151
Geevarghese AV, Kasmani FB, Dolatyabi S (2023). Curcumin and curcumin nanoparticles counteract the biological and managemental stressors in poultry production: An updated review. Res. Vet. Sci., https://doi.org/10.1016/j.rvsc.2023.104958
Kumar M, Barbhai MD, Hasan M, Dhumal S, Singh S, Pandiselvam R, Rais N, Natta S, Senapathy M, Sinha N, Amarowicz R (2022). Onion (Allium cepa L.) peel: A review on the extraction of bioactive compounds, its antioxidant potential, and its application as a functional food ingredient. J. Food Sci., 87(10): 4289–4311. https://doi.org/10.1111/1750-3841.16297
Reisinger N, Ganner A, Masching S, Schatzmayr G, Applegate TJ (2012). Efficacy of a yeast derivative on broiler performance, intestinal morphology and blood profile. Livest. Sci., 143(2–3): 195–200. https://doi.org/10.1016/j.livsci.2011.09.013
Scherer R, Godoy HT (2009). Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem., 112(3): 654–658. https://doi.org/10.1016/j.foodchem.2008.06.026
Sjofjan O, Adli DN (2021). The effect of replacing fish meal with fermented sago larvae (FSL) on broiler performance. Livest. Res. Rural Dev., 33: 2-7.
Sjofjan O, Adli DN, Natsir MH, Nuningtyas YF, Bastomi I, Amalia FR (2021). The effect of increasing levels of palm kernel meal containing α-β-mannanase replacing maize to growing-finishing hybrid duck on growth performance, nutrient digestibility, carcass trait, and VFA. J. Indones. Trop. Anim. Agric., 46: 29-39. https://doi.org/10.14710/jitaa.46.1.29-39
Stoica F, Aprodu I, Enachi E, Stanciuc N, Condurache NN, Bahrim GE, Rapeanu G (2021). Bioactive’s characterization, biological activities, and in silico studies of red onion (Allium cepa L.) skin extracts. J. Plants, 10: 1–17. https://doi.org/10.3390/plants10112330
Yang SJ, Paudel P, Shrestha S, Seong SH, Jung HA, Choi JS (2019). In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: A comparative study. Food Sci. Nutr., 7(1): 205–215. https://doi.org/10.1002/fsn3.863
To share on other social networks, click on any share button. What are these?






