In-vitro Ruminal Ecosystem in Buffaloes on Concentrates and Fat Supplementation
Research Article
In-vitro Ruminal Ecosystem in Buffaloes on Concentrates and Fat Supplementation
Amirul Faiz Mohd Azmi1,4, Hafandi Ahmad1, Norhariani Mohd Nor1, Goh Yong Meng1, Mohd Zamri Saad2, Md Zuki Abu Bakar1, Norafizah Abdul Rahman5,6, Agung Irawan7,9, Anuraga Jayanegara8,9, Hasliza Abu Hassim1,3,9*
1Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia; 2Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia; 3Laboratory of Sustainable Animal Production and Biodiversity, Institute of Tropical Agriculture and Food Security, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia; 4Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia; 5Agro Biotechnology Institute, National Institutes of Biotechnology Malaysia, Serdang 43400, Selangor, Malaysia; 6Faculty of Agriculture and Life Sciences, Lincoln University, Lincoln 7647, Canterbury, New Zealand; 7Vocational School, Universitas Sebelas Maret, Surakarta 57126, Indonesia; 8Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia; 9Animal Feed and Nutrition Modelling (AFENUE) Research Group, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia.
Abstract | The use of dietary supplementation such as concentrate and bypass fat to improve the buffalo performance warrants further investigations, especially in an in-vitro study. Even though several studies have reported the potential of supplementation in enhancing the growth of buffaloes, the effect on a different breeds of buffaloes, the potential to reduce methane production, and the changes in microbial populations remained unclear following different ratios of forage: supplementation feeding regime. This study described the effects of supplementing Brachiaria decumbens grass (G) received either concentrate (C) or mixed with bypass fat (B) supplement on the in-vitro rumen fermentation and microbial ecosystem of Murrah cross and Swamp buffaloes. Three males Murrah cross and Swamp buffaloes consuming 100% DM of fresh B. decumbens were used as rumen contents donors. The in-vitro ruminal fermentation and microbial population profiles were investigated. The study revealed that Diet C had the highest ether extract and gross energy, with optimum value of crude proteins but low in crude fiber compared to diets B and A. Total volatile fatty acids (TVFA) and their molars proportion, gas production, total fatty acids, total bacteria count, and total protozoa count increased in parallel with the concentrate levels in Diets B and C (P < 0.05) in both breeds. The result also revealed that Murrah cross and Swamp buffaloes showed comparable rumen fermentation patterns when treated with the same dietary treatments, but Swamp buffalo were significantly (P < 0.05) higher in Ruminococcus albus and total fatty acid. This study showed that supplementing concentrates solely or a mixture with bypass fat into a grass-based diet could decrease methane production, as well as methanogens without giving a detrimental effect on rumen fermentation but also increase the degree of fatty acids saturation partially via increasing the abundance of fibrolytic bacteria. Thus, both dietary treatments are highly recommended to enhance optimal rumen fermentation and eventually support production performance.
Keywords | Buffaloes; Bypass Fat; Concentrate; In-vitro Rumen Fermentation; Microbial Population
Received | October 16, 2022; Accepted | April 25, 2023; Published | June 15, 2023
*Correspondence | Hasliza Abu Hasim, Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia; Email: [email protected]
Citation | Azmi AFM, Ahmad H, Nor NM, Meng GY, Saad MZ, Abu Bakar MZ, Rahman NA, Irawan A, Jayanegara A, Hassim HA (2023). In-vitro ruminal ecosystem in buffaloes on concentrates and fat supplementation. Adv. Anim. Vet. Sci. 11(8): 1313-1331.
DOI | https://doi.org/10.17582/journal.aavs/2023/11.8.1313.1331
ISSN (Online) | 2307-8316

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Indigenous Swamp and introduced Murrah cross are the most common buffalo breeds in Malaysia (Mohd Azmi et al., 2021a). They play important roles to produce high-quality animal proteins for growing populations, particularly meat and milk (Kusriatmi et al., 2014). In Malaysia, the buffalo industry contributed approximately 11.5% of total ruminant meat production, which is equivalent to 21% of protein sources (Cruz, 2010). Therefore, Malaysia is continuously trying to improve buffalo production to achieve 50% self-sufficiency in meat by 2025. However, the buffalo population between 2013 and 2017 progressively decreased by an average of 1.97% per annum (Department of Veterinary service, 2018). As a result, the local demand for meat remains inadequate, especially with the increasing numbers of the human population (Mohd Azmi et al., 2021b; Shahudin et al., 2018; Zamri-Saad and Azhar, 2015). It is reported that poor feeding management mainly contributed to lower buffalo production (Mohd Azmi et al., 2021c; Mohd Azmi et al., 2021b). The lack of high-quality feedstuffs and imbalanced nutrition provided to buffaloes are constraining productivity and performance in developing countries. The ration contains largely forage with no/fewer supplements (Othman et al., 2014; Suharti et al., 2011). Therefore, new sources, approaches, and technology are needed to improve the energy supply and feeding systems that are important in buffalo production (Van Dung et al., 2014). A recent study revealed that adding feed supplements to ruminant diets could improve the animals’ performance traits and health, and this is being practiced in many dairy farms (Duvvu et al., 2018). Feed supplementations, particularly concentrate and bypass fat are commonly practiced in buffalo farms in Brazil, India, and the Philippines. Indeed, high nutrients in feed namely energy and protein, which are provided by the supplements could enhance buffaloes’ growth performance and production (Mohd Azmi et al., 2021b). Therefore, the beneficial influences of adding supplements to the buffalo basal diet in Malaysia need further investigation.
The concentrate is well known for being the main supplement used by farmers to increase the energy content of diets. However, feeding an improper ratio of concentrate with insufficient fiber is likely to cause metabolic problems such as subacute ruminal acidosis (Natnael et al., 2020; Jaramillo-López et al., 2017). In addition, bypass fat supplement has been proven to improve fiber digestibility and protect the essential fatty acids from rumen biohydrogenation while avoiding methanogenesis (Behan et al., 2019) without adverse effect on the ruminal microbial population. Thus, additions of high-fat and protein concentrates and bypass fat supplements are the most common strategies used in ruminant nutrition to optimize the ruminal fermentation (Tahir et al., 2013) and the microbial population (Behan et al., 2019; Haque, 2018). We hypothesize that the addition of concentrates and bypass fat into the diets of Malaysian buffaloes could improve the in-vitro ruminal fermentation. Nevertheless, previous studies suggested that the type of supplement and their ratios affected the characteristics of ruminal fermentation and microbial populations differently (Angeles-Hernandez et al., 2020; Moallem, 2018). This is because each supplement differs in its physical and chemical properties (Behan et al., 2019; Li et al., 2019). Furthermore, there might be inconsistent effects of supplementation due to the slow release of fatty acids into the rumen resulting in an acid-hydrogenated product that impairs microbial activities (Behan et al., 2019; Jiao et al., 2014).
Evidence of the effects of concentrate and fat inclusion in dairy and beef cows, and small ruminants are abundantly available (Angeles-Hernandez et al., 2020; Rafiee-Yarandi et al., 2016; Noviandi et al., 2014; Patra, 2014). To our knowledge, little information is available on the effect of concentrate and protected fat supplementations on ruminal metabolism, particularly ruminal biohydrogenation and methane production in buffaloes. Therefore, this study evaluated the effects of concentrate and bypass fat supplements in buffalo basal diet on the in-vitro rumen fermentation, the kinetics of gas production, fatty acid compositions, and ruminal microbial populations using ruminal fluid from Murrah cross and Swamp buffaloes.
MATERIALS AND METHODS
Ethical statement and experimental station
The study was conducted at Sabah Meat Technology Centre, Sabah, Malaysia. Selected buffaloes for this experiment were kept according to the Animal Utilization Protocol of the Institution Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (Approval No. UPM/IACUC/AUP-017/2018). Sample collection from the animals was strictly performed under veterinary supervision and certified butcher.
Experimental rations and their nutritional analysis
A total of three experimental rations were prepared from three major components, consisting of (1) Brachiaria decumbens (G), (2) a commercial concentrate (C), and commercial bypass fat (B). The composition of concentrate was previously described by Mohd Azmi et al. (2021c) which mostly contained energy sources, macro mineral, and vitamin-mineral premix (composition: corn grain (25.0%), palm kernel cake (32.0%), rice bran (18.0%), soya bean meal (19.7%), calcium carbonate (1.0%), molasses (2.8%), vitamin-mineral premix (0.3%), sodium chloride (0.6%),
Table 1: Nutritional and fatty acid compositions of feedstuffs.
| Feedstuffs |
|||
|
Nutritional composition1 |
Grass | Concentrate | Bypass fat |
| DM (%) | 90.34 | 90.36 | 99.75 |
| Organic matter (% DM) | 5.090 | 5.44 | - |
| Crude fibre (% DM) | 26.03 | 7.49 | - |
| Ether extract (% DM) | 2.03 | 5.46 | 100.00 |
| Crude protein (% DM) | 6.09 | 18.15 | - |
| Neutral detergent fibre (% DM) | 64.27 | 56.87 | - |
| Acid detergent fibre (% DM) | 33.86 | 17.38 | - |
| Acid detergent lignin (% DM) | 3.55 | 2.96 | - |
| Non-fibrous carbohydrates (% DM) | 22.05 | 13.85 | - |
| Gross energy (MJ/kg) | 11.07 | 15.74 | 37.65 |
| Hemicellulose (% DM) | 30.41 | 39.49 | - |
| Cellulose (% DM) | 30.32 | 14.42 | - |
| Fatty acids compositions (% total FA) | |||
| C12:0 (Lauric Acid) | 0.75 | 6.70 | 0.51 |
| C14:0 (Myristic Acid) | 0.30 | 1.96 | 0.16 |
| C16:0 (Palmitic Acid) | 2.03 | 51.5 | 51.2 |
| C18:0 (Stearic Acid) | 8.43 | 10.1 | 0.43 |
| C18:1 n-9, (Oleic Acid) | 45.9 | 19.9 | 47.4 |
| C18:2 n-6, (Linoleic Acid) | 40.4 | 8.10 | 0.10 |
|
C18:3 n-3, (α-Linolenic Acid) |
1.93 | 1.37 | 0.10 |
|
C20:0 (Eicosanoic Acid) |
0.24 | 0.38 | 0.05 |
|
Σ SFA2 |
11.7 | 70.6 | 52.4 |
|
Σ UFA3 |
88.3 | 29.4 | 47.6 |
|
Σ PUFA n-34 |
1.93 | 1.37 | 0.10 |
|
Σ PUFA n-65 |
40.4 | 8.10 | 0.10 |
|
Σ PUFA6 |
42.4 | 9.47 | 0.20 |
| n-6/n-3 ratio | 20.9 | 5.90 | 1.05 |
|
Σ MUFA7 |
45.9 | 19.9 |
47.4 |
Abbreviations: 1g/kg (DM basis), except specified; 2SFA (saturated fatty acids) =C12:0+C14:0+C16:0+ C18:0+C+20:0; 3UFA (unsaturated fatty acids) = C18:1 + C18:2 + C18:3; 4PUFA (polyunsaturated fatty acids) n-3 = C18:3 n-3; 5PUFA (polyunsaturated fatty acid) n-6 = C18:2 n-6; 6Total PUFA = PUFA n-3 +PUFA n-6; 7MUFA (monounsaturated fatty acids) = C18:1. Retrieved with permission from Mohd Azmi et al. (2021c).
Table 2: Nutritional and fatty acid compositions of dietary treatments (DM basis).
| Dietary treatments |
|||
| Ingredient (%) | Diet A | Diet B |
Diet C |
|
Brachiaria decumbens (G) |
100 | 70 | 70 |
| Concentrate (C) | - | 30 | 26 |
| Bypass fat (B) | - | - | 4 |
| Total | 100 | 100 | 100 |
|
Nutritional composition1 |
|||
| DM (%) | 90.34 | 90.35 | 90.72 |
| Organic matter (% DM) | 5.09 | 5.20 | 4.98 |
| Crude fibre (% DM) | 26.03 | 20.47 | 20.17 |
| Ether extract (% DM) | 2.03 | 3.06 | 6.84 |
| Crude protein (% DM) | 6.09 | 9.71 | 8.98 |
|
Neutral detergent fibre (% DM) |
64.27 | 62.05 | 59.78 |
| Acid detergent fibre (% DM) | 33.86 | 28.92 | 28.22 |
| Acid detergent lignin (% DM) | 3.55 | 3.37 | 3.25 |
| Non-fibrous carbohydrates (% DM) | 22.05 | 19.59 | 19.04 |
| Gross energy (MJ/kg) | 11.07 | 12.47 | 13.35 |
| Hemicellulose (% DM) | 30.41 | 33.13 | 31.55 |
| Cellulose (% DM) | 30.32 | 25.55 | 24.97 |
| Fatty acids compositions (% total FA) | |||
| C12:0 Lauric Acid | 0.75 | 2.60 | 2.13 |
| C14:0 Myristic Acid | 0.30 | 1.01 | 0.76 |
| C16:0 Palmitic Acid | 2.03 | 11.0 | 59.6 |
|
C18:0 Stearic Acid |
8.43 | 55.9 | 18.8 |
| C18:1 n-9, Oleic Acid | 45.9 | 13.3 | 9.58 |
| C18:2 n-6, Linoleic Acid | 40.4 | 13.7 | 8.65 |
|
C18:3 n-3, α-Linolenic Acid |
1.93 | 1.04 | 0.46 |
|
C20:0 Eicosanoic Acid |
0.24 | 0.22 | 0.04 |
|
Σ SFA2 |
11.7 | 70.7 | 81.3 |
|
Σ UFA3 |
88.3 | 29.2 | 18.4 |
|
Σ PUFA n-34 |
1.93 | 1.04 | 0.46 |
|
Σ PUFA n-65 |
40.4 | 13.7 | 8.65 |
|
Σ PUFA6 |
42.4 | 14.7 | 9.11 |
| n-6/n-3 ratio | 20.9 | 13.2 | 18.9 |
|
Σ MUFA7 |
45.9 | 13.3 |
9.58 |
1g/kg (DM basis), except specified; 2SFA (saturated fatty acids) =C12:0+C14:0+C16:0+ C18:0+C+20:0; 3UFA (unsaturated fatty acids) = C18:1 + C18:2 + C18:3; 4PUFA (polyunsaturated fatty acids) n-3 = C18:3 n-3; 5PUFA (polyunsaturated fatty acids) n-6 = C18:2 n-6; 6Total PUFA = PUFA n-3 +PUFA n-6; 7MUFA (monounsaturated fatty acids) = C18:1. Retrieved with permission from Mohd Azmi et al. (2021c).
dicalcium phosphate (0.6%), and (3) a commercial bypass fat (B). The commercial bypass was a fractionated palm oil fat in a calcium salt form, formulated without containing trans-fatty acids (OPTI-FAT F8016RXP). The B. decumbens grass was freshly collected from the pasture of Telupid Buffalo Breeding and Research Centre, Sabah, Malaysia. The concentrate and bypass fat supplements were purchased from an authorized manufacturer (Lipidchem Sdn Bhd, Masai, Johor, Malaysia). Dietary treatments were formulated according to the previous study (Mohd Azmi et al., 2021c). Three experimental rations were diet A = 100% B. decumbens which was assigned as control with low energy and protein levels; diet B = 70% B. decumbens and 30% concentrate, and diet C = 70% B. decumbens and 26% concentrate, and 4% bypass fat. Diets B and C were formulated to meet the optimum nutrient requirement protein (9-10% DM) for growing buffalo according to Paengkoum et al. (2021), in which diet C was formulated to increase the ether extract (6-7% DM) (Basra, 2003) and gross energy. Approximately 10 g of feedstuffs and formulated diets were used to determine the nutritional and fatty acid composition. The dry matter (DM, Method: 950.05), Ash (Method: 942.05), crude protein (CP, Method: 984.13), crude fiber (CF, Method: 978.10), and ether extract (EE, 920.39) of feedstuff and formulated diets were analyzed according to AOAC (2000), gross energy was determined by bomb calorimeter (IKA C2000 Basic, IKA-Werke, Staufen, Germany), acid detergent fiber (ADF), and neutral detergent fiber (NDF) were analyzed according to Van Soest et al. (1991), and acid detergent lignin (ADL, Method: 973.18), hemicellulose, and cellulose were analyzed according to method AOAC (2012). Non-fibrous carbohydrate (NFC) was calculated according to Bava et al. (2019) as follows: NFC= 100- Ash-CP-EE-NDF. The fatty acid compositions for the feedstuffs and formulated diets were determined according to the method described by Folch et al. (1957). The nutritional profiles and fatty acid compositions of feedstuffs and formulated diets were analyzed in triplicate and the results were tabulated as a means. The nutritional and fatty acid composition of the feedstuffs and dietary groups are shown in Tables 1 and 2, respectively.
Collection of rumen fluids
This study was organized into two identical in-vitro fermentation trials (runs). For each trial, three male Murrah cross and Swamp buffaloes (n=6) with an average live weight of 330 ± 4 kg were used as rumen contents donors. The animals were slaughtered in a commercial slaughterhouse (Sabah Meat Technology Centre, Sabah, Malaysia) for production purposes, after being fed with 100% DM of fresh B. decumbens grass for 3 weeks. The buffaloes were healthy, housed in the same barn located 20 km from the slaughterhouse and they received standard operational procedures for feeding and management.
On the farm, all buffaloes were given a thorough physical check-up and were treated for ecto- and endoparasites. Buffaloes were housed in individual stalls with free access to feed, clean drinking water, and mineral blocks. The buffalo was fed twice a day (9 am and 5 pm). The area was 30 m2 per animal, mostly with a compacted dirt floor, while the area close to the feeder was covered with concrete. The stalls were cleaned twice daily (8 am and 4 pm) using water rush for removing all the litter. The natural was adequate to monitor the animals at the day, while industrial dim lighting was used at night. Mechanical ventilation tools (using fans with diameter 20 cm and velocity 1.5 ms-1) from the eastern side and opened west ventialtion were used in the farm for removing carbon dioxide produced by the animals and emitted from the manure. The average daily temperature, relative humidity and annual rainfall in the farm recorded were between 29 °C to 30 °C, 65.5% to 68.5% and 200 and 400 mm/year, respectively. In the present study, the commercialize fly repellent was used in the farm which contained 5% deltamethrin, highly active pyrethroid and odour free (Butox Swish). The feeders were vinyl type and were placed transversely on the upper part of the stalls, while drinkers were located at the divider between two stalls. To provide greater comfort and well-being, the buffaloes were released into a paddock in the morning and evening, one h before feeding, throughout the experimental period. Besides, they were submitted daily to sprinkler baths for thermal comfort.
The animals were moved to the slaughterhouse twoh before being slaughtered where they had free access to fresh. After slaughtering, approximately 500 ml of the rumen fluids were immediately collected from all the animals in equal amounts and then were coarsely filtered through a four-layered cheesecloth, the liquids bulked together and were transferred immediately into an insulated, prewarmed vacuum flask that was already flushed with carbon dioxide. The flask was then sealed tightly while the temperature was conditioned at 39°C before the rumen samples were transported to the laboratory.
In-vitro gas production
The in-vitro incubation of the rumen fluid was performed in a 100 mL syringe according to the published method of Menke (1998) with a slight modification by Rahman et al. (2011). Equal volumes of rumen fluid collected from the selected 3 buffaloes for each breed were mixed. The strained rumen fluid was mixed with the bicarbonate phosphate buffer saline in a ratio of 1:4 (v/v) under continuous CO2 flushing to maintain the anaerobic condition and was placed in a prewarmed water bath at 39°C. The bicarbonate phosphate buffer saline contained macro- and micromineral nutrients and the pH of the mixed buffers were adjusted using the pH meter (mettle-Toledo, Switzerland) to 6.8. The incubation medium (1:1) was composed of phosphate buffer and bicarbonate buffer. The phosphate buffer was made by dissolving 28.8 g Na2HPO4.12H2O, 6.1 g NaH2PO4.H2O, and 1.4 g NH4Cl into 1 L of distilled water while the bicarbonate buffer was prepared with 39.2 g NaHCO3 into 1 L of distilled water. The dried substrates from the dietary treatments (0.25 g) were placed into a 100 mL syringe fitted with a rubber tube and a 25 mL rumen fluid-buffer medium containing 5 mL of rumen fluid with 20 mL bicarbonate phosphate buffer was added. This experiment was conducted in two runs and total for each breed, there were 30 syringes with three formulated diets and 10 biological replicates (n=10). In addition, three blank syringes without served to correct gas production. The cumulative volume of produced gas was recorded at 0, 2, 4, 6, 8, 12, 18, 24, 31, 36, and 48 h of incubation. The Orskov and McDonald (1979) model were used for non-linear fitting gas production data:
GP = a + b (1– e – c (t)) (1)
where GP is the cumulative gas production at a given time ‘t’ (mL/time); a is the potential gas produced from an immediately soluble fraction (mL), b is the gas production produced from the insoluble fraction (mL); a + b is the potential extent of gas production (mL); c is the gas production rate constant from the insoluble fraction (mL/h) and t is the fermentation time (h).
The sampling of rumen fluid and determination of pH
Five syringes (out of 10 replicates) were taken out from the water bath after 24 h of incubation for pH measurement. The pH was measured with Mettler- Toledo pH meter (Mettler- Toledo, Ltd, Switzerland). The samples of fermented rumen fluid were filtered with filter bags and collected into a 50-mL centrifuge tube and fixed using 25% of metaphosphoric acid in the ratio 4:1 (v/v) to prevent fermentation. Two sets of a 2-mL aliquot of the filtrate were collected to determine volatile fatty acid (VFA) and fatty acid concentrations using gas chromatography. Another two sets of a 5-ml aliquot of the filtrate were stored at -20°C for quantification of rumen microbial population and ammonia analysis.
In-vitro degradability
The fermentation residues in the filter bag were dried at 105 °C overnight and then incinerated in a muffle furnace at 550°C for 6 h. Loss in weight after incineration was used as a measure of organic matter. The in-vitro organic matter degradability (IVOMD) at 24 h of incubation was calculated as equation below:
IVOMD (%) = [(OM sample – OM residue – blank)/OM sample]x 100
Volatile fatty acid analysis
Aliquoted rumen samples as described in the previous section were centrifuged at 4℃ at 15,000× g for 10 min to obtain the supernatant. The supernatant (0.5 mL) was added to 0.5 mL of 20 mmol/L 4-methyl-N-valeric acids (C6:0) (Sigma, St. Louis, Mo., USA) as the internal standard for volatile fatty acids (VFA) assay. The rumen VFA composition (acetic acid (C2), propionic acid (C3), butyric acid (C4), and others (isobutyric acid, valeric acid, and isovaleric acid) were determined using a Quadrex 007 Series of gas chromatography (Quadrex Corporation, New Haven, CT 06525 USA). A capillary column (15 m, 0.32 mm ID, 0.25 μm film thickness; Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) was used to detect the peaks. The setting method was previously described (Mohd Azmi et al., 2021c); the injector temperature was set at 220 °C and the detector at 230 °C. The column temperature was initially set at 70 °C to 150 °C with an increasing rate of 7 °C /min to achieve optimum separation of peaks (Ebrahimi et al., 2017).
Methane and apparent rumen degradable carbohydrate determination
The levels of C2, C3, and C4 (% molar proportion) were used to estimate the methane (CH4) level according to the equation of Moss et al. (2000): CH4 (%) = (0.45 × C2) – (0.275 × C3) + (0.4 × C4). The equation of Rahman et al. (2011) was used to calculate the apparent rumen degradable carbohydrate (ARDC) where C2, C3, and C4 were expressed in micro-molar with 162 as the molecular weight of 1 mol of fermented carbohydrates (Demeyer, 1991).
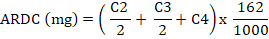 (2)
(2)
Ammonia assay
Rumen ammonia (NH3) concentration was determined using the colorimetric method of Parsons et al. (1984). In brief, the preserved rumen fluid at 24 h incubation sample was thawed and centrifuged at 12,000 x g for 20 min before 5 mL of the supernatant was used to assess the ammonia content. A standard solution of ammonia-N was prepared by dissolving 1.908 g of NH4Cl with 500 mL distilled water which resulted in a concentration of 100 mg/L. A series of standard solutions was prepared to give 0.2, 0.5, 1.0, and 2.0 ppm concentrations by dissolving the stock solution (0.02, 0.05, 0.10, 0.20 mL) with 100 mL purified water standard solution or purified water (blank). Approximately 0.5 mL of rumen fluid sample was mixed with 0.2 mL of phenol solution and transferred into a conical flask. Sequentially, 0.5 mL of oxidizing solution and 0.2 mL of nitroprusside were added into the flask. The flask was swirled until the mixture was well-mixed and incubated for 1 h at room temperature before the measurement. The NH3 content of the reaction solution was then measured using a spectrophotometer (Thermo Fisher Scientific, GENESYS 20, USA) at a wavelength of 640 nm. The regression equation was calculated from the standard and blank samples before estimating the NH3 level in the samples.
Fatty acid compositions
The fatty acid (FA) compositions of experimental rations and the rumen fluid at 24 h post-incubation were identified using GC as described previously by Mohd Azmi et al. (2021c). FA extraction was performed in a mixture of chloroform: methanol (2:1, v/v) and subsequently followed by methylation to obtain the fatty acid methyl esters (FAME). The FAME was injected into GC for FA separation (Gas Chromatography, Model 6890 Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and a splitless injector with the aid of 100 m Supelco SP-2560 capillary column. High-purity nitrogen was used as the carrier gas at the flow rate of 40 mL/min. Compressed air and high-purity H2 were used for the FID in the chromatograph. The oven temperature was initially set at 100°C for 2 min and increased to 170°C at 10°C/min, held for 2 min. Following this, it was further increased to 230°C at the rate of 5°C/min and then held for 20 min. The FA was identified by comparing the peaks output with the relative FAME peak retention times of FA methyl standards. A reference standard (mix C4-C24 methyl esters; Sigma-Aldrich, Inc., St. Louis, Missouri, USA) was used to determine individual fatty acid composition. Heneicosanoic acid (C21:0) was used as an internal standard to correct the identified peak area (Sigma, St. Louis, Mo., USA).
Quantification of rumen microbial population
For each treatment, the preserved rumen fluid at 24 h incubation was thawed and centrifuged at 10,000 x g for 15 min. The precipitate was collected and used for the analysis of the microbial population. Briefly, DNA was extracted
Table 3: The primers used for qPCR to quantify the rumen microbial population.
| Microorganism |
Primer sequence 5’ – 3’ |
Amplicon (base pair) |
Reference |
| Total Bacteria |
1F:CGGCAACGAGCGCAACCC 2R:CCATTGTAGCACGTGTGTAGCC |
145 | Koike and Kobayashi (2001) |
| Ruminococcus albus |
F:CCCTAA AAGCAGTCTTAGTTCG R:CCTCCTTGCGGTTAGAACA |
175 | |
|
Fibrobacter succinogenes |
F:GTTCGGAATTACTGGGCGTAAA R:CGCCTGCCCCTGAACTATC |
122 | Lane (1991) |
| Methanogens |
F:CCGGAGATGGAACCTGAGAC R:CGGTCTTGCCCAGCTCTTATTC |
160 |
Zhou et al. (2009) |
| Total Protozoa |
F: CTTGCCCTCYAATCGTWCT R:GCTTTCGWTGGTAGTGTATT |
223 | Sylvester et al. (2004) |
1F: forward; 2R: reverse; bp: base pair (product size).
from the precipitate using the QIAamp DNA Stool Mini Kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s procedures. The extracted DNA was kept at -20°C before analysis. The concentration and purity of DNA were then measured using a Nanodrop spectrophotometer (Thermo Fisher, ND100, Branchburg, New Jersey, USA) before microbial quantification using quantitative real-time polymerase chain reaction (qPCR). The primers used are provided in Table 3 from the BioRad CFX96 Touch (BioRad, Hercules, California, USA). 25 μL of Maxima SYBR Green Mastermix (Thermo Scientific, Waltham, Massachusetts, USA) was used for the qPCR reactions where it consisted of 12.5 μL SYBR Green Supermix, 1 μL of each reverse and forward primer, 2 μL of the extracted DNA sample, and 8.5 μL of DNase-free water. In all samples, the following qPCR conditions were applied: an initial incubation at 94°C for 5 min, followed by 40 cycles of denaturation at 94°C for 20 s, annealing for 30 s, and then extension at 72 °C for 20 s. The temperature setting for the annealing of the primers of the total bacteria, total fungi, total protozoa, methanogens, Butyrivibrio fibrisolvens, Ruminococcus albus, and Fibrobacter succinogenes was 55°C (Zhang et al., 2020). The standards used in this study were prepared following the protocol established by Atika et al. (2018). The standard used for each microbe’s parameters underwent DNA extraction using normal PCR and was purified by using MEGA quick spin (Intron Biotechnology Incorporated, Gyeonggi-do, Incheon, Korea). The standard curve was constructed for each microbial group using DNA plasmid serial dilution (Atika et al., 2018).
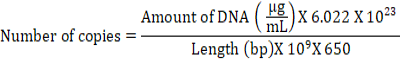 (3)
(3)
Statistical analysis
The experiment was designed as a completely randomized design (CRD) involving three experimental groups and replicates. All data obtained from this experiment were subjected to ANOVA analysis using the following statistical model:
Yij = µ + αi + βj + (α × β)ij + eij, (4)
in which, Yij = observation from dietary treatments, µ = overall mean, αi = effect of dietary treatments (i = diet A, diet B, diet C), βj = effect of breeds, (α × β)ij = interaction effect between experimental diets and breeds on in vitro rumen fermentation, rumen fatty acid compositions, and rumen microbial population, and ɛij = random error. The analysis was performed using the general linear model (GLM) procedure available in the SPSS (Statistical Package for the Social Science 25.0, Inc., Chicago, IL, USA), declaring the dietary treatments and breed as fixed effects while the replicates and sampling times as random effects in the model. In addition, gas production kinetics data were analyzed using repeated measure analysis to compare the difference between observation times and dietary treatments. For all parameters, Tukey’s test was used to identify significant differences among the treatments, and the means were considered significant at P < 0.05.
RESULTS
Fermentation kinetics
As shown in Table 4, significant interaction effects (P < 0.05) were observed in the immediately soluble (a) and insoluble (b) fractions of the rations, most notably in the Diet B of Murrah cross which resulted in the greatest (b) value (57.36 mL; P < 0.05). This was the opposite of what we observed in Swamp buffalo which had the greatest (a) value and lowest (b) value, numerically. Those opposite results might be related to the distinct fractional fermentation rate between Murrah and Swamp buffaloes (0.05 vs 0.07 h-1) since the (c) values were similar among diets. Consequently, the estimated IVOMD was different from diet B resulting in the highest IVOMD (69.79%) that was similar between Murrah cross and Swamp buffaloes. Overall, diet C had lower gas production and IVOMD when compared to diet B but it is similar to diet A.
Table 4: Kinetics of gas production of dietary concentrate and bypass supplement on Murrah cross vs Swamp buffalo
|
Diets
|
Breed
|
a (mL)1 |
b (mL)2 |
a + b (mL)3 |
c (h-1)4 |
IVOMD5 |
|
A6 |
MC9 |
6.05d |
52.40e |
58.45 | 0.05 |
64.50e |
|
B7 |
6.03d |
57.36d |
63.39 | 0.06 |
69.96d |
|
|
C8 |
5.72e |
45.05f |
50.77 | 0.05 |
65.98e |
|
| A |
SW10 |
6.45i |
48.55g |
55.00 | 0.06 |
63.55i |
| B |
9.19g |
46.03h |
55.22 | 0.06 |
69.55g |
|
| C |
8.97h |
49.82g |
58.78 | 0.07 |
65.69h |
|
| Diets | A |
6.25p |
50.47q |
56.72 | 0.06 |
64.02q |
| B |
7.61q |
51.69p |
59.30 | 0.06 |
69.76p |
|
| C |
7.34q |
47.44r |
54.78 | 0.06 |
65.83q |
|
| Breed | MC |
5.93x |
51.60x |
57.54 |
0.05x |
66.81 |
| SW |
8.20y |
48.13y |
56.33 |
0.07y |
66.24 | |
|
SEM11 |
||||||
| 1.66 | 0.05 | 22.23 | 0.11 | 2.44 | ||
| P-value | ||||||
| Diet | 0.031 | 0.025 | 0.107 | 0.076 | 0.039 | |
| Breed | 0.048 | 0.034 | 0.314 | 0.048 | 0.090 | |
| Interaction | 0.047 | 0.049 | 0.244 | 0.278 |
0.395 |
|
d,e,f Different superscript within the column indicates a significant means different from Murrah cross buffalo fed different dietary treatment at p<0.05. g,h,i Different superscript within the column indicates a significant means different from Swamp buffalo fed different dietary treatment at p<0.05. p,q,r Different superscript within the column indicates a significant means different in dietary treatment at p<0.05 x, y Breeds effect is significantly different when the superscripts are different between the column at p<0.05.1 a = soluble fraction; 2b = insoluble fraction; 3a + b = gas production potential; 4c = fractional rate constant of gas production for the insoluble fraction; 5IVOMD: estimated organic matter digestibility (in vitro); 6A: 100% Brachiaria decumbens; 7B: Brachiaria decumbens supplemented with 30% concentrate; 8C: Brachiaria decumbens supplemented with 26 % concentrate + 4 % bypass fat; 9MC: Murrah cross buffalo; 10SW: Swamp buffalo. 11SEM = standard error of the means (SEM reported herein is the pooled SEM from interaction means of Diet × Breed).
Table 5: Rumen fermentation profile and methane production of diets received concentrate and bypass supplement under rumen fluid of Murrah cross and Swamp buffaloes.
|
Diets
|
Breed
|
CTGP1 (mL/250 mg DM) |
pH
|
TVFA2 |
C23 |
C34 |
C45 |
Others6 |
CH47 |
C2: C38
|
ARDC9 (mg) |
NH310 (mg/dL) |
|||
| (mmol/L incubation) |
(molar proportion %) |
||||||||||||||
|
A11 |
MC14 |
12.07c |
6.69 |
40.64c |
52.43a |
27.40b |
9.38b |
10.79 |
19.81a |
1.91a |
3.25c |
6.96b |
|
||
|
B12 |
18.07a |
6.52 |
86.02a |
48.78b |
31.95a |
11.97a |
7.30 |
17.95b |
1.53b |
7.29a |
9.40a |
||||
|
C13 |
14.40b |
6.55 |
55.54b |
48.88b |
30.87a |
10.45a |
9.80 |
17.69b |
1.58b |
4.53b |
7.42a |
||||
| A |
SW15 |
9.30f |
6.67 |
54.88f |
57.91d |
22.82e |
6.01e |
13.26 |
22.19d |
2.54d |
4.12f |
6.85f |
|||
| B |
14.50d |
6.53 |
86.09d |
43.95f |
32.56d |
13.43d |
10.06 |
15.00f |
1.35e |
7.21d |
9.53d |
||||
| C |
10.20e |
6.55 |
71.18e |
46.88e |
28.73d |
10.66d |
13.73 |
18.66r |
1.63e |
5.59e |
8.43e |
||||
| Diets | A |
10.69i |
6.68 |
47.76i |
55.17g |
25.11h |
7.70i |
12.02 |
21.00g |
2.20g |
3.70i |
6.91i |
|||
| B |
16.29g |
6.53 |
86.05g |
46.37h |
32.26g |
12.70g |
8.67 |
16.48i |
1.44h |
7.25g |
9.46g |
||||
| C |
12.30h |
6.55 |
63.36h |
47.88h |
29.80g |
10.56g |
11.76 |
18.17h |
1.61h |
5.07h |
7.93h |
||||
| Breed | MC | 14.85 | 6.59 |
60.73y |
50.03 | 30.07 | 10.60 | 9.30 | 18.48 | 1.66 | 4.98 | 7.93 | |||
| SW | 13.33 | 6.58 |
70.72x |
49.58 | 28.04 | 10.03 | 12.35 | 18.61 | 1.77 | 5.60 | 8.27 | ||||
|
SEM16 |
|||||||||||||||
| 1.66 | 0.05 | 2.18 | 2.72 | 2.10 | 1.45 | 1.67 | 1.32 | 0.11 | 0.20 | 0.13 | |||||
|
p-value |
|||||||||||||||
| Diet | 0.041 | 0.135 | 0.022 | 0.035 | 0.029 | 0.036 | 0.059 | 0.028 | 0.041 | 0.047 | 0.043 | ||||
| Breed | 0.059 | 0.362 | 0.038 | 0.091 | 0.075 | 0.107 | 0.099 | 0.057 | 0.117 | 0.123 | 0.261 | ||||
| Interaction | 0.084 | 0.144 | 0.249 | 0.063 | 0.076 | 0.770 | 0.243 | 0.063 | 0.063 | 0.338 |
0.314 |
||||
a,b,c Different superscript within the column of Murrah cross buffalo indicates a significant means different from dietary treatment at p<0.05. d,e,f Different superscript within the column of Swamp buffalo indicates a significant means different from dietary treatment at p<0.05. g,h,i Different superscript within the column indicates a significant means different in dietary treatment at p<0.05 x, y Breeds effect is significantly different when the superscripts are different between the column at p<0.05.
1CTGP: cumulative total gas production at 48 h; 2TVFA: Total volatile fatty acids; 3C2: acetic acid; 4C3: propionic acid; 5C4: butyric acid; 6Others (isobutyric acid, valeric acid and isovaleric acid); 7CH4: methane production; 8C2:C3: acetic:propionic acid ratio; 9ARDC: Apparent rumen degradable carbohydrate; 10NH3: Ammonia; 11A: 100% Brachiaria decumbens; 12B: Brachiaria decumbens supplemented with 30% concentrate; 13C: Brachiaria decumbens supplemented with 26 % concentrate + 4 % bypass fat; 14MC: Murrah cross buffalo; 15SW: Swamp buffalo; 16SEM: standard error of the means, the SEM reported here is the pooled SEM from interaction means of Diet × Breed.
In-vitro rumen fermentation pattern
Figure 1 shows the kinetics of gas production (mL/250mg DM) for all treatments up to 48-h incubation in the in-vitro ruminal fermentation model. Total gas production was significantly (P < 0.05) influenced by dietary treatments and breeds (Table 5). Diet B showed a significant increase compared with other diets after 12-h post-incubation, leading to the highest gas production (18.07 mL and 14.50 mL for Murrah cross and Swamp buffalo, respectively). Bypass fat contained in diet C lowered gas production compared with diet B (P < 0.05), although it was higher than diet A which only contained grass as a substrate. Furthermore, Murrah cross exhibited higher gas production (P < 0.05) compared to Swamp buffalo.
Table 5 showed the effect of diets on total gas production at 48 h incubation, and TVFA, VFA proportion, ARDC, CH4, and NH3 productions, and on the pH of Murrah cross and Swamp buffaloes during the 24-h incubation. The pH and i-C4 were similar to the effects of diet and breed. Furthermore, CTGP, TVFA, C2, C3, C4 C2:C3 ratio, ARDC, CH4, and NH3 levels were influenced (P < 0.05) by the diets. Both Murrah cross and Swamp buffaloes showed significantly (P < 0.05) lower levels of CTGP, TVFA, C3, C4, ARDC, and NH3, while higher in the proportion of C2, C2:C3, and CH4 levels when treated with the control diet (diet A). Nevertheless, diets B and C also produced significantly (P < 0.05) higher and a similar pattern in C3 and C4 but lower in C2 and C2:C3 ratio as compared to the control diet. Meanwhile, diet B produced significantly (P < 0.05) higher in CTGP, TVFA, ARDC, and NH3 but lower in CH4 proportion compared to diet C. Furthermore, the production of TVFA was also significantly (P < 0.05) affected by the breeds, although all parameters were similar between both breeds.
Fatty acid compositions
The effects of dietary supplements with concentrate and bypass fat on in-vitro ruminal fatty acid production at 24 h incubation was shown in Table 6. Significant differences (P < 0.05) were observed in the mean concentrations of C16:0, C18:0, and SFA among the treatment diets. Numerically, C18:0 and C16:0 demonstrated the highest value of fatty acid composition in the in-vitro ruminal fermentation, while C18:3 showed the lowest values. Buffaloes fed with diet C recorded significantly (P < 0.05) higher in C16:0 while lower in C18:0 composition when compared to diets B and A.
Table 6: Fatty acid compositions of rumen fluid at 24 h post-incubation.
|
Fatty acid composition (% total FA) |
Murrah cross |
Swamp |
Diet |
Breed |
SEM |
p-value |
|||||||||
|
A1 |
B2 |
C3 |
A | B | C | A | B | C | MC | SW | Diet | Breed |
Diet× Breed |
||
| C12:0 |
0. 82 |
3. 03 |
2.64 | 1.34 | 0.44 |
1. 35 |
1.08 | 1.74 | 2.00 | 2.16 | 1.04 | 0.56 | 0.102 | 0.074 | 0.136 |
| C14:0 |
7. 27 |
5. 07 |
2.26 | 2.00 | 0.60 |
1. 88 |
4.64 | 2.84 | 2.07 | 4.87 | 1.49 | 1.69 | 0.059 | 0.062 | 0.171 |
| C15:1 |
4. 52 |
4. 17 |
4.24 | 4.61 | 4.34 |
4. 18 |
4.57 | 4.26 | 4.21 | 4.31 | 4.38 | 0.03 | 0.073 | 0.097 | 0.251 |
| C16:0 |
15 .06c |
18. 44b |
21. 22a |
18.31e |
16. 55f |
29. 18d |
16.69i |
17.50h |
25.20g |
18. 24 |
21. 35 |
1.55 | 0.048 | 0.055 | 0.094 |
| C18:0 |
68. 28a |
65. 42c |
66. 41b |
72.80e |
77. 39d |
62. 00f |
70.54g |
71.41g |
64.21h |
66. 70 |
70. 73 |
2.01 | 0.021 | 0.080 | 0.137 |
| C18:1 |
2. 35 |
3. 14 |
1.85 | 0.52 | 0.56 | 0.59 | 1.44 | 1.85 | 1.22 | 2.45 | 0.56 | 0.95 | 0.067 | 0.082 | 0.106 |
| C18:2 |
1. 51 |
0. 51 |
0.69 | 0.21 | 0.06 | 0.41 | 0.86 | 0.29 | 0.55 | 0.90 | 0.23 | 0.34 | 0.070 | 0.114 | 0.085 |
| C18:3 |
0. 19 |
0. 22 |
0.69 | 0.21 | 0.06 | 0.41 | 0.20 | 0.14 | 0.55 | 0.37 | 0.23 | 0.07 | 0.061 | 0.256 | 0.166 |
|
SFA4 |
91. 43 |
91. 96 |
92. 53 |
94.45 |
94. 98 |
94. 41 |
92.94 | 93.47 | 93.47 |
91. 97 |
94. 61 |
1.32 | 0.075 | 0.149 | 0.212 |
|
UFA5 |
8. 57 |
8. 04 |
7.47 | 5.55 | 5.02 | 5.59 | 7.06 | 6.53 | 6.53 | 8.03 | 5.39 | 1.32 | 0.087 | 0.059 | 0.173 |
|
PUFA6 |
1. 70 |
0. 73 |
1.38 | 0.42 | 0.12 | 0.82 | 1.06 | 0.43 | 1.10 | 1.27 | 0.45 | 0.41 | 0.080 | 0.094 | 0.129 |
|
MUFA7 |
6. 87 |
7. 31 |
6.09 | 5.13 | 4.90 | 4.77 | 6.00 | 6.11 | 5.43 | 6.76 | 4.93 | 0.98 | 0.122 | 0.061 |
0.112 |
a,b,c Different superscript within the row of Murrah cross buffalo indicates a significant means different from dietary treatment at p<0.05. d,e,f Different superscript within the row of Swamp buffalo indicates a significant means different from dietary treatment at p<0.05. g,h,i Different superscript within the row indicates a significant means different in dietary treatment at p<0.05 x, y Breeds effect is significantly different when the superscripts are different between the column at p<0.05.
1A = basal diet (100% Brachiaria decumbens); 2B: basal diet supplemented with 30% concentrate; 3C: basal diet supplemented with 26 % concentrate + 4 % bypass fat; 4SFA = saturated fatty acids); 5UFA = unsaturated fatty acids; 6PUFA= polyunsaturated fatty acids; 7MUFA = monounsaturated fatty acids; 8SEM= Standard error of the means (the SEM reported here is the pooled SEM from interaction means of Diet × Breed).
Table 7: Rumen microbial population (Log11 copy number /mL) after 24 h of incubation of dietary treatments under different breed of buffaloes.
| Breed |
Murrah cross |
Swamp |
Diet |
Breed |
SEM |
p-value |
|||||||||
| Diet | A | B | C | A | B | C | A | B | C | Murrah cross | Swamp | Diet | Breed |
Diet× Breed |
|
|
Total Bacteria |
11. 35b |
11. 74a |
11. 75a |
11. 40e |
11. 90d |
11. 25f |
11.38i |
11. 82g |
11. 50h |
11.61 | 11.52 | 0.04 | 0.004 | 0.327 | 0.027 |
|
Total Protozoa |
2. 54b |
3. 25a |
3. 38a |
2. 70e |
4. 78d |
2. 77e |
2.62i |
4. 02g |
3. 08h |
3.06 | 3.42 | 0.18 | <0.001 | 0.290 | 0.154 |
| Methanogens |
5. |
4. 99b |
5. 12b |
5. 67d |
4. 83f |
5. 02e |
5.61g |
4. 91h |
5. 07h |
5.22 | 5.17 | 0.05 | 0.032 | 0.164 | 0.340 |
| Fibrobacter succinogens |
4. 02c |
4. 72a |
4. 54b |
4. 77e |
4. 92d |
4. 13f |
4.40h |
4. 82g |
4. 34h |
4.43 | 4.61 | 0.09 | <0.001 | 0.258 | <0.001 |
| Ruminococcus albus |
8. 21b |
8. 35a |
8. 31a |
10. 95e |
12. 33d |
9. 62f |
9. 58h |
10. 34g |
8. 97i |
8.29y |
10.97x |
1.34 | <0.001 | <0.001 |
<0.001 |
a,b,c Different superscript within the row of Murrah cross buffalo indicates a significant means different from dietary treatment at p<0.05. d,e,f Different superscript within the row of Swamp buffalo indicates a significant means different from dietary treatment at p<0.05. g,h,i Different superscript within the row indicates a significant means different in dietary treatment at p<0.05 x, y Breeds effect is significantly different when the superscripts are different between the column at p<0.05.
A = basal diet (100% Brachiaria decumbens); B: basal diet supplemented with 30% concentrate; C: basal diet supplemented with 26 % concentrate + 4 % bypass fat; SEM= Standard error of the means (the SEM reported here is the pooled SEM from interaction means of Diet × Breed
In-vitro rumen microbial population
The effects of supplementary concentrate and bypass fat on the rumen microbial populations are summarized in Table 7. The dietary treatments were found to significantly influence (P < 0.05) the total populations of bacteria, protozoa, methanogens, R. albus, and F. succinogens. Significant interactions (P < 0.05) between diets and breeds were observed in the total population of bacteria, R. albus, and F. succinogens, although there was breed effect only for R. albus.
Highest (P < 0.05) population of total bacteria, total protozoa, F. succinogens, and R. albus population were observed in diet B which was supplemented with 30% concentrates. Meanwhile, the microbes were slightly reduced (P < 0.05) in diet C which was supplemented with 26% concentrate and 4 % bypass fat. Both diets B and C showed significantly (P < 0.05) lower compared to diet A. Furthermore, the number of R. albus was significantly higher (P < 0.05) in Swamp than in Murrah cross buffalo.
DISCUSSION
Effects of supplemented diets on in-vitro ruminal kinetics and fermentation
Supplemented diet provides adequate nutrients that are required for animal production (Sheikh et al., 2018). Liu et al. (2011) and Makkar (2004) described the most common methods to study the effects of the diet on in-vitro ruminal fermentation and gas production. There were positive correlations between in-vitro gas production with in-vivo fermentation and feed digestibility (Moss et al., 2000). Thus, in-vitro gas production measurement was intended to assess the impact of different formulated diets on the in-vitro ruminal fermentation characteristics of buffaloes. Preceding experiments have shown that increasing readily fermentable carbohydrate and fat are a valid strategy to promote better ruminal fermentation. However, specific report on energy supplementation in the form of concentrate and bypass fat with different breeds of buffaloes is limited.
The major indicators of ruminal fermentation are TVFA, VFA proportion, pH, ARDC, and CH4. It was observed that there was a general similarity between Murrah cross and Swamp breeds in the levels of gas production and rumen fermentation. However, Murrah cross displayed slightly higher gas production than Swamp buffaloes. In ruminants, distinctive rumen microbiomes among breeds of animals have been reported to influence the fermentation pattern and metabolism efficiency (Fregulia et al., 2021).
As expected, our study confirmed that across the treatments, concentrate inclusion resulted in the highest gas production potential, indicating the enhancement of rumen fermentation. Although bypass fat inclusion increased soluble fraction degradation compared to the control, the degradability was lower than that of concentrate supplementation. It is reasonable because bypass fat inclusion changes the proportion of concentrate in diet C, which proportionally decreased non-fibrous carbohydrates (NFC). The increase in gas production in diet B was also supported by higher TVFA and ARDC values as well as lower CH4 production. This could be due to two reasons. Firstly, the diet containing high concentrate contributed to an increase in non-fibrous carbohydrates, which subsequently enhanced the ruminal fermentation activities, resulting in higher production of gas, C3, C4, and ARDC and concomitantly in lower CH4 production (Irawan et al., 2020). Greater branch chain fatty acids as reflected by the higher VFA production, especially butyrate is known as a good predictor for increasing the cellulolytic and non-cellulolytic bacterial growth responsible for degrading fiber and the starch fraction of feed (Paula et al., 2017). Our study also showed the same pattern that diet B which has been tested in the in-vitro study revealed higher C4 proportion and cellulolytic bacteria compared to diets A and C. A previous study reported an increase in cumulative gas production, and the TVFA levels in the basal diet containing a high ratio of concentrate following a 24-hour incubation (Serment et al., 2016). Other reports also showed an increase in cumulative gas production with an increased ratio of concentrates to hay (Nagadi et al., 2000). This is because the digestibility of concentrate is faster than forage, resulting in higher total gas production (Kim et al., 2018). Secondly, the pH in the syringes was not low enough to influence methanogenesis as suggested by Serment et al. (2016). They revealed that the inhibitory effects on methanogenesis only occurred at a pH of less than 6.0. Following treatment with diets B and C, the C4 proportion was significantly elevated, suggesting the possibility of acetate as a precursor to butyrate (Serment et al., 2016). In this analysis, the high and low VFA levels with high and low diet concentrate levels were in agreement with Kim et al. (2018). Furthermore, Ogata et al. (2019) also showed that Japanese black beef cattle fed with a high-concentrate diet showed a significantly increased level of VFA.
The high carbohydrate level (starch and sugars) in the concentrate usually contributes to the faster fermentation process leading to increased levels of C3 and lowering the ration of C2:C3 (Kim et al., 2018). In our study, high levels of VFA, especially C3, and lower levels in the C2:C3 ratio were observed in the diets supplemented with high concentrates (Diets B and C). The proportion of VFA in this study was comparable to that of the study by Calabro et al. (2008), who studied buffalo ruminal fermentation using the in-vitro gas production technique. The study showed that the rumen fluid of buffaloes fed with forage and concentrate produced C2, C3, and C4 between 50% and 70%, 15% and 30%, and 5% and 15%, respectively.
The supplementation ratio in diet C contributed to changes in the molar proportion of VFA. During fermentation, the sugars and glycerol that were released following the fermentation of glycolipids were converted into VFA. The lower TVFA and VFA proportions in diet C reflect little influence of diet C on the ruminal fermentation of buffalo process since the high melting point of bypass fat makes it less soluble in the rumen. However, a pH of 2.5 makes the fat easily assimilated in the ruminant abomasum (Saparin et al., 2012). Similarly, the lower production of gas by diet C had been observed in an earlier study (Norliza et al., 2014) and was attributed to the supplementation of bypass fat in the diet.
Our study examined the potential of reducing CH4 production from in-vitro rumen study by adding supplements to the buffalo basal diet that supplied long-chain fatty acids varying in their degree of saturation and ruminal availability. It seemed that methane was less generated from treatment groups, which was 21.52% and 13.48% from diets B and C, respectively, lower than the control group. Furthermore, some studies reported that the inclusion of high concentrate level reduced methane proportion in in-vitro ruminal fermentation (Hook et al., 2010), but the reduction occurred only when the concentrate ratio was more than 40% (Kang and Wanapat, 2013; Phesatcha et al., 2020). A study with the ratio of roughage: concentrate (R: C) of 80:20 to 60:40 showed similar CH4 production with 30.6 mM and 30.3 mM, respectively (Phesatcha et al., 2020). However, the increment ratio of R: C (40:60 and 20:80) drastically reduced the methane production to 28.3 mM and 22.4 mM, respectively. Furthermore, studies by Kang and Wanapat (2013) showed that a slight reduction of methane proportion (29.7% to 28%) occurred with the diet R: C ratio of 75:25 to 25:75 on in-vitro ruminal fermentation of buffalo. In addition, Dohme et al. (2000) used a cattle ruminal fluid in a simulation technique and showed that compared with no added fat supplementation, adding 8.1% of the dietary DM as calcium salts of long-chain fatty acid lowered CH4 emission by 5.4% because of a decrease in organic matter fermented in the rumen. Surprisingly, this study showed that buffaloes fed with 30% concentrate have a greater reduction in methane proportion compared to the inclusion of 26% concentrate. Moreover, buffaloes and cattle are different species, and this has affected the rate of methane production (Kang and Wanapat, 2013).
In the process of ruminal fermentation, pH is an important factor that affects gas and methane production, VFA proportion, and rumen microbial population (Kim et al., 2018). The rumen microorganisms would be reasonably stable when the rumen pH remained between 6.2 and 7.0 and this could ensure safe fermentation in the rumen (Zhang et al., 2013). In this study, the pH for all diets indicated the adequate buffering ability of the buffalo’s ruminal fluid to maintain a pH between 6.2 and 6.8. This is important in providing a suitable environment for cellulolytic bacterial activity such as F. succionogen and R. albus (Calabrò et al., 2008). Similar findings were reported on the rumen pH of buffaloes fed a diet containing 75% roughage and 15% concentrate (Hook et al., 2010). The cellulolytic bacteria would not be functioning effectively at a pH of less than 6.0 and the fibrolytic activity decreases gradually at low pH (Russell and Wilson, 1996).
Kim et al. (2018) concluded that the rumen pH supplemented with a low concentrate level is much higher. In our study, diet C (pH 6.55) was slightly higher than diet B (pH 6.52 to 6.53) due to: i) a low amount of concentrate supplementation and ii) the bypass fat supplement was able to escape the digestion process in the rumen as the bypass fat was insoluble at pH 6 to 7 (Hartati et al., 2012). Therefore, concentrate and bypass fat supplementations failed to stimulate acidic conditions in the buffalo rumen ecosystem below the threshold of pH 6.0 (Liu et al., 2019). The results were also in agreement with Liu et al. (2019) who indicated that one of the factors that influenced the rumen pH was the type and the amounts of supplements added to the basal diet.
Supplemented feed was found to alter the level of NH3. The effect significantly increased the NH3 concentration when the inclusion of solely concentrate and the mixture of concentrate and bypass fat in the basal diet by 47.90% and 35.61%, respectively. This was comparable with a study by Wang et al. (2020) who used a 30% to 52% concentrate ratio and found an increase in NH3 contents. As reported by Sun et al. (2021), the inclusion of bypass fat supplements also increased NH3 levels in the ruminal fluid of dairy cows. Furthermore, the addition of concentrate (26%) and bypass fat (4%) in this study slightly decrease the amount of NH3 in both breeds compared to diet B, which is due to the different proportion of concentrate in the basal diet. A similar finding was also reported in a study by Atikah et al. (2018), who showed a reduced amount of NH3 in the rumen by a fat-enriched diet. It seemed that the rumen bypass fat formed a layer that protected the fat molecules, allowing direct passage across the rumen without involvement in rumen fermentation (Naik, 2013). Therefore, the abomasum digests the bypass fat, and the small intestine efficiently absorbed the fat.
The efficiency of ruminant digestion is primarily dependent on its indigenous rumen microbes. Thus, understanding the rumen microbial population and functions is crucial for the fermentation process. This study showed that the TVFA levels in Swamp buffalo were much higher than in Murrah cross buffalo fed with diets A and C, but not much difference between both breeds fed with diet B. It seemed that total VFA levels were affected by the diets and the breed of buffaloes. Similar findings were also reported in previous studies where the TVFA and short-chain fatty acid levels and the C2:C3 ratio were affected by the different supplementation diets and breeds (Chen et al., 2021; Iqbal et al., 2018).
Effect of supplemented diets on in-vitro rumen fatty acid profiles
The different types of diets gave different patterns of long-chain fatty acid composition in the rumen fluid after 24 h incubation through an in-vitro study. In this study, the concentration of stearic acid (C18:0) had shown dominant fatty acid present in the rumen fluid in all diets after 24 h incubation. The study by Kim et al. (2020) was shown comparable with our finding where the in-vitro study was done after 24 h by using rumen fluid from a dairy cow tested with the diet contained basal diet with concentrate supplement only and with a mixture of concentrate and different types of bypass fat which showed stearic acid was high in the composition followed by linoleic, and α -linolenic acid with an average value of 41.91, 1.49, and 0.57%, respectively.
The previous researcher analyzed the different proportions of grass ratio used on the Kacang goat breed tested with 45 and 30% of Brachiaria decumbens through in-vitro methods, where the result showed a significantly higher C18:0 SFA percentage and biohydrogenation rate in the grass ratio of 45% when compared to 30% after the 24 h incubation period (Makmur et al., 2020). The presence of a high percentage of B. decumbens in all diets significantly increased the concentration of C18:0 SFA contents, but the slight increment in SFA contents recorded in treatment diets as compared to the control group (Diet A) was due to the additional supplement in the basal diet. Nevertheless, the previous study also reported that the basal diet content whether solely concentrates or a mixture of bypass fat showed a comparable value of stearic acid composition (Kim et al., 2020); thus, the study agreed with our finding. The high composition in C16:0 and C18:0 in treatment diet groups (Diets B and C) contributed to the high SFA contents in ruminal fluid. This might be due to the high SFA composition in diets B and C when compared to Diet A. Furthermore, the content of SFA in in-vitro ruminal fermentation had been reported to be closely related to the composition of the basic ingredients of the rations (Makmur et al., 2020), where it was in line with our findings that showed the comparable composition of SFA in treatment diets. The same pattern could be clarified due to the rate of biohydrogenation by microbes being the same in trend (Wu et al., 2016); since the supplement ratio did not vary between diets. Until now, there is no study has reported on fatty acid composition in the different breeds of buffaloes. Moreover, no significant difference was recorded between breeds, indicating that the blood fatty acid composition of both breeds was similar.
Indeed, there was considerable variability in the degree of metabolism or types of supplements used, as protection supplements potentially lower the deleterious effects of the “free fat” on fiber digestibility and potentially increase the ruminal microbial metabolism (Palmquist, 1991). The study by Kim et al. (2020) claimed that the biohydrogenation study on in-vitro analysis for the different fat supplements is possibly higher than what is expected from the in-vivo study. Therefore, the in-vitro methods tend to overestimate the degree of rumen inertness. The inconsistency in fermentation findings from different studies in analyzing fatty acid using an in-vitro approach would be described based on the different types of supplements and proportion, period of sampling, and the type of breed and species of animal the studies used (Wu et al., 2016; Kucuk et al., 2001). Nevertheless, data retrieved from this experiment potentially can be a value-added in the prediction of biohydrogenation and rumen metabolism patterns on actual live animals. However, further investigation needs to be done to study the effect of dietary concentrate and bypass fat supplements on in vivo fermentation, thus a better understanding can be achieved of the actual buffalo rumen physiology, particularly on the changes of long-chain fatty acid.
Effect of supplemented diets on in vitro rumen microbial population
Ruminal microbial communities are mainly affected by the diet (Ramos et al., 2021). The rumen microorganisms such as the protozoa, and the cellulolytic, proteolytic, and amylolytic bacteria play significant roles in the digestion of fiber and nutrients in the feed (Ivan et al., 2013). When fed with a different diet, there would be a noticeable change in the number and type of bacteria, methanogens, protozoa, and cellulose-digesting bacteria such as F. succinogen, and R. albus.
Changes in VFA concentration are attributed to the microbial population of the rumen, which is affected by the type and component of the diet. In the process of fermentation, rumen microbes contribute to VFA production which is influential in generating subsequent microbial mass (Atikah et al., 2018). It has been documented that rumen microbial biomass is a source of protein that supports animal growth (Albores-Moreno et al., 2019), and the bacterial and protozoal biomasses are influenced by the compositions of the diets, the rumen pH, and the efficiency of feed consumption (Faniyi et al., 2019). Our findings showed that the pH was negatively correlated with the VFA concentration, and the low pH disturbed the dynamic of the ruminal microbial population. This happens when the amount of VFA available is higher than the amount of VFA being absorbed (Plaizier et al., 2008). Surprisingly, a slight reduction of pH in diets B and C did not significantly alter the total bacterial number. This was also observed by Atika et al. (2018), who attributed it to the ratio of concentrate in the diet. In addition, the differences in fermentation characteristics and rumen microbial populations are influenced by the animal breeds and species. In comparison to cattle, buffaloes are better in buffering capacity to sustain a high pH level that allows the rumen microbial to adapt to the different energy and protein ratio in the diet (Paengkoum et al., 2020).
Besides archaea, methanogens are also the main producers of methane. The methanogen was significantly lower when diets were changed from non-supplemented (diet A, control) to supplemented diet (diets B and C). There was a positive correlation reported previously between the number of methanogens and the level of methane production (Kim et al., 2018), and our study showed a similar result. The increase in the number of concentrate diets may promote the reduction of methane proportion because the cell wall components of concentrate feed are lower than forage (Kim et al., 2013). It is also suggested that the rumen’s methanogen density is influenced by the high level of the concentrate in the diet and is significantly affected the diversity of the methanogens (Hook et al., 2009). Previous studies showed that the dominant methanogen which is Methanobrevibacter was found to be correlated with a high ratio of concentrate in diet (Hook et al., 2009, Zhou et al., 2009). Furthermore, there are similar effects of dietary treatments on the methanogens population of Murrah cross and Swamp buffaloes. Numerically, concentrate (diet B) or concentrate + bypass fat (diet C) supplementation decreases methanogens, without a significantly different between those supplements, which is supported by the previous study that showed bypass fat does not affect methanogenesis (Behan et al., 2019).
On the other hand, other ruminants that were fed a basal diet supplemented with concentrate recorded a high population of methanogens (Candyrine et al., 2018), suggesting a low BH activity leading to lower production of the end product of C18:0 or intermediates products of C18:11n – 9, C18:2n – 6 or C18:3n – 3 in the rumen. Thus, the decrease of CH4 in this study was most likely due to the indirect effect of VFA production rather than the direct effect on methanogens inhibition. However, the nutrient contents of concentrates may slightly differ from each other and thus, resulted in variations in methane production (Kim et al., 2013; Hansen et al., 2022). Also, the methanogenesis activities are positively correlated with the C2:C3 ratio (Kim et al., 2013), and the low methane proportion by Diet C in this study was corresponding with the low C2:C3 ratio of the diet. Kim et al. (2013) also showed that methane proportion was moderate in the medium-containing concentrate. However, methane proportion may vary following prolonged incubation and substrate utilization during fermentation (Kim et al., 2013). Furthermore, the different breeds might show different C2:C3 ratios (Paz et al., 2016), resulting in different fermentation characteristic variations in the rumen.
The total protozoa population was recorded as similar among the breed but significantly affected by the diet. The total protozoa population was recorded higher for the basal diet supplemented with solely concentrate and the mixture of concentrate and bypass fat (Diet B and C) when compared to a diet without supplementation (Diet A). This study agreed with previous studies in ewes, steers, and buffalo fed with solely concentrate and a mixture of concentrate and bypass fat increased the number of rumen protozoa (Bhatt et al., 2013; Fiorentini et al., 2015; Sinha et al., 2017). Moreover, the protozoa population may vary in the rumen ecosystem according to the type and source of diet (Franzolin et al., 2010).
The most dominant cellulolytic species in the rumen are R. albus and F. succinogenes. They break down cellulose in the ruminal digestive system and thus, alterations in their population might affect the ruminal fiber metabolism and VFA concentrations (Liu et al., 2012; Ibrahim et al., 2021). Significant increases in the populations of F. succinogenes and R. albus were observed for diet B in this study since the cellulolytic bacterial population corresponded to the fiber content in the diets.
R. albus is a cellulose- and starch-degrading bacterium producing acetic and succinic acids (Sun et al., 2016). The succinic acid is subsequently transformed into C3 (Li et al., 2015) to be a source of energy for protein synthesis by rumen microbes. The abundance of R. albus and F. succinogens in the treatment groups in this study might be due to the presence of starch in corn, soybean meal, and rice bran in the concentrate, promoting the degradation of complex carbohydrates into a simple monomer (monosaccharides) during fermentation (Li et al., 2019), resulting in a greater bacterial proliferation and promoting bacterial nitrogen in the rumen (Guo et al., 2022). This was also in agreement with a study of Dorper sheep fed with a 5% rumen bypass fat (Behan et al., 2019).
Rumen microbes interact with methanogens via the incorporation of the methanogens into the bacterial biofilms using hydrogen transfer on feed particles. In this interaction, carbon dioxide and hydrogen are produced by the ruminal bacteria as substrates for the methanogens (Lan and Yang, 2019; Morgavi et al., 2010). This interaction between methanogens and rumen bacteria affects the conservation of energy, volatile fatty acid profiles, and the level of methane (Patra et al., 2017). However, in-depth analyses such as metagenomic and meta-transcriptomic are required to understand the microbial interactions in the rumen, especially of Murrah cross and Swamp buffaloes.
CONCLUSION
This study revealed that supplementing rapidly digestible carbohydrates into a grass-based diet could enhance rumen fermentation and decrease methane production both in Murrah and Swamp buffaloes partially via increasing the abundance of fibrolytic bacteria, F. succinogens, and R. albus. This leads to increase gas production, ARDC, and TVFA production. Concentrate supplementation did inhibit methanogens bacteria, indicating that the reduced methane production and methanogens might be achieved via a direct modulatory effect on rumen fermentation. Adding bypass fat generally did not favorably improve overall rumen fermentation but had a minor effect on the ruminal bacterial population. Thus, dietary bypass fat incorporation might be re-considered in buffaloes while concentrate supplementation is highly recommended to enhance optimal rumen fermentation and eventually support production performance. Although the higher degree of saturation of fatty acids in the rumen was found by supplementing bypass fat, a higher flow of PUFA might be expected in the animal tissue as most of them were protected. Moreover, both breeds showed similar rumen fermentation characteristics, but Swamp buffaloes have a slightly higher R albus population in rumen fluid compared to the Murrah cross. This research can be used as a model aimed at forecasting the rumen functions of the different breeds of buffaloes. Further studies using high-throughput sequencing are recommended for a deeper understanding of the microbial communities’ interaction and functions in vitro.
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the Department of Veterinary Service Sabah (JPHPT), the Buffalo Breeding and Research Centre, Telupid Sabah, the Sabah Meat Tech Technology Centre (SMTC), and the postgraduates and staff from the Nutrition Laboratory, Faculty of Veterinary Medicine, Universiti Putra Malaysia for their assistance in this study. This study was financially supported by the Flagship Research grant ABI/FS/2016/01 of the Ministry of Science, Technology and Innovation Malaysia, the Higher Institutions Centres of Excellence (HiCoE) grant of the Ministry of Higher Education Malaysia, and Universiti Putra Malaysia.
CONFLICTS OF INTEREST
The author declares that they have no competing interests.
NOVELTY STATEMENT
The author declares that there are no copyright issues with the study.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiment: HAH, MZAB, ZMS, GYM, NMN, and HA. Provide supervision for animal health assessment and feed formulation during the experiment: HAH, MZAB, ZMS. Experimented and analyzed the data: AFMA. Data interpretation and scientific discussion: AFMA, NAR, AI, and AJ. Contributed materials and reagents: HAH, HA. Writing the manuscript: AFMA and HAH. All authors have read and approved the final manuscript.
REFERENCES
Albores-Moreno S., Alayón-Gamboa J. A., Miranda-Romero L. A., Alarcón-Zúñiga B., Jiménez-Ferrer G., Ku-Vera J. C., Piñeiro-Vázquez A. T. (2019). Effect of tree foliage supplementation of tropical grass diet on in vitro digestibility and fermentation, microbial biomass synthesis and enteric methane production in ruminants. Trop. Anim. Health Prod., 51(4): 893-904. https://doi.org/10.1007/s11250-018-1772-7
Angeles-Hernandez J.C., Vieyra Alberto R., Kebreab E., Appuhamy J.A.D.R.N., Dougherty H.C., Castelan-Ortega O, Gonzalez-Ronquillo M. (2020) Effect of forage to concentrate ratio and fat supplementation on milk composition in dairy sheep: A meta-analysis. Livest. Sci., 238: 104069. https://doi.org/10.1016/j.livsci.2020.104069
AOAC. (2000) Official Methods of Analysis of AOAC International, 17th edition. AOAC International, MD.
AOAC. (2012) Official Methods of Analysis of AOAC International, 19th edition. AOAC International, MD.
Atikah I.N., Alimon A.R., Yaakub H., Abdullah N., Jahromi M.F., Ivan M., Samsudin A.A. (2018). Profiling of rumen fermentation, microbial population and digestibility in goats fed with dietary oils containing different fatty acids. BMC Vet. Res., 14: 344. https://doi.org/10.1186/s12917-018-1672-0
Basra M.J., Nisa M., Khan M.A., Riaz M., Tuqeer N.A., Saeed M.N. (2003). Nili-ravi buffalo III. (2003) Energy and protein requirements of 12–15 months old calves. Int. J. Agric. Biol., 5: 382-383.
Bava L., Jucker C., Gislon G., Lupi D., Savoldelli S., Zucali M., Colombini S. (2019). Rearing of Hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals., 9: 289. https://doi.org/10.3390/ani9060289
Behan A.A., Loh T.C., Fakurazi S., Kaka U., Kaka A., Samsudin A.A. (2019). Effects of supplementation of rumen protected fats on rumen ecology and digestibility of nutrients in sheep. Animals., 9: 400. https://doi.org/10.3390/ani9070400
Bhatt R.S., Karim S.A., Sahoo A., Shinde A.K. (2013). Growth performance of lambs fed diet supplemented with rice bran oil as such or as calcium soap. Asian-Aust. J. Anim. Sci., 26: 812. https://doi.org/10.5713/ajas.2012.12624
Calabrò S., Moniello G., Piccolo V., Bovera F., Infascelli F., Tudisco R,. Cutrignelli M.I. (2008). Rumen fermentation and degradability in buffalo and cattle using the in vitro gas production technique. J. Anim. Physiol. Anim. Nutri., 92: 356-362. https://doi.org/10.1111/j.1439-0396.2007.00799.x
Candyrine S.C.L., Mahadzir M.F., Garba S., Jahromi M.F., Ebrahimi M., Goh Y.M. (2018). Effects of naturally-produced lovastatin on feed digestibility, rumen fermentation, microbiota and methane emissions in goats over a 12-week treatment period. PloS ONE., 13: e0199840. https://doi.org/10.1371/journal.pone.0199840
Chen Y., Gong X., Yang T., Jiang M., Wang L., Zhan K., Zhao G. (2021). Ginkgo Biloba L. Residues Partially Replacing Alfalfa Hay Pellet in Pelleted Total Mixed Ration on Growth Performance, Serum Biochemical Parameters, Rumen Fermentation, Immune Function and Meat Quality in Finishing Haimen White Goats. Animals., 11(11): 3046 https://doi.org/10.3390/ani11113046.
Cruz LC. (2010). Recent Developments in the Buffalo Industry of Asia. Rev. Vet., 21: 7-19.
Demeyer D.I. (1991). Quantitative aspects of microbial metabolism in the rumen and hindgut. Rumen microbial metabolism and ruminant digestion., 217-237.
Department of Veterinary Services (DVS): Livestock statistic 2016/2017. Unit Pengesanan dan Penilaian, Bahagian Perancangan, Department of Veterinary Services. Kuala Lumpur: Ministry of Agriculture, Malaysia (2018). http:// www.dvs.gov.my/dvs/resources/user_1/DVS%20pdf/ perancangan/ 2018/ Perangkaan%202016%202017/3.
Dohme F., Machmüller A., Wasserfallen A., Kreuzer. M. (2000). Comparative efficiency of various fats rich in medium chain fatty acids to suppress ruminal methanogenesis as measured with RUSITEC. Can. J. Anim. Sci., 80, 473–482. https://doi.org/10.4141/A99-113
Duvvu M.V., Rao K.A., Seshaiah C.V., Kumar D.S. (2018) Effect of garlic supplementation on the blood biochemical profile of Murrah Buffalo Calves. Int. J. Curr. Microbiol. App. Sci., 3: 2973-83. https://doi.org/10.20546/ijcmas.2018.703.344
Ebrahimi M., Rajion M.A., Adeyemi K.D., Jafari S., Jahromi F., Oskoueian E., Goh Y.M., Ghaffari M.H. (2017). Dietary n-6:n-3 Fatty Acid Ratios Alter Rumen Fermentation Parameters and Microbial Populations in Goats. J. Agric. Food Chem., 65: 737–744. https://doi.org/10.1021/acs.jafc.6b04704
Faniyi T. O., Adegbeye M. J., Elghandour M. M. M. Y., Pilego A. B., Salem A. Z. M., Olaniyi T. A., Adewumi M. K. (2019). Role of diverse fermentative factors towards microbial community shift in ruminants. J. Appl. Microbiol., 127(1): 2-11. https://doi.org/10.1111/jam.14212
Fiorentini G., Carvalho I.P., Messana J.D., Canesin R.C., Castagnino P.S., Lage J.F., Arcuri P.B., Berchielli. T.T. (2015) Effect of lipid sources with different fatty acid profiles on intake, nutrient digestion and ruminal fermentation of feedlot nellore steers. Asian-Aust. J. Anim. Sci., 28: 1583. https://doi.org/10.5713/ajas.15.0130
Folch J., Lees M., Sloane-Stanley G. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 226: 497– 509. https://doi.org/10.1016/S0021-9258(18)64849-5
Franzolin R., Rosales F. P., Soares W. V. B. (2010). Effects of dietary energy and nitrogen supplements on rumen fermentation and protozoa population in buffalo and zebu cattle. Rev. Bras. Zootec., 39: 549-555. https://doi.org/10.1590/S1516-35982010000300014
Fregulia P., Neves A.L.A., Dias R.J.P., Campos M.M. (2021). A review of rumen parameters in bovines with divergent feed efficiencies: What do these parameters tell us about improving animal productivity and sustainability? Livest. Sci., 254: 104761. https://doi.org/10.1016/j.livsci.2021.104761
Guo Y., Hassan F.U., Li M., Tang Z., Peng L., Peng K., Yang C. (2022). Effect of Hydrogen-Consuming Compounds on In Vitro Ruminal Fermentation, Fatty Acids Profile, and Microbial Community in Water Buffalo. Curr. Microbiol., 79(8): 1-18. https://doi.org/10.1007/s00284-022-02904-7
Hansen N.P., Kristensen T., Johansen M., Wiking L., Poulsen N. A., Hellwing A. L. F., Weisbjerg M.R. (2022). Effects on feed intake, milk production, and methane emission in dairy cows fed silage or fresh grass with concentrate or fresh grass harvested at early or late maturity stage without concentrate. J. Dairy Sci., 105(10): 8036-8053. https://doi.org/10.3168/jds.2022-21885
Hartati L., Agus A., Widyobroto B.P., Yusiati L.M. (2012). In vitro digestibilities of six rumen protected fat-protein supplement formulas. J. Anim. Prod., 14: 1-5.
Haque M.N. (2018). Dietary manipulation: a sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol., 60: 1-10. https://doi.org/10.1186/s40781-018-0175-7
Hook S.E., Northwood K.S., Wright A.D.G., McBride B.W. (2009). Long-term monensin supplementation does not significantly affect the quantity or diversity of methanogens in the rumen of the lactating dairy cow. Appl. Environ. Microbiol., 75: 374–380. https://doi.org/10.1128/AEM.01672-08
Hook S.E. Wright A.D., McBride B.W. (2010). Methanogens: methane producers of the rumen and mitigation strategies. Archaea., 11. https://doi.org/10.1155/2010/945785
Ibrahim N.A., Alimon A.R., Yaakub H., Samsudin A.A., Candyrine S.C.L., Wan Mohamed W. N., Mookiah S. (2021). Effects of vegetable oil supplementation on rumen fermentation and microbial population in ruminant: A review. Trop. Anim. Health Prod., 53(4): 1-11. https://doi.org/10.1007/s11250-021-02863-4
Iqbal M.W., Zhang Q., Yang Y., Li L., Zou C., Huang C., Lin B. (2018) Comparative study of rumen fermentation and microbial community differences between water buffalo and Jersey cows under similar feeding conditions. J. Appl. Anim. Res., 46: 740-8. https://doi.org/10.1080/09712119.2017.1394859
Irawan A., Noviandi C.T., Kustantinah W.B.P., Astuti A., Ates S. (2020). Effect of leucaena leucocephala and corn oil on ruminal fermentation, methane production and fatty acid profile: An in vitro study. Anim. Prod. Sci., 61: 459–469. https://doi.org/10.1071/AN20003
Ivan M., Petit H.V., Chiquette J., Wright A.D. (2013) Rumen fermentation and microbial population in lactating dairy cows receiving diets containing oilseeds rich in C-18 fatty acids. Brit. J. Nutri., 109: 1211-8. https://doi.org/10.1017/S0007114512003030
Jaramillo-López E., Itza-Ortiz M.F., Peraza-Mercado G., Carrera-Chávez J.M. (2017). Ruminal acidosis: strategies for its control. Austral. J. Vet. Sci., 49: 139-148. https://doi.org/10.4067/S0719-81322017000300139
Jiao H.P., Dale A.J., Carson A.F., Murray S., Gordon A.W., Ferris C.P. (2014). Effect of concentrate feed level on methane emissions from grazing dairy cows. J. Dairy Sci., 97(11): 7043-7053. https://doi.org/10.3168/jds.2014-7979
Kang S., Wanapat M. (2013). Using plant source as a buffering agent to manipulating rumen fermentation in an in vitro gas production system. Asian-Aust. J. Anim. Sci., 26: 1424. Kim S.H., Mamuad L.L., Jeong C.D., Choi Y.J., Lee S.S., Ko J.Y., Lee S.S. (2013). In vitro evaluation of different feeds for their potential to generate methane and change methanogen diversity. Asian-Aust. J. Anim. Sci., 26, 1698–1707. https://doi.org/10.5713/ajas.2013.13260
Kim S.H., Mamuad L.L., Kim E.J., Sung H.G., Bae G.S., Cho K.K., Lee S.S. (2018). Effect of different concentrate diet levels on rumen fluid inoculum used for determination of in vitro rumen fermentation, methane concentration, and methanogen abundance and diversity. Ital. J. Anim. Sci., 17: 359-367. https://doi.org/10.1080/1828051X.2017.1394170
Kim T.B., Lee J.S., Cho S.Y., Lee H.G. (2020). In vitro and in vivo studies of rumen-protected microencapsulated supplement comprising linseed oil, vitamin E, Rosemary extract, and hydrogenated palm oil on rumen fermentation, physiological profile, milk yield, and milk composition in dairy cows. Animals., 10: 1631. https://doi.org/10.3390/ani10091631
Koike S., Kobayashi Y. (2001). Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett., 204: 361-366. https://doi.org/10.1111/j.1574-6968.2001.tb10911.x
Kucuk O., Hess B.W., Ludden P.A., Rule D.C. (2001). Effect of forage:concentrate ratio on ruminal digetsion and duodenal flow of fatty acids in ewes. J. Anim. Sci., 79: 2233–2240 https://doi.org/10.2527/2001.7982233x
Kusriatmi R.O., Syaukat Y., Said A. (2014). Analysis of the effects of beef import restrictions policy on beef self-sufficiency in Indonesia. J. ISSAAS., 20: 115-30.
Lan W., Yang C. (2019). Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ., 654: 1270-1283. https://doi.org/10.1016/j.scitotenv.2018.11.180
Lane D.J. (1991). 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics, 115-175.
Li X.Z., Park B.K., Shin J.S., Choi S.H., Smith S.B., Yan C.G. (2015). Effects of dietary linseed oil and propionate precursors on ruminal microbial community, composition, and diversity in yanbian yellow cattle. PLoS One, 10: e0216473 https://doi.org/10.1371/journal.pone.0126473
Li R., Teng Z., Lang C., Zhou H., Zhong W., Ban Z., Lou Y. (2019). Effect of different forage-to-concentrate ratios on ruminal bacterial structure and real-time methane production in sheep. PloS one., 14(5). https://doi.org/10.1371/journal.pone.0214777
Liu S.J., Bu D.P., Wang J.Q., Liu L., Liang S., Wei H.Y., Zhou L.Y., Li D., Loor J.J. (2012). Effect of incremental levels of fish oil supplementation on specific bacterial populations in bovine ruminal fluid. J. Anim. Physiol. Anim. Nutr., 96: 9-16. https://doi.org/10.1111/j.1439-0396.2010.01113.x
Liu. H., Xu T., Xu S., Ma L., Han X., Wang X., Pi L. (2019) Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on the Qinghai-Tibetan plateau. Peer Reviewed J., 7: 7462. https://doi.org/10.7717/peerj.7462
Liu H., Vaddella V., Zhou D. (2011). Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J. Dairy Sci., 94: 6069-6077. https://doi.org/10.3168/jds.2011-4508
Makkar H.P. (2004). Recent advances in the in vitro gas method for evaluation of nutritional quality of feed resources. Assessing quality and safety of animal feeds. FAO Anim. Prod. Health Paper., 16: 55-88. https://doi.org/10.14710/jitaa.45.2.124-135
Makmur M., Zain M., Marlida Y., Khasrad K., Jayanegara A. (2020). In vitro ruminal biohydrogenation of C18 fatty acids in mixtures of Indigofera zollingeriana and Brachiaria decumbens. J. Indonesian Trop. Anim. Agric., 45: 124-135.
Menke K.H. (1988). Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev., 28: 7-55.
Moallem U. (2018). Invited review: Roles of dietary n-3 fatty acids in performance, milk fat composition, and reproductive and immune systems in dairy cattle. J. Dairy Sci., 101, 8641–8661. https://doi.org/10.3168/jds.2018-14772
Mohd Azmi A.F., Abu Hassim H., Mohd Nor N., Ahmad H., Meng G.Y., Abdullah P., Zamri-Saad M. (2021a). Comparative Growthand Economic Performances between Indigenous Swamp and Murrah Crossbred Buffaloes in Malaysia. Animals., 11: 957. https://doi.org/10.3390/ani11040957
Mohd Azmi A.F., Ahmad H., Mohd Nor N., Meng G.Y., Zamri-Saad M., Abu Bakar M.Z., Salleh A., Abdullah P., Jayanegara A., Abu Hassim H. (2021b). The Impact of Feed Supplementations on Asian Buffaloes: A Review. Animals., 11: 2033. https://doi.org/10.3390/ani11072033
Mohd Azmi A.F., Ahmad H., Mohd Nor N., Meng G.Y., Saad M.Z., Abu Bakar M.Z., Abu Hassim H. (2021c). Effects of concentrate and bypass fat supplementations on growth performance, blood profile, and rearing cost of feedlot buffaloes. Animals., 11: 2105. https://doi.org/10.3390/ani11072105
Morgavi D., Forano E., Martin C., Newbold C. (2010). Microbial ecosystem and methanogenesis in ruminants. Animal., 4: 1024–36. https://doi.org/10.1017/S1751731110000546
Moss A.R., Jouany J.P., Newbold J. (2000). Methane production by ruminants: its contribution to global warming. Ann. Zootech., 49: 231-253. https://doi.org/10.1051/animres:2000119
Naik P.K. (2013). Bypass fat in dairy ration-a review. Anim. Nutri. Feed Tech., 13: 147-163.
Nagadi S., Herrero M., Jessop N.S. (2000). The influence of diet of the donor animal on the initial bacterial concentration of ruminal fluid and in vitro gas production degradability parameters. Anim. Feed Sci. Technol., 87: 231-239. https://doi.org/10.1016/S0377-8401(00)00197-8
Norliza S., Zahariah I., Mohd S.A., Shawaluddin T., Mardhati M., Wong H. (2014). In vitro digestibility study of fractionated Refined, Bleached and Deodorized Palm Stearin. Malaysian J. Vet. Res., 5: 1-4.
Noviandi C.T., Eun J., Peel M.D., Waldron B.L., Min B.R., Zobell D.R., Miller R.L. (2014). Effects of energy supplementation in pasture forages on in vitro ruminal fermentation characteristics in continuous cultures. Prof. Anim. Sci., 30: 13–22. https://doi.org/10.15232/S1080-7446(15)30077-2
Natnael D.A., Tao W., Gui-Xin Q., Yu-Guo Z., Xue-Feng Z., Xue C., Seidu A. (2020) Effects of physically effective fiber on rumen and milk parameters in dairy cows: A review. Indian J. Anim. Res., 54(11): 1317-1323.
Ogata T., Makino H., Ishizuka N., Iwamoto E., Masaki T., Ikuta K., Sato S. (2019). Long-term high-grain diet altered the ruminal pH, fermentation, and composition and functions of the rumen bacterial community, leading to enhanced lactic acid production in Japanese Black beef cattle during fattening. PloS one., 14(11): e0225448. https://doi.org/10.1371/journal.pone.0225448
Palmquist D.L. (1991). Influence of source and amount of dietary fat on digestibility in lactating cows. J. Dairy Sci., 74: 1354-1360. https://doi.org/10.3168/jds.S0022-0302(91)78290-8
Paengkoum S., Tatsapong P., Taethaisong N., Sorasak T., Purba R.A.P., Paengkoum P. (2021). Empirical evaluation and prediction of protein requirements for maintenance and growth of 18–24 months old thai swamp buffaloes. Animals., 11(5): 1405. https://doi.org/10.3390/ani11051405
Patra A., Park T., Kim M., Yu Z. (2017). Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol., 8: 13 https://doi.org/10.1186/s40104-017-0145-9.
Paula E.M., Monteiro H.F., Silva L.G., Benedeti P.D.B., Daniel J.L.P., Shenkoru T., Broderick G.A., Faciola A.P. (2017). Effects of replacing soybean meal with canola meal differing in rumen-undegradable protein content on ruminal fermentation and gas production kinetics using 2 in vitro systems J. Dairy Sci., 100: 5281-5292. https://doi.org/10.3168/jds.2016-12301
Paz H.A., Anderson C.L., Muller M.J., Kononoff P.J., Fernando S.C. (2016). Rumen bacterial community composition in Holstein and Jersey cows is different under same dietary condition and is not affected by sampling method. Front. Microbiol., 7: 1206. https://doi.org/10.3389/fmicb.2016.01206
Phesatcha K., Phesatcha B., Wanapat M., Cherdthong A. (2020) Roughage to Concentrate Ratio and Saccharomyces cerevisiae Inclusion Could Modulate Feed Digestion and In Vitro Rumin. Ferment.. Vet. Sci., 7: 151. https://doi.org/10.3390/vetsci7040151
Plaizier J.C., Krause D.O., Gozho G.N., McBride B.W. (2008). Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J., 176: 21–31. https://doi.org/10.1016/j.tvjl.2007.12.016
Orskov E.R., McDonald I. (1979). The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci., 92: 499-503. https://doi.org/10.1017/S0021859600063048
Othman R., Bakar M.Z., Kasim A, Zamri–Saad M. (2014). Improving the reproductive performance of buffaloes in Sabah, Malaysia. J. Anim. Health Prod., 2: 1-4. https://doi.org/10.14737/journal.jahp/2014/2.1.1.4
Paengkoum S., Tatsapong P., Taethaisong N., Sorasak T., Purba R.A.P., Paengkoum P. (2021). Empirical evaluation and prediction of protein requirements for maintenance and growth of 18–24 months old thai swamp buffaloes. Animals., 11: 1405. https://doi.org/10.3390/ani11051405
Parsons T.R., Maita Y., Lalli C.M. (1984). Determination of chlorophylls and total carotenoids: spectrophotometric method. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford., 101-112. https://doi.org/10.1016/B978-0-08-030287-4.50032-3
Patra A.K. (2014). A meta-analysis of the effect of dietary fat on enteric methane production, digestibility and rumen fermentation in sheep, and a comparison of these responses between cattle and sheep. Livest. Sci., 162: 97–103. https://doi.org/10.1016/j.livsci.2014.01.007
Rafiee-Yarandi H., Ghorbani G.R., Alikhani M., Sadeghi-Sefidmazgi A., Drackley J.K. (2016). A comparison of the effect of soybeans roasted at different temperatures versus calcium salts of fatty acids on performance and milk fatty acid composition of mid-lactation Holstein cows. J. Dairy Sci., 99: 5422–5435. https://doi.org/10.3168/jds.2015-10546
Rahman M.M., Lourenço M., Hassim H.A., Baars J.J., Sonnenberg A.S., Cone J.W., Fievez V. (2011). Improving ruminal degradability of oil palm fronds using white rot fungi. Anim. Feed Sci. Technol., 169: 157-166. https://doi.org/10.1016/j.anifeedsci.2011.06.014
Ramos S.C., Jeong C.D., Mamuad L.L., Kim S.H., Kang S.H., Kim E.T., Lee S.S. (2021). Diet transition from high-forage to high-concentrate alters rumen bacterial community composition, epithelial transcriptomes and ruminal fermentation parameters in dairy cows. Animals., 11(3): 838. https://doi.org/10.3390/ani11030838
Russell J.B., Wilson D.B. (1996). Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci., 79: 1503–1509. https://doi.org/10.3168/jds.S0022-0302(96)76510-4
Saparin N., Ismail Z., Yusoff M.S.A., Tahiruddin S. (2012). Fractionated RBD palm stearin as potential rumen bypass supplements. Malaysian J. Vet. Res., 3: 65-70.
Serment A., Giger‐Reverdin S., Schmidely P., Dhumez O., Broudiscou L.P., Sauvant D. (2016). In vitro fermentation of total mixed diets differing in concentrate proportion: relative effects of inocula and substrates. J. Sci. Food Agri., 96: 160-168. https://doi.org/10.1002/jsfa.7076
Shahudin M.S., Ghani A.A.A., Zamri-Saad M., Zuki A.B., Abdullah F.F.J., Wahid H., Hassim H.A. (2018). The Necessity of a Herd Health Management Programme for Dairy Goat Farms in Malaysia. Pertanika J. Trop. Agri. Sci., 41: 1-18.
Sheikh G.G., Ganai A.M., Reshi P.A., Bilal S., Mir S., Masood D. (2018). Improved paddy straw as ruminant feed: A review. Agricult. Rev., 39(2). https://doi.org/10.18805/ag.R-1667
Sinha S. K., Chaturvedi V. B., Singh P., Chaudhary L. C., Ghosh M., Shivani S. (2017). Effect of high and low roughage total mixed ration diets on rumen metabolites and enzymatic profiles in crossbred cattle and buffaloes. Vet. World., 10: 616. https://doi.org/10.14202/vetworld.2017.616-622
Suharti. S., Astuti D.A., Wina E., Toharmat T. (2011). Rumen microbial population in the in vitro fermentation of different ratios of forage and concentrate in the presence of whole lerak (Sapindus rarak) fruit extract. Asian-Aust. J. Anim. Sci., 24: 1086-1091. https://doi.org/10.5713/ajas.2011.10409
Sun B., Wang X., Bernstein S., Huffman M.A., Xia D.P., Gu Z., Chen R., Sheeran L.K., Wagner R.S., Li J. (2016). Marked variation between winter and spring gut microbiota in free-ranging Tibetan Macaques (Macaca thibetana). Sci. Rep., 6: 26035. https://doi.org/10.1038/srep26035
Sun C. H., Lee J.S., Nejad J.G., Kim W.S., Lee H.G. (2021). Effect of a rumen-protected microencapsulated supplement from linseed oil on the growth performance, meat quality, and fatty acid composition in Korean native steers. Animals., 11: 1253. https://doi.org/10.3390/ani11051253
Sylvester J.T., Karnati S.K., Yu Z., Morrison M., Firkins J.L. (2004). Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr., 134: 3378-3384. https://doi.org/10.1093/jn/134.12.3378
Tahir M.N., Hetta M., Larsen M., Lund P., Huhtanen P. (2013). In vitro estimations of the rate and extent of ruminal digestion of starch-rich feed fractions compared to in vivo data. Anim. Feed Sci. Technol., 179: 36-45. https://doi.org/10.1016/j.anifeedsci.2012.11.006
Van Dung D., Shang W., Yao W. (2014). Effect of crude protein levels in concentrate and concentrate levels in diet on in vitro fermentation. Asian-Aust. J. Anim. Sci., 27: 797. https://doi.org/10.5713/ajas.2013.13560
Van Soest P.J., Roberson J.B, Lewis V.A. (1991). Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci., 74: 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Wang L., Zhang G., Li Y., Zhang Y. (2020). Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen. Animals., 10(2): 223. https://doi.org/10.3390/ani10020223
Wu D., Xu L., Tang S., Guan L., He Z., Guan Y., Wang M. (2016). Influence of oleic acid on rumen fermentation and fatty acid formation in vitro. PLoS One., 11: e0156835 https://doi.org/10.1371/journal.pone.0156835.
Zamri-Saad M., Azhar K. (2015). Issues of ruminant integration with oil palm plantation. J. Oil Palm Res., 27: 299-305.
Zhang G.W., Wang C., Du H.S., Wu Z. Z., Liu Q., Guo G., Zhang S.L. (2020). Effects of folic acid and sodium selenite on growth performance, nutrient digestion, ruminal fermentation and urinary excretion of purine derivatives in Holstein dairy calves. Livest. Sci., 231: 103884. https://doi.org/10.1016/j.livsci.2019.103884
Zhang T., Zhuang S., Dong W., Chang G., Yan R., Wang T. (2013). Effects of different dietary concentrate to forage ratios on rumen fluid pH and VFA levels and blood VFA levels in dairy goats. J. Anim. Husbandry Vet. Med., 4: 5-10.
Zhou M., Hernandez-Sanabria E., Guan L.L. (2009). Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl. Environ. Microbiol., 75: 6524–6533. https://doi.org/10.1128/AEM.02815-08
To share on other social networks, click on any share button. What are these?





