Hepatoprotective Effects of Tamarix dioica Leaf Extracts on Paracetamol-Induced Hepatotoxicity in Mice
Hepatoprotective Effects of Tamarix dioica Leaf Extracts on Paracetamol-Induced Hepatotoxicity in Mice
Tahani Ahmad Al-Matrafi1, Zuhair M. Mohammedsaleh2, Mamdoh S. Moawadh2, Waheeb S. Aggad3, Rawabi Mohamed almuhimed4, Zamzam Alhuwaymil5, Aishah E Albalawi6, Ifat Alsharif7, Hailah M. Almohaimeed8, Fatima S. Alaryani9 and Mona H. Soliman10,11*
1Anatomy Department, College of Medicine, King Saudi University, Saudi Arabia
2Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences,University of Tabuk, Tabuk 71491, Saudi Arabia
3Department of Anatomy, College of Medicine, University of Jeddah, P.O. Box 8304, Jeddah23234, Saudi Arabia
4Saudi Authority for Intellectual Property (SAIP), Saudi Arabia
5Organic Department, College of Science and Humanities at Al-Quway’iyahl, Shaqra University, Saudi Arabia
6Faculty of Science, Department of Biology, University of Tabuk, Tabuk 47913, Saudi Arabia
7Department of Biology, Jamoum University College, Umm Al-Qura University, 21955, Makkah, Saudi Arabia
8Department of Basic Science, College of Medicine, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
9University of Jeddah, College of Science, Department of Biological Sciences, Jeddah 21589, Saudi Arabia
10Botany and Microbiology Department, Faculty of Science, Cairo University, Giza 12613,Egypt
11Biology Department, Faculty of Science, Taibah University, Al-Sharm, Yanbu El-Bahr,Yanbu 46429, Kingdom of Saudi Arabia
ABSTRACT
This study aimed to evaluate the hepatoprotective effects of aqueous, methanolic, and ethanolic extracts of Tamarix dioica leaves against paracetamol-induced toxicity. In this study, 36 albino mice were randomly grouped into six groups, each consisting of six mice: Group I (normal control), Group II (paracetamol-toxified), Group III (positive control with Silymarin @ 200 mg/Kg), Group IV (aqueous T. dioica extract @ 400 mg/Kg), Group V (methanolic T. dioica extract @ 400 mg/Kg), and Group VI (ethanolic T. dioica extract @ 400 mg/Kg). Hepatoprotective potential was assessed through liver function indicators (ALT, AST, ALP, total bilirubin, and total protein in blood serums), hepatic antioxidants (SOD, CAT, GSH, and GPx in liver homogenate), and inflammatory markers (IL-6, TNF-α, COX2), along with other liver biomarkers. Histopathological alterations in the liver were evaluated using Hematoxylin and Eosin staining. The leaf extracts effectively restored liver function indicators and hepatic antioxidants to normal levels, demonstrating a significant improvement compared to the elevated levels observed in the paracetamol control group (P < 0.001). Furthermore, a reversal of hepatoarchitecture was recorded. The study highlights the pronounced hepatoprotective effects of T. dioica leaf extracts against paracetamol-induced toxicity in albino mice. The extracts not only successfully normalized liver function indicators and hepatic antioxidants but also exhibited a significant reversal of hepatoarchitecture. These findings suggest the potential therapeutic value of T. dioica in mitigating liver disorders, emphasizing its promising role as a natural hepatoprotective agent.
Article Information
Received 24 September 2023
Revised 05 December 2023
Accepted 26 December 2023
Available online 27 February 2024
(early access)
Published 19 July 2024
Authors’ Contribution
Conceptualization: TA and ZM. Methodology: MM, ZM, IA and WA. Software and Visualization: RM, ZA, IA and AA. Formal analysis: RM, ZA, FSA, IF and AA. Investigation: TA, HM, MH and ZM. Writing original draft preparation: FSA, IA, TA and HM. Editing: MM, ZM, TA, and WA. Supervision: MM, FSA, ZM, IF and WA. Project administration: HMA and MH. Funding acquisition, Hailah M. Almohaimeed. Submission, Waheeb S. Aggad. All authors have read and agreed to the published version of the manuscript.
Key words
Tamarix dioica, Hepatoprotective, Silymarin, IL6, TNF-α, COX2
DOI: https://dx.doi.org/10.17582/journal.pjz/20230924082821
* Corresponding author: [email protected]
0030-9923/2024/0005-2195 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The liver, a central organ for detoxification, metabolism, and excretion, holds a pivotal role in maintaining physiological balance within the human body (Ayenew and Wasihun, 2023). The production of highly reactive molecules, known as free radicals, by the liver poses a threat to tissue integrity, with the covalent linkage of free radicals to cell membrane lipids leading to tissue injury (Pramod et al., 2008). Across all age groups, the liver is indispensable for sustaining metabolic and physiological equilibrium (Narici et al., 2021). Hepatitis, cirrhosis, and alcoholic liver problems can be attributed to exposure to various stressors, including free radicals, alcohol, xenobiotics, food additives, and pollution (Wahyuningsih et al., 2021). Approximately 10% of the global population suffers from liver ailments, encompassing conditions like fibrosis, cirrhosis, chronic hepatitis, hepatocellular cancer, and alcoholic steatosis (Zhang et al., 2013). The associated morbidity and mortality of liver diseases present a notable public health concern, particularly in economically disadvantaged nations (Ornos et al., 2023). The treatment of liver diseases in modern medicine faces challenges, with corticosteroids and immunosuppressive medications being the only approved options, albeit accompanied by various negative side effects (Ayenew and Wasihun, 2023). In response, there has been an increasing reliance on complementary and alternative medicine, particularly herbal treatments, for liver conditions, given the recognized efficacy of plant medicines (Alqasoumi, 2012). Paracetamol (acetaminophen), a commonly used non-narcotic analgesic and antipyretic, can become a potent hepatoxin leading to fulminated hepatic and renal tubular necrosis when ingested in lethal quantities. This transformation poses a fatal threat to humans and various animal species, including mice (He et al., 2012).
Liver disease treatment is important and requires careful attention to detail. Although several traditional medications have been shown to boost liver function, provide hepatic protection, or aid in the regeneration of hepatic cells, these same medications have also been shown to be hepatotoxic when used in certain dosages (Stahl et al., 2018). Among the many common experimental models used to assess the hepatoprotective properties of plant extracts, acetaminophen (N-acetyl-p-aminophenol, paracetamol) induced toxicity in rats and mices have been observed (Subramanya et al., 2018). Paracetamol is safe when used in therapeutic dosages (Caparrotta et al., 2018). However, in people and experimental animals, overdosing on it might result in hepatic necrosis, nephrotoxicity, extra hepatic diseases, and even death (Offfor et al., 2022). Natural substances should be tested to see whether they can replace synthetic ones.
Antioxidants have been shown to lessen the likelihood of developing liver disease by blocking the oxidative damage produced by free radicals (Shahidi and Zhong, 2010). Several illnesses have been treated with herbal remedies for decades. Yet, natural products continue to play a significant role as sources for the creation of numerous medications used to treat a broad range of disorders, including cancer and liver disease (Ji et al., 2009). Many types of trees, shrubs, and bushes from antiquity belong to the genus Tamarix, which is simply referred to it by its common name. Tamarix, also known as Tamarisk and salt cedar, is a genus of over 60 halophyte plants grown worldwide and distributed widely in Saudi Arabia. Salt glands cover the needlelike leaves of these plants (Samadi et al., 2013). Primitive species like T. dioica Roxb. Roth and T. ericoides Rotti et Wild may be found in India and its neighboring regions (Arianmanesh et al., 2016).
Tamarix dioica, widely utilized in herbal medicine, holds significance for the treatment and prevention of various diseases (Komal et al., 2021). Belonging to the family Tamaricaceae, T. dioica has a rich history in both ancient and contemporary herbal remedies (Atanasov et al., 2015). Acknowledged for its diverse properties such as anti-fungal, anti-diabetic, anti-dermatosis, anti-infective, carminative, anti-diuretic and anti-inflammatory, T. dioica has been valued in medicinal applications (Bahramsoltani et al., 2020). Despite its traditional use, there is a scarcity of clinical studies examining the therapeutic effectiveness of T. dioica (Samejo et al., 2013). Many studies on the phytochemistry of several Tamarix species have shown a wide variety of substances, the most notable of which are polyphenolic compounds such phenolic acids, flavonoids, and tannins. In addition, the natives of Asian and African nations, including Saudi Arabia, Pakistan, India, and Algeria, use this plant due to its medicinal properties, where tamarisk grows naturally (Alnugaydan and Rah, 2019). In the leaves, many chemical compounds of therapeutic value, such as tamarixetin, kaempferide, D-mannitol, rhamnetin, polyphenols, flavanols, and β-sitosterol, were identified (Samejo et al., 2013). Tamarix dioica served a number of practical applications for the economy, including production of fuel and baskets (Percival et al., 2014). T. dioica is used medicinally as carminative, antifungal, anti-infectious (Rehecho et al., 2011), for treating ring worms and gonorrhoea, aphrodisiac (Aziz et al., 2018; Urumarudappa et al., 2019), utilized in cough and flu treatments, and diuretic for liver and spleen inflammation in ancient and modern herbal medicines. External applications of an ointment made from the bark of this plant were used to treat piles and ulcers (Post-White et al., 2007).
The milk thistle (Silybum marianum) plant is the source of silymarin, a flavonolignan that is used almost exclusively for the purpose of hepatoprotection (Valková et al., 2020; Pradhan and Girish, 2006). Instead of dealing with the difficulties of standardization, quality control, and contamination with heavy metals or bacterial toxins, silymarin might be used instead, potentially replacing polyherbal formulations. Flavonolignan isomers silybin, isosilybin, silydianin, and silychristin make up silymarin. The most popular and effective among all is silybin (Radko and Cybulski, 2007). Silymarin is mostly eliminated as sulphates and conjugates in bile after being ingested orally. Silymarin provides effective protection in a number of animal studies using toxic liver disease models (Radko and Cybulski, 2007). Mechanisms of action include antioxidant defense, lipid peroxidation defense, fibrosis resistance, inflammation suppression, membrane stabilization, immune modulation, and liver regeneration (Domitrović and Potočnjak, 2016).
However, the therapeutic efficacy of T. dioica has seen little clinical testing too far. In light of these considerations, the purpose of the current investigation is to determine the hepatoprotective activity of aqueous, methanolic and ethanolic extracts of the T. dioica plant using Swiss albino mice having the acute liver damage caused by paracetamol. Efficacy in protecting against aracetamol-induced hepatotoxicity was compared to that of silymarin, a well-established hepatoprotective drug.
Materials and Methods
Chemicals
Paracetamol was purchased from local supplier in Saudi Arabia (Panadol, GSK), and liver functions (ALT, AST, ALP and total bilirubin) were detected by detection kits (Verisana). All the other chemicals of analytical grades were purchased from the chemical market of Riyadh, Saudi Arabia (Merck).
Plant collection and preparation of extracts
Tamarix dioica leaves were bought at an open market of Riyadh, Saudi Arabia, washed twice with tap water to remove the dust particles. Plants were then kept at Oven at 45 ℃ to dry the washed leaves, cut, and crushed into a powder (using pestle mortar and mechanical blender). The powder was stored in an airtight container until required.
Aqueous, methanolic and ethanolic extracts were prepared by following the protocol of Samejo et al. (2013) with minor modifications. The dried T. dioica plant leaf powder was soaked in solvent (water, 80% methanol, absolute ethanol) overnight at a concentration of 20g/200ml heated for 48 h at 40℃. The extract was then cooled to room temperature and filtered off using Whatman filter paper no 42 to separate the supernatant. The prepared extracts were initially subjected to the qualitative phytochemical analysis.
Qualitative phytochemical analysis
To assess contents, the aqueous, methanolic, and ethanolic extracts and powdered leaf samples of Tamarix dioica were analyzed for proteins, terpenoids, alkaloids, steroids, amino acids, saponins, flavonoids, glycosides, tannins, phenols and phlobatannins according to published literatures (Domitrović and Potočnjak, 2016; Trease and Evans, 1989; Zar-Pasha et al., 2022).
Tannins and phenols gave blue-black precipitate with 0.1% FeCl3 alkaloid precipitated reddish-brown, with a few drops of Dragendorff’s reagent. Blueish green ring after addition of chloroform and a few drops of concentrated H2SO4 indicated terpenoids. Foam foamation after boiling distilled water with dried powdered sample indicated the presence of saponins. Formation of red color after boiling ethanol treated sample with a few bits of magnesium ribbon and a few drops of strong HCl showed the presence of flavoriods.
The appearance of a green/blue precipitate after adding 1 ml of glacial acetic acid, a few drops of FeCl3, and a few drops of concentrated H2SO4 to 2 ml of 1% extracts, suggested the existence of glycosides. Formation of green ring after addition of acetic anhydride and few drops of concentrated H2SO4 shows presence of steroids. Deposition of a red hue after addition of 1% HCl and boiling indicated the presence of phlobatannins. The presence of amino acid was shown by appearance of purple tint after adding ninhydrin reagent and heating.
The presence of proteins was revealed by their violet color, after adding a few drops of 5% NaOH and a few drops of 1% Cu(SO4)2.
Experimental plan
Healthy Swiss albino mices (male), 65-75g were mentioned in an animal house with a temperature of 25 ± 2 °C, a relative humidity range of 40–50 percent, and a light: dark cycle of 12:12 h. They were fed on normal standard diet.
A total of 36 albino mices were divided into 6 groups each containing six mice. Where, Group I (Normal Control) received only distilled water @ 5ml/Kg of body weight (P.O) for 7 days. Group II (Paracetamol Control) received distilled water @ 5ml/Kg of body weight (P.O) for 7 days except the 5th day when paracetamol @ 2g/Kg of body weight was given. Group III (Positive Control) received only Silymarin @ 200 mg/Kg of body weight (P.O) for 7 days except the 5th day when paracetamol @ 2g/Kg of body weight was given. Group IV (Test group I) received only aqueous T. dioica extract @ 400 mg/Kg of body weight (P.O) for 7 days except the 5th day when paracetamol @ 2g/Kg of body weight was given. Group V (Test group II) received only methanolic T. dioica extract @ 400 mg/Kg of body weight (P.O) for 7 days except the 5th day when paracetamol @ 2g/Kg of body weight was given. Group VI (Test group III) received only ethanolic T. dioica extract @ 400 mg/Kg of body weight (P.O) for 7 days except the 5th day when paracetamol @ 2g/Kg of body weight was given.
Biochemical analysis of blood serum
At the end of experimental period blood and liver samples were taken. Blood samples were centrifuged at 12,000 rpm for 5 min for isolation of serum (Zar-Pasha et al. 2022), for assessment of key biomarkers like alkaline phosphate (ALP), alanine aminotransferase (ALT), aspartate transaminase (AST), total bilurubin and total proteins, albumin, triglycerides, urea , creatinine, total chlolestrol and lipid as per specifications of kits used for determination. Percentage protection can be calculated by the following formula:
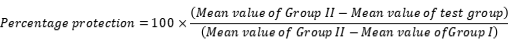
Assessment of key oxidative stress biomarkers
A portion of the liver tissue taken from the animals that were used in the experiment was washed and then homogenized (1:10, weight-for-weight) in an ice-cold solution of 50 mmol/L Tris buffer with a pH of 7.4. The homogenate was centrifuged at 10,000 rpm for 20 min at 4 oC. The supernatant was used for determination of superoxide dismutase (SOD) according to Marklund and Marklund (1974), catalase (CAT), glutathione (GSH), and glutathione peroxidase (GPx), malondialdehyde (MDA) is a result of lipid peroxidation that was tested in the form of thiobarbituric acid reactive (TBARS) material.
Quantification of IL-6, TNF-α and COX2
ELISA procedure, using kits of Elabscience®, China for the estimation of interlink-6 (IL-6), tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX2) was followed.
Histopathological assessment
For hietopathological assessment, the liver tissue was fixed in 10% formaline and then proceeded further for cutting 5 µm thick section. The histological sections were stained with a hematoxylin and eosin (H and E).
Statistical analysis
The statistical analysis was performed using GraphPad Prism version 5.0, considering mean ± standard deviation (SD) values from a sample size of six (n = 6). A two-way analysis of variance (ANOVA) was initially conducted to assess the overall variability, followed by post hoc Dunnett’s multiple comparison test. The significance levels were set at P ≤ 0.05, indicating statistical significance, P ≤ 0.01 for a higher degree of significance (most significant), and P ≤ 0.001 denoting a highly significant difference.
Results and Discussion
Qualitative phytochemical analysis
Table I shows the qualitative phytochemical analysis of leaf extract of Tamarix dioica. Water is the most common solvent used for plant extracts and traditional medicine relies heavily on water extract (Tiwari et al., 2011). The results of phytochemical screening of Tamarix dioica crude leaf, aqueous, methanolic and ethanolic extracts showed that proteins, amino acids, alkaloids and glycosides were not found in all extracts. Steroids and phenols were additionally not found in methanolic extracts, while tannins and saponins were additionally absent in the ethanolic extract of Tamarix dioica. Phytochemicals in T. dioica may be medicinal. Flavonoids have antioxidant, anti-allergic, anti-inflammatory, hepatoprotective, anti-carcinogenic, anti-viral, and anti-thrombotic properties (Najafi et al., 2010). Anti-hemorrhoidal, anti-diarrheal, and hemostatic medications mostly include tannins. Saponins, steroid glycosides, produce a waxy protective layer on plant skins. Antioxidant and anti-inflammatory saponins reduce cholesterol level (Shah et al., 2014). Terpenoids are a broad and diversified class of naturally occurring organic compounds present in all living things. They possess antibacterial and skin thickening properties, boost wound antioxidants, and recover inflammatory tissues by improving blood flow (Krishnaiah et al., 2009). Phenolics protect against cardiovascular disease, apoptosis, inflammation, aging, atherosclerosis, cancer, endothelial function, and angiogenesis and cell proliferation.
Table I. Qualitative phytochemical analysis of Tamarix dioica raw leaf, aqueous, methanolic and ethanolic extracts.
|
S. No |
Compound |
Raw leaf extract |
Aqueous extract |
Methanolic extracts |
Ethanolic extracts |
|
1 |
Proteins |
̶ |
̶ |
̶ |
̶ |
|
2 |
Amino acids |
̶ |
̶ |
̶ |
̶ |
|
3 |
Steroids |
+ |
+ |
̶ |
+ |
|
4 |
Tannins |
+ |
+ |
+ |
̶ |
|
5 |
Phlobatannins |
+ |
+ |
+ |
+ |
|
6 |
Alkaloids |
̶ |
̶ |
̶ |
̶ |
|
7 |
Terpenoids |
+ |
+ |
+ |
+ |
|
8 |
Flavonoids |
+ |
+ |
+ |
+ |
|
9 |
Saponins |
+ |
+ |
+ |
̶ |
|
10 |
Phenols |
+ |
+ |
̶ |
+ |
|
11 |
Glycosides |
̶ |
̶ |
̶ |
̶ |
They also coagulate red blood cells, having bitter, hemolytic, cholesterol-binding, and foam forming activities. They are also significant because they interact with sex hormones and have antimicrobial effects (Yadav and Agarwala, 2011). The presence of flavonoids, terpenoids, and phenolics in T. dioica suggests its potential medicinal value, with documented antioxidant, anti-inflammatory, and various other beneficial properties. These findings contribute to a better understanding of the plant’s chemical composition, laying the groundwork for potential therapeutic applications and further exploration of its pharmacological effects.
Toxicity tolerance
Animals showed a high degree of tolerance to experimental doses of aqueous, methanolic, and ethanolic extracts of the leaf from T. dioica that were as high as 2,000 mg/kg of body weight. This was due to the fact that the animals were given the extracts in one of the three different solvents. After 15 days of treatment with the extracts, there were no visible signs of toxicity or mortality, which led the researchers to the conclusion that the substance was not dangerous. This was true even at the maximum dose. In most cases, 1/10th and 1/5th of the lethal dosage is utilized for the calculation of the effective dose. As a consequence of this, 400 mg/kg of body weight has been assessed as test doses for each of the samples. In addition, utilizing the specific test doses that have been selected up to the point where the research has been done, not a single poisonous indication has been found. This is the case even though the study has been completed. Medicinal plants in different extract forms have hepatoprotective effect (Yu et al., 2002). Some plants have ability to decrease the hepatotoxic effects produced by hepatotoxins via their antioxidant potential (Belfield and Goldberg, 1971). During a study by Sangameswaran et al. (2008) on rats it was observed that methanolic and aqueous extracts of Andrographis lineata leaf @ 845 mg/Kg of the body mass have hepatoprotective effects against the liver damage caused by CCl4 in rodents. Similarly, the leaves of C. hystrix and C. maxima were used to make their ethanolic extracts which were then used to measure their hepatoprotective against paracetamol induced toxicity (Marklund and Marklund, 1974). An investigation was conducted by Tiwari et al. (2011) to determine the effect of methanolic leaf extract of Ginkgo biloba in reducing the damage to the liver caused by lantadenes in guinea pigs. According to the results of this study, the use of G. biloba as a possible hepatoprotectant against the liver damage. Present study on liver protective action showed that T. dioica leaves extracts (aqueous methanolic and ethanolic) have given promising results against experimentally paracetamol induced loss of liver’s structure and functions in albino mice, and ratio of improvement relatively comparable to the standard hepatoprotective drug, silymarin. Numerous studies have been conducted to investigate the antihepatotoxic properties of silymarin and silybin, the component of silymarin that is considered to be the most active. silymarin is derived from the plant Silybum marianum (Najafi et al., 2010).
Effect of serum biomarkers of liver
The measurement of enzymes in the serum is a valuable quantitative marker that may be used to identify the degree of hepatocellular damage as well as the kind of damage. This can be done by comparing the levels of enzymes in the blood before and after exposure to the toxin. A significant amount of liver damage and necrosis of cells was observed in rats after they were given an overdose of paracetamol at a dose of 2 g/kg. This was demonstrated by an increase in the serum levels of hepatic enzymes (ALT, AST, and ALP), a decrease in the level of protein, and an increase in the level of total bilirubin. The serum levels of hepatic enzymes (AST, ALT and ALP) are pictorially represented in Figure 1A while distribution of total bilirubin and total proteins among the test groups and controlled groups is represented in Figure 1B. The findings of the current study substantiate the hypothesis that paracetamol has hepatotoxic effects, as evidenced by a significant increase in the activity of liver function marker enzymes ALT, AST, ALP, and bilirubin in the serum of mice. These results align with related research (Ayenew and Wasihun, 2023; Olaleye et al., 2014; Sait et al., 2014; Senthilkumar et al., 2014), providing further support for the notion of paracetamol-induced hepatotoxicity.
When administered orally to mice, extracts of T. dioica leaves in aqueous, methanolic, and ethanolic solvents were shown to be practically devoid of harmful effects. Changes in serum marker enzymes revealed that mice who were given paracetamol at a hazardous dosage had significant liver damage. This was proved by the fact that the enzyme levels changed. Mice that had been pretreated with the leaves of Tamarix dioica (in aqueous, methanolic, and ethanolic extracts) exhibited a significant level of hepatoprotective activity against paracetamol, as well as a marked armor against the hepatotoxicity that was caused by paracetamol to the liver cells. This level of activity was comparable to that of the standard silymarin.
Hepatotoxicity is proven and typically confirmed by elevated levels of AST, ALT, ALP, and bilirubin (Shah et al., 2022; Krishnaiah et al., 2009). Increases in ALT, ALP, and bilirubin were blocked by coadministration of extracts from all of the plants tested. The regeneration of liver cells is responsible for the decrease in blood levels of alanine aminotransferase, alanine aminotransferase, and alanine aminopeptidase (Yadav and Agarwala, 2011).
Effect of T. dioica and Silymarin on the key serum biomarkers of liver
Percentage protection among serum biomarkers after silymarin usage for AST, ALT, ALP, TBIL an T.P were 98.98 %, 96.30 %, 97.35 %, 95.09 % and 85.86 % respectively which indicates the reversal of hepatotoxicity by the liver cells after the use of known and control antihepatotoxic agent. The use of extracts (aqueous, methanolic and ethanolic) of T. dioica showed reversal in toxicity produced by paracetamol at its lethal dose (2000 mg/kg of body weight) but the aqueous extracts of T. dioica showed maximum protection (Table II) among the all used extracts of T. dioica. Overall protection of treated groups in Table II also showed that the aqueous extract of T. dioica showed the best results among the other extracts used.
Table II. Percentage protection of serum biomarkers of LFTs among different treated groups.
|
Serum biomarker |
Group III |
Group IV |
Group V |
Group VI |
|
AST |
98.98 |
97.85 |
92.69 |
91.91 |
|
ALT |
96.30 |
91.83 |
89.96 |
86.75 |
|
ALP |
97.35 |
88.26 |
76.96 |
71.82 |
|
TBIL |
95.09 |
87.73 |
79.14 |
69.33 |
|
Total protein |
85.86 |
67.49 |
51.61 |
42.18 |
|
Overall protection |
||||
|
94.71 |
86.63 |
78.07 |
72.40 |
|
AST, aspartate transaminase; ALT, alanine aminotransferase; ALP, alkaline phosphate; TBIL, total bilirubin.
Increased level of TNF α (230 ± 2.5), COX-2 (455 ± 3.09) and IL-6 (59.9 ±1.85) in group II was observed to be decreased in all the treated groups of T. dioica extracts as well as silymarin treated group and comparative results are shown in graphical form in Figure 1D. Proteomics studies have identified IL-6, COX-2, and TNF-α as indicators of inflammation. They play a role in the systemic inflammatory response. In addition to its involvement in controlling inflammation and apoptosis, TNF-α also has a role in regulating a variety of other physiological events (Alkhudayari et al., 2021). It has been observed before that several types of experimental and clinical liver damage are associated with elevated levels of inflammatory cytokine activity (Fadhel and Amran, 2002). Extreme inflammation and consequent upregulation of TNF-α, COX-2, and IL-6 were seen in albino mice treated with paracetamol in the present investigation. Our findings show that a group treated with T. dioica extracts was able to reduce the severity of paracetamol-induced liver damage, which may be attributed to the extracts ability to reduce inflammation
Table III. Comparison of oxidative biomarkers in test groups.
|
Treated groups |
Treatment |
SOD (U/mg protein) |
CAT (µmol of H2O2 decomposed/mg protein) |
GPx (U/mg protein) |
GSH (µg/mg protein) |
MDA (nmol MDA/mg protein) |
|
Group I |
Normal control |
31.13 ± 0.48 |
115.17 ± 0.89 |
3.61 ± 0.06 |
6.37 ± 0.13 |
1.65 ± 0.09 |
|
Group II |
Paracetamol control |
14.81 ± 0.46 |
59.33 ± 1.19 |
0.75 ± 0.03 |
3.37 ± 0.17 |
5.24 ± 0.16 |
|
Group III |
Positive control |
27.17 ± 0.97 |
107.19 ± 1.76 |
3.57 ± 0.02 |
5.95 ± 0.09 |
1.71 ± 0.02 |
|
Group IV |
Test group I |
25.11 ± 0.48 |
102.85 ± 0.34 |
3.47 ± 0.03 |
5.31 ± 0.09 |
1.84 ± 0.11 |
|
Group V |
Test group II |
23.18 ± 0.59 |
97.69 ± 0.41 |
3.03 ± 0.18 |
5.13 ± 0.07 |
2.12 ± 0.11 |
|
Group VI |
Test group III |
19.85 ± 0.51 |
91.49 ± 0.55 |
2.88 ± 0.15 |
4.87 ± 0.12 |
2.28 ± 0.18 |
CAT, catalase; GSH, glutathione; GPx, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
by restoring normal levels of inflammatory mediators. In terms of hepatoprotection, its impact was on par with that of the gold standard, silymarin (Abirami et al., 2015). From the Table III it is obvious that the usage of extracts (aqueous, methanolic and ethanolic) from leaves of T. dioica brought the liver biochemical markers also known as oxidative stress markers back to the normal range. Aqueous extract of T. dioica again found to be the post potent treatment other than the control treatment (silymarin treatment).
Effect of T. dioica extract on oxidative biomarkers
Paracetamol induced toxification @ 2000mg/kg in mices (Group II) caused the decrease in biochemical parameters of liver (SOD, CAT, GPx and GSH) while increase in MDA level was observed as compared to the normal mices (Group I) (Figure 2). Detailed comparison of liver biochemical parameters between different treated groups (Group II to Group VI) and normal mices (Group I) has been summarized in Table III. SOD, CAT, and GPx are just a few examples of antioxidant enzymes (Ighodaro and Akinloye, 2018) that play a crucial role in protecting organisms against reactive oxygen species (Miguez et al., 1994). SOD is a protective enzyme that breaks down harmful superoxide radicals into harmless hydrogen peroxide. Eukaryotic cells’ peroxisomes include catalase, a hemeprotein that catalyses the breakdown of hydrogen peroxide into harmless water and oxygen. GPx is essential for preventing the oxidative degradation of lipids and proteins caused by chemical agents (Akther et al., 2013), and for keeping the redox state of animals in check during periods of acute oxidative stress. It has been hypothesised that lipid peroxidation is the damaging mechanism in paracetamol-induced liver damage (Ezzat et al., 2019). An uptick in liver MDA levels is indicative of increased lipid peroxidation, tissue damage, and a breakdown in antioxidant defence systems. Reactive oxygen species created by toxicants may be toxic, as shown by a reduction in glutathione, GPx, SOD, and catalase enzyme activity. Paracetamol-treated test animals may have had lower GSH levels because glutathione was being conjugated with NAPQ1 to generate mercapturic acid (Balkwill, 2002).
Histopathological analysis
When compared to the group of normal mice (Group I), the results of the liver histological examination revealed substantial alterations in the liver segment that had been subjected to paracetamol toxification (Group II). Toxic effects of paracetamol on liver sections showed aberrant morphological features, including vacuolated hepatocytes, fat buildup, mitotic figures, and the degree of hepatic damage.
According to the findings of a histological investigation that looked at liver slices from the group that had been treated with silymarin (Group III), the mitotic figures, the vacuolated hepatocytes, and the fat deposits were all significantly reduced (Abirami et al., 2015; Ayenew and Wasihun, 2023; Parimoo et al., 2014; Shareef et al., 2022). In comparison to the group of mice that was given paracetamol (Group II), the groups of mice that were pretreated with aqueous extract (Group IV), methanolic extract (Group V), and ethanolic extract (Group VI) at doses of 400 mg/kg of body weight were found to have significantly organized liver tissues, highly significant lower fat accumulation, and vacuolated hepatic cells. The level of liver protection was shown to be highest, in the group of mices, that had been administered an aqueous extract at a dosage of 400 mg/kg. In addition, when a high dose of T. dioica aqueous, methanolic, and ethanolic extracts was supplied orally over the course of 24 h, there was no indication of any toxicity symptoms in comparison to the group that served as the control. The dosage was 2000 mg/kg body weight of each extract. Furthermore, inflammation score and fibroblast score in all the test group is also calculated and recorded in Figure 3.
No prior research has shown that Tamarix dioica leaf extract has any anti-hepatotoxic action against paracetamol toxicity. Because extracts significantly decrease the elevated levels of certain blood indicators, hence Tamarix dioica extracts may be utilized to treat liver damage.
Conclusion
Extracts of Tamarix dioica leaves at a dosage of 400mg/kg were shown to have the most hepatoprotective qualities and to prevent paracetamol-induced toxicity on serum biochemical parameters to the greatest extent. Maximum and analogous activity against paracetamol-induced hepatotoxicity was seen by the aqueous extract. Extracts of Tamarix dioica leaves may be purified to yield chemicals with hepatoprotective action. For the purpose of employing the separated active lead compounds in studies, clinical trials, and retesting using various animal models, NMR spectroscopy and other spectrum analyses may aid the researcher in determining the molecular formula of the compounds.
ACKNOWLEDGMENT
The author are grateful for the support from Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R213), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
The study was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R213), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Ethical approval
This study was approved by Medical Research Ethical Committee from Health Science Research Center -Kaauh at Princess Nourah bint Abdulrahman University under (IRB Log Number: 23-0030), Riyadh, KSA, guided by the Guide for the Care and Use of Laboratory Animals issued by US National Institutes of Health (NIH publication No.85-23, revised 1996).
The research follows the international ethical committee from the institutional bioethics. The research was conducted in accordance with OECD guidelines-423 (acute toxic class method) (OECD, 2001).
Statement of conflicts of interest
The authors have declared no conflict of interest.
References
Abirami, A., Nagarani, G. and Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Fd. Sci. Hum. Wellness, 4: 35–41. https://doi.org/10.1016/j.fshw.2015.02.002
Akther, N., Shawl, A.S., Sultana, S., Chandan, B.K. and Akhter, M., 2013. Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J. Pharm. Res., 7: 565–570. https://doi.org/10.1016/j.jopr.2013.06.023
Ali, S.S., Ahsan, H., Zia, M.K., Siddiqui, T. and Khan, F.H., 2020. Understanding oxidants and antioxidants: Classical team with new players. J. Fd. Biochem., 44. https://doi.org/10.1111/jfbc.13145
Alkhudhayri, D.A., Osman, M.A., Alshammari, G.M., Al-Maiman, S.A. and Yahya, M.A., 2021. Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats. Saudi J. biol. Sci., 28: 3333–3342. https://doi.org/10.1016/j.sjbs.2021.02.078
Alnuqaydan, A.M. and Rah, B., 2019. Tamarix articulata (T. articulata). An important halophytic medicinal plant with potential pharmacological properties. Curr. Pharma. Biotech., 20: 285–292. https://doi.org/10.2174/1389201020666190318120103
Alqasoumi, S.I., 2012. Okra Hibiscus esculentus L.: A study of its hepatoprotective activity. Saudi Pharma. J., 20: 135–141. https://doi.org/10.1016/j.jsps.2011.10.002
Arianmanesh, R., Mehregan, I., Assadi, M. and Nejadsattari, T., 2016. Comparative morphology of the genus Tamarix (Tamaricaceae) in Iran. Int. Lett. natl. Sci., 60: 1–12. https://doi.org/10.56431/p-6s8gxp
Atanasov, A.G., Waltenberger, B., Pferschy-Wenzig, E.M., Linder, T., Wawrosch, C., Uhrin, P., Temml, V., Wang, L., Schwaiger, S. and Heiss, E.H., 2015. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv., 33: 1582–1614. https://doi.org/10.1016/j.biotechadv.2015.08.001
Ayenew, K.D. and Wasihun, Y., 2023. Hepatoprotective effect of methanol extract of Agave americana leaves on paracetamol induced hepatotoxicity in Wistar albino rats. BMC Complement. Med. Ther., 23: 99. https://doi.org/10.1186/s12906-023-03931-y
Aziz, M.A., Khan, A.H., Adnan, M. and Ullah, H., 2018. Traditional uses of medicinal plants used by Indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J. Ethnobiol. Ethnomed., 14: 11. https://doi.org/10.1186/s13002-018-0212-0
Bahramsoltani, R., Kalkhorani, M., Zaidi, S.M.A., Farzaei, M.H. and Rahimi, R., 2020. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol., 246: 112245. https://doi.org/10.1016/j.jep.2019.112245
Balkwill, F., 2002. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev., 13: 135–141. https://doi.org/10.1016/S1359-6101(01)00020-X
Belfield, A. and Goldberg, D.M., 1971. Normal ranges and diagnostic value of serum 5’ nucleotidase and alkaline phosphatase activities in infancy. Arch. Dis. Child., 46: 842–846. https://doi.org/10.1136/adc.46.250.842
Bratovcic, A., 2020. Antioxidant enzymes and their role in preventing cell damage. Acta Sci. Nutr., 4: 1–7. https://doi.org/10.31080/ASNH.2020.04.0659
Caparrotta, T.M., Antoine, D.J. and Dear, J.W., 2018. Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature. Eur. J. clin. Pharmacol., 74: 147–160. https://doi.org/10.1007/s00228-017-2356-6
Domitrović, R. and Potočnjak, I., 2016. A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch. Toxicol., 90: 39–79. https://doi.org/10.1007/s00204-015-1580-z
Ezzat, S., Abo Rabia, N., Khalaf, G. and El-Morsy, Dina, 2019. Histological study on the possible protective role of Moringa oleifera leaves extract on paracetamol induced liver damage in adult male albino rats. Egypt. J. Histol., 42: 712-729. https://doi.org/10.21608/ejh.2019.7258.1069
Fadhel, Z.A. and Amran, S., 2002. Effects of black tea extract on carbon tetrachloride-induced lipid peroxidation in liver, kidneys, and testes of rats. Phytother. Res., 16: 28–32. https://doi.org/10.1002/ptr.793
Fujii, J., Homma, T. and Osaki, T., 2022. Superoxide radicals in the execution of cell death. Antioxidants, 11: 501. https://doi.org/10.3390/antiox11030501
He, M., Zhang, S., Jiao, Y., Lin, X., Huang, J., Chen, C., Chen, Z. and Huang, R., 2012. Effects and mechanisms of rifampin on hepatotoxicity of acetaminophen in mice. Fd. Chem. Toxicol., 50: 3142–3149. https://doi.org/10.1016/j.fct.2012.06.020
Ighodaro, O.M. and Akinloye, O.A., 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med., 54: 287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Ji, H., Li, X. and Zhang, H., 2009. Natural products and drug discovery: Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep., 10: 194–200. https://doi.org/10.1038/embor.2009.12
Kaur, P., Shergill, R., Mehta, R.G., Singh, B. and Arora, S., 2021. Biofunctional significance of multi-herbal combination against paracetamol-induced hepatotoxicity in Wistar rats. Environ. Sci. Pollut. Res., 28: 61021–61046. https://doi.org/10.1007/s11356-021-15019-6
Komal, S., Malik, A., Akhtar, N., Kazmi, S.A.J., Anjum, F. and Rida, A., 2021. Tamarix dioica (Ghaz) protective potential in the carbon tetrachloride-induced hepatotoxicity animal model. Proc. Shaikh Zayed Medical Complex, 35: 37–43. https://doi.org/10.47489/PSZMC-806-35-3-37-43
Krishnaiah, D., Devi, T., Bono, A. and Sarbatly, R., 2009. Studies on phytochemical constituents of six Malaysian medicinal plants. J. med. Pl. Res., 3: 67–72.
Marklund, S. and Marklund, G., 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem., 47: 469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Miguez, M.P., Anundi, I., Sainz-Pardo, L.A. and Lindros, K.O., 1994. Hepatoprotective mechanism of silymarin: No evidence for involvement of cytochrome P450 2E1. Chemico-Biol. Interact., 91: 51–63. https://doi.org/10.1016/0009-2797(94)90006-X
Najafi, S., Sanadgol, N., Nejad, B.S., Beiragi, M.A. and Sanadgol, E., 2010. Phytochemical screening and antibacterial activity of Citrullus colocynthis (Linn.) Schrad against Staphylococcus aureus. J. med. Pl. Res., 4: 2321–2325.
Narici, M., Vito, G.D., Franchi, M., Paoli, A., Moro, T., Marcolin, G., Grassi, B., Baldassarre, G., Zuccarelli, L., Biolo, G., di Girolamo, F.G., Fiotti, N., Dela, F., Greenhaff, P. and Maganaris, C., 2021. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci., 21: 614–635. https://doi.org/10.1080/17461391.2020.1761076
Naskar, S., Mazumder, U.K., Pramanik, G., Gupta, M., Suresh Kumar, R.B., Bala, A. and Islam, A., 2011. Evaluation of antihyperglycemic activity of Cocos nucifera Linn. on streptozotocin induced type 2 diabetic rats. J. Ethnopharmacol., 138: 769–773. https://doi.org/10.1016/j.jep.2011.10.021
Offor, S.J., Amadi, C.N., Chijioke-Nwauche, I., Manautou, J.E. and Orisakwe, O.E., 2022. Potential deleterious effects of paracetamol dose regime used in Nigeria versus that of the United States of America. Toxicol. Rep., 9: 1035–1044. https://doi.org/10.1016/j.toxrep.2022.04.025
Olaleye, M.T., Amobonye, A.E., Komolafe, K. and Akinmoladun, A.C., 2014. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J. biol. Sci., 21: 486–492. https://doi.org/10.1016/j.sjbs.2014.06.005
Ornos, E.D., Murillo, K.J. and Ong, J.P., 2023. Liver diseases: Perspective from the Philippines. Annls Hepatol., 28: 101085. https://doi.org/10.1016/j.aohep.2023.101085
Parimoo, H.A., Sharma, R., Patil, R.D., Sharma, O.P., Kumar, P. and Kumar, N., 2014. Hepatoprotective effect of Ginkgo biloba leaf extract on lantadenes-induced hepatotoxicity in guinea pigs. Toxicon, 81: 1–12. https://doi.org/10.1016/j.toxicon.2014.01.013
Percival, G., Schaffert, E. and Hailey, L., 2014. Trees in the rural landscape. In: Horticulture: Plants for people and places (eds. G.R. Dixon and D.E. Aldous). Volume 2. Springer Netherlands, Dordrecht, pp. 713–730. https://doi.org/10.1007/978-94-017-8581-5_6
Post-White, J., Ladas, E.J. and Kelly, K.M., 2007. Advances in the use of milk thistle (Silybum marianum). Integr. Cancer Ther., 6: 104–109. https://doi.org/10.1177/1534735407301632
Pradhan, S.C. and Girish, C., 2006. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. med. Res., 124: 491–504.
Pramod, K., Deval, R.G. and Ramachandra, S.S., 2008. Antioxidant and hepatoprotective activity of tubers of Momordica tuberosa Cogn. against CCl4 induced liver injury in rats. Indian J. exp. Biol., 46: 510–513.
Radko, L. and Cybulski, W., 2007. Application of silymarin in human and animal medicine. J. Pre-Clin. clin. Res., 1: 22–26.
Rehecho, S., Uriarte-Pueyo, I., Calvo, J., Vivas, L.A. and Calvo, M.I., 2011. Ethnopharmacological survey of medicinal plants in Nor-Yauyos, a part of the landscape reserve Nor-Yauyos-Cochas, Peru. J. Ethnopharmacol., 133: 75–85. https://doi.org/10.1016/j.jep.2010.09.006
Riddle, E.S., Campbell, M.S., Lang, B.Y., Bierer, R., Wang, Y., Bagley, H.N. and Joss-Moore, L.A., 2014. Intrauterine growth restriction increases TNF α and activates the unfolded protein response in male rat pups. J. Obesity, 2014: 1–9. https://doi.org/10.1155/2014/829862
Sait, I.I., Harindran, J., Vahab, A.A., Jeena, J.L., Nasli, S. and John, J., 2014. Potential hepatoprotective effect and antioxidant role of methanol extract of Morinda tinctoria in carbon tetrachloride induced hepatotoxicity in albino rats. Int. J. Phar., 4: 363–368.
Sallie, R.W., Reed, W.D. and Shilkin, K.B., 1991. Confirmation of the efficacy of hepatic tissue iron index in differentiating genetic haemochromatosis from alcoholic liver disease complicated by alcoholic haemosiderosis. Gut, 32: 207–210. https://doi.org/10.1136/gut.32.2.207
Salmeri, F.M., Laganà, A.S., Sofo, V., Triolo, O., Sturlese, E., Retto, G., Pizzo, A., D’Ascola, A. and Campo, S., 2015. Behavior of tumor necrosis factor-α and tumor necrosis factor receptor 1/tumor necrosis factor receptor 2 system in mononuclear cells recovered from peritoneal fluid of women with endometriosis at different stages. Reprod. Sci., 22: 165–172. https://doi.org/10.1177/1933719114536472
Samadi, N., Ghaffari, S.M. and Akhani, H., 2013. Meiotic behaviour, karyotype analyses and pollen viability in species of Tamarix (Tamaricaceae). Willdenowia, 43: 195–203. https://doi.org/10.3372/wi.43.43121
Samejo, M.Q., Sumbul, A., Shah, S., Memon, S.B. and Chundrigar, S., 2013. Phytochemical screening of Tamarix dioica Roxb. ex Roch. J. Pharma. Res., 7: 181–183. https://doi.org/10.1016/j.jopr.2013.02.017
Sangameswaran, B., Reddy, T.C. and Jayakar, B., 2008. Hepatoprotective effect of leaf extracts of Andrographis lineata nees on liver damage caused by carbon tetrachloride in rats. Phytother. Res., 22: 124–126. https://doi.org/10.1002/ptr.2250
Senthilkumar, R., Chandran, R. and Parimelazhagan, T., 2014. Hepatoprotective effect of Rhodiola imbricata rhizome against paracetamol-induced liver toxicity in rats. Saudi J. biol. Sci., 21: 409–416. https://doi.org/10.1016/j.sjbs.2014.04.001
Shah, N.A., Khan, M.R., Sattar, S., Ahmad, B. and Mirza, B., 2014. HPLC-DAD analysis, antioxidant potential and anti-urease activity of Asparagus gracilis collected from District Islamabad. BMC Complement. Altern. Med., 14: 347. https://doi.org/10.1186/1472-6882-14-347
Shahidi, F. and Zhong, Y., 2010. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev., 39: 4067. https://doi.org/10.1039/b922183m
Shareef, S.H., Ibrahim, A.A.I., Alzahrani, A.R., Al-Medhtiy, M.H. and Abdulla, A.M., 2022. Hepatoprotective effects of methanolic extract of green tea against thioacetamide-induced liver injury in sprague dawley rats. Saudi J. biol. Sci., 29: 564–573. https://doi.org/10.1016/j.sjbs.2021.09.023
Stahl, E.C., Haschak, M.J., Popovic, B. and Brown, B.N., 2018. Macrophages in the aging liver and age-related liver disease. Front. Immunol., 9: 2795. https://doi.org/10.3389/fimmu.2018.02795
Subramanya, S., Venkataraman, B., Meeran, M., Goyal, S., Patil, C. and Ojha, S., 2018. Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int. J. mol. Sci., 19: 3776. https://doi.org/10.3390/ijms19123776
Tiwari, P., Kaur, M. and Kaur, H., 2011. Phytochemical screening and extraction: A review. Int. Pharma. Sci., 1: 98-106
Trease, G.E. and Evans, W.C., 1989. Pharmacognsy 11th edition. Bralliar Tridel Can Macmillian Publishers.
Urumarudappa, S.K.J., Krishna, C.M.J., Semotiuk, A.J. and Krishna V., 2019. Indigenous knowledge on medicinal plants used by ethnic communities of South India. Ethnobot. Res. App., 18: 1–112. https://doi.org/10.32859/era.18.4.1-112
Valková, V., Ďúranová, H., Bilčíková, J. and Habán, M., 2020. Milk thistle (Silybum marianum): A valuable medicinal plant with several therapeutic purposes. J. Microb. Biotech. Fd. Sci., 9: 836–843. https://doi.org/10.15414/jmbfs.2020.9.4.836-843
Wahyuningsih, S.P.A., Mwendolwa, A.A., Winarni, D., Anggreini, R.W. and Mamuaya, B.K.K., 2021. Protective effect of red okra (Abelmoschus esculentus (L.) Moench) pods against sodium nitrite-induced liver injury in mice. Vet. Med. Int., 2021: 1–11. https://doi.org/10.1155/2021/6647800
Yadav, R.N.S. and Agarwala, M., 2011. Phytochemical analysis of some medicinal plants. J. Phytol., 3: 10-14.
Yu, C., Wang, F., Jin, C., Wu, X., Chan, W. and McKeehan, W.L., 2002. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am. J. Pathol., 161: 2003–2010. https://doi.org/10.1016/S0002-9440(10)64478-1
Zar-Pasha, A., Bukhari, A.S., El-Enshasy, A.H., El-Adawi, H. and Al-Obaid, S., 2022. Compositional analysis and physicochemical evaluation of date palm (Phoenix dactylifera L.) mucilage for medicinal purposes. Saudi J. biol. Sci., 29: 774–780. https://doi.org/10.1016/j.sjbs.2021.10.048
Zhang, A., Sun, H. and Wang, X., 2013. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. med. Chem., 63: 570–577. https://doi.org/10.1016/j.ejmech.2012.12.062
To share on other social networks, click on any share button. What are these?









