Enhancing Rice Bran Stability with Moringa oleifera Leaf Antioxidants During Extended Storage
Research Article
Enhancing Rice Bran Stability with Moringa oleifera Leaf Antioxidants During Extended Storage
Martina Tri Puspita Sari1, Muhammad Ridla2,3*, Heri Ahmad Sukria2,3
1Study Program of Nutrition and Feed Science, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Kampus IPB Dramaga Bogor, Indonesia; 2Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Kampus IPB Dramaga Bogor, Indonesia; 3Center for Tropical Animal Sciences (CENTRAS), IPB University Bogor, Indonesia.
Abstract | Rice bran, a valuable energy source for animal feed and abundant in Indonesia, is prone to rancidity, which affects its quality and shelf life. Moringa oleifera leaves, which grow easily in diverse environmental conditions and are widely available in Indonesia, contain high levels of bioactive compounds with numerous benefits. This study evaluated Moringa oleifera leaves as a stabilization method to preserve rice bran quality over a 30 days of storage. A randomized block design was employed with ten treatments and four replications: no stabilization with no storage (Control-0) and 30 days of storage (Control-30), heat treatment without storage (Heated-0) and with 30 days of storage (Heated-30), addition of Moringa oleifera leaf powder (MOLP) without storage (MOLP-0) and with 30 days of storage (MOLP-30), addition of Moringa oleifera leaf extract (MOLE) without storage (MOLE-0) and with 30 days of storage (MOLE-30), addition of synthetic antioxidant Butylated hydroxytoluene without storage (BHT-0) and with 30 days of storage (BHT-30). Data were analyzed using ANOVA and post-hoc Duncan tests to determine significant differences (P < 0.05). Results indicated that MOLE and MOLP significantly (P < 0.05) reduced water activity and fat content, increased compacted bulk density, and preserved protein content of rice bran after storage. Heat treatment effectively reduced moisture content and free fatty acid levels (P < 0.05). In conclusion, the use of natural Moringa oleifera leaves as an antioxidant can replace synthetic antioxidants, as their functional benefits help maintain rice bran quality after 30 days of storage.
Keywords | Antioxidant, Heating, Moringa oleifera leaf, Rice bran, Stabilization, Storage
Received | September 27, 2024; Accepted | November 13, 2024; Published | December 31, 2024
*Correspondence | Muhammad Ridla, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Kampus IPB Dramaga Bogor, Indonesia; Email: hmridla@apps.ipb.ac.id
Citation | Sari MTP, Ridla M, Sukria HA (2025). Enhancing rice bran stability with Moringa oleifera leaf antioxidants during extended storage. Adv. Anim. Vet. Sci. 13(1): 189-197.
DOI | https://dx.doi.org/10.17582/journal.aavs/2025/13.1.189.197
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Rice is a primary food source for people in many regions around the world, with several Asian countries serving as major rice producers to meet global demand. In 2023, Indonesia ranked as the fourth-largest rice producer, following China, India, and Bangladesh, contributing approximately 10.28% of global rice production. According to the Indonesian Central Statistics Agency, Indonesia’s paddy production in 2022 was 54.75 million tons of milled dry grain, with rice production reaching 31.54 million tons, which increased from 2021 (BPS, 2023). Consequently, the by-products of rice milling are substansial, particularly rice bran, which constitutes about 8%-10% of the total paddy processed into rice. Rice bran includes the germ layer and the outer layer of rice, making it a rich source energy. The gross energy content of rice bran is 4,500 kcal kg-1 (Zhang et al., 2020). Given its high energy content and abundant availability in Indonesia, rice bran holds significant potential as a local ingredient for animal feed and as an energy source for livestock, particularly as a partial substitute for corn in poultry rations. Additionally, rice bran has favourable nutritional qualities, with crude protein ranging from 11%-17%, crude fiber from 6%-14%, and crude fat from 15%-22% (Sharif et al., 2014), along with bioactive compounds such as phenolic compounds that serve as antioxidants. However, challenges remain in optimizing the use of rice bran due to its tendency to spoil and develop rancid odors, attributed to its high fat content and fat-digesting enzymes, such as lipase.
Due to its susceptibility to lipid oxidation and degradation, rice bran stabilization is crucial for deactivating fat-digesting enzymes. Stabilization is particularly important when rice bran is stored for an extended period before use as feed ingredient. This practice is common in the feed industry, where large quantities of feed ingredients are stored for some time before usage, often managed using the FIFO (first-in, first-out) principle. With appropriate stabilization methods, the shelf life of rice bran can be extended, which helps maintain its quality during storage until it is processed into feed. Although stabilization requires significant effort, specialized equipment, and financial resources, the resulting extended shelf life supports the production of high-quality feed products. Additionally, stabilization methods that prolong shelf life help mitigate challenges posed by varying environmental conditions around storage facilities, as this condition is the greatest challenge to using rice bran as feed ingredient (Mujahid et al., 2005).
High-temperature heating is a commonly used stabilization method because it effectively deactivates fat-digesting enzymes, is efficient, and can be applied in small to medium-scale operations (Thanonkaew et al., 2012). However, rice bran stabilization through heat treatment may reduce its nutrient content. The addition of antioxidants is another approach to prevent lipid oxidation and stabilize rice bran. Natural antioxidants offer a safer alternative to synthetic antioxidants, which can pose adverse effects and toxicity, including carcinogenic potential (Carocho and Ferreira, 2013).
Several previous studies have examined comparisons between the use of natural and synthetic antioxidants. Besides Moringa oleifera leaves, several other natural materials have also been studied for their antioxidant potential. Jennings and Akoh (2009) found that carnosic acid and rosemary extract as natural antioxidant were as effective as the synthetic antioxidant TBHQ in improving the oxidative stability index of rice bran oil. Similarly, research by Sultana et al. (2008) demonstrated that extracts from agricultural by-products, such as pomegranate, apple, orange, and banana peels, were even more effective than the synthetic antioxidant BHT in reducing peroxide values, conjugated dienes and trienes, and p-anisidine values in corn oil over 30-day storage period. Their findings suggest that natural antioxidants may serve as alternatives to reduce or replace synthetic antioxidants. Various extraction methods have been studied, enabling the selection of affordable and easily accessible techniques. Consequently, there is strong justification for reducing synthetic antioxidant use and shifting to healthier, natural antioxidants.
Moringa oleifera leaves are a natural material with potential as a natural antioxidant. Moringa oleifera leaves are rich source of phenolic compounds such as flavonoids and tannins, along with other beneficial compounds like terpenoids, alkaloids, and saponins, which offer various advantages, including antioxidant, antibacterial, antihypertensive (Rivai, 2020; Yuliyana et al., 2023). Previous studies have demonstrated that Moringa oleifera leaf extract possesses high phenolic content and strong antioxidant activity (Sadiah et al., 2022). Moringa oleifera leaves also provide a high-quality nutrient profile, containing over 20% crude protein and serving as a rich source of vitamins and minerals (Masih et al., 2019). Moringa oleifera leaves contain 27.3%-30.17% crude protein content (Selim et al., 2021; Aditama et al., 2021). Vitamin C, which also functions as an antioxidant, is present in Moringa oleifera leaves significantly higher than the vitamin C content in oranges (Sultana, 2020). Furthermore, Moringa oleifera plants are easy to cultivate across diverse environmental conditions, making them widely available. Not only the leaves, but all parts of the Moringa oleifera plant can be utilized for their health benefits. These attributes have drawn considerable research interest to Moringa oleifera.
Research on the addition of Moringa oleifera leaves to animal feed has been widely conducted to assess its effects on growth, performance, digestibility, and product quality in both ruminants and poultry. However, studies specifically investigating the use of Moringa oleifera leaves as an antioxidant to preserve feed quality are limited. Therefore, this study aims to evaluate the use of Moringa oleifera leaves, in both powder and extract forms, as a natural antioxidant and stabilization method to maintain nutrient quality and reduce lipid degradation indicators in rice bran.
MATERIAL AND METHODS
Rice Bran Preparation
The fresh rice bran was sourced daily from a rice mill in Situ Daun Village, Tanjolaya District, Bogor Regency, West Java, Indonesia, and stored in a freezer until treatment. Particle size distribution was measured using a sieve shaker, following ASAE (1983), and purity was tested with phloroglucinol solution. The geometric mean particle diameter was 0.43 mm, with a standard deviation of 1.9. The rice bran contained 10%-15% husk contamination.
Preparation of Moringa oleifera Leaf Powder (MOLP) and Moringa oleifera Leaf Extract (MOLE)
Moringa leaves were dried in a dome dryer at 30-40°C for 3-4 days, followed by 30 minutes in a cabinet dryer at 50-60°C. The dried Moringa leaves were ground with a hammer mill (3 mm screen) and sieved (30-mesh, 0.6 mm) to obtain MOLP. MOLE was prepared via maceration method, modified from Moo-Huchin et al. (2019), using a 1:10 (w/v) ratio of MOLP to ethanol-water (1:1) solvent. The extraction process lasted 92 hours with intermittent stirring for 30 minutes, and the solution was concentrated at 40°C using a rotary evaporator.
Rice Bran Stabilization and Storage
This study involved ten treatments, utilizing different stabilization methods and storage conditions for rice bran (RB). The stabilization treatments included no stabilization (control), heating, Moringa oleifera leaf extract (MOLE) and powder (MOLP) as natural antioxidants, and Butylated hydroxytoluene (BHT) as a synthetic antioxidant. Each stabilization treatment was either not stored (day 0) or stored for 30 days (day 30), resulting in a total of 10 treatments: Control-0, Control-30, Heated-0, Heated-30, MOLP-0, MOLP-30, MOLE-0, MOLE-30, BHT-0, and BHT-30. After stabilization, the rice bran was placed in plastic sack at an average room temperature of 29.5°C and humidity of 58.6%. Aerobic storage using plastic sacks is a common method of feed storage in field practice, allows for contact between the feed material and air, which facilitates lipid oxidation and degradation. This aerobic storage presents a challenge for various stabilization methods employed, providing an opportunity to identify of the most effective stabilization method for preserving the quality of rice bran during storage. Rice bran samples, both those not stored and those stored for 30 days, were immediately placed in a freezer until testing.
A commonly used heating method for stabilization was employed as a positive control. Dry heating in an oven at 110°C for 20 minutes was selected, as it represents the optimal temperature and heating duration to reduce lipase activity and increase ABTS and DPPH antioxidant activities without damaging the nutrient content of rice bran (Meral, 2021). The concentration of MOLE used in this study was 600 ppm, based on Sultana et al. (2008), who demonstrated that applying natural extracts with a similar total phenolic content at 600 ppm effectively reduced the oxidation rate of corn oil. Meanwhile, the concentration of MOLP used in this study was calculated to be equivalent to 600 ppm of MOLE, at 0,45%. Additionally, synthetic antioxidants were compared with MOLP and MOLE, which served as natural antioxidants. A concentration of 200 ppm was selected in accordance with the maximum allowable usage of synthetic antioxidants, as stipulated by Indonesia’s Food and Drug Supervisory Agency Regulation.
Determination of the Total Phenolic Content (TPC) of Moringa oleifera leaf
The TPC was determined following the Indonesian Herbal Pharmacopeia (Ditjen Farmalkes, 2011). A 10 mg sample or 5 ml volume extract was dissolved in 1 ml of p.a. methanol in a 25 ml volumetric flask. Then, 5 ml of 7.5% Folin-Ciocalteu reagent was added, and the mixture was vortexed and incubated in the dark for 8 minutes. After adding 4 ml of 1% NaOH, and the mixture was vortexed again and incubated in 1 hour in the dark. The TPC was measured using a spectrophotometer at a wavelength 0f 730 nm. Gallic acid was used as the standard for testing total phenolic content.
Determination of DPPH Radical Scavenging Activity of Moringa oleifera leaf
The DPPH radical scavenging activity was determined according to Brand-Williams et al. (1995). A 0.2 g sample was vortexed with 5 ml of p.a. methanol for extraction for 30 seconds or until homogeneous. Then, 0.2 ml of the extract was mixed with 0.8 ml of 0.1 mM DPPH reagent and incubated in the dark at room temperature for 1 hour. Absorbance was measured at 515 nm using a spectrophotometer. A blank was prepared with 0.2 ml of methanol and 2.8 ml of 0.1 mM DPPH reagent.
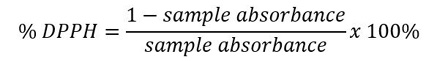
Determination of Water Activity (Aw) and Compacted Bulk Density (CBD) of Rice Bran
Aw was determined using an Aw meter. CBD was measured according to The United States Pharmacopeial Convention (2014). The measurement was conducted using a 100 ml plastic measuring cylinder. First, the empty cylinder was weighted. After recording this weight, the scale was zeroed, and rice bran was added until the volume reached 100 ml, allowing for the measurement of the rice bran’s weight at this volume. The rice bran was then compacted by tapping or gently shaking the cylinder for 10 minutes. Following compaction, the final volume was recorded, and the compacted bulk density was calculated using the following formula:
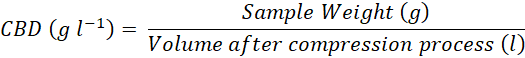
Determination of Moisture Content (MC), Crude Protein (CP), and Crude Fat (CF) Content of Rice Bran
The determination MC, CP, and CF was conducted using proximate analysis according to the guidelines outlined by AOAC (1980), as this method is common, cost-effective, and easy to use, requiring no advanced technology in the process. However, it can provide a broad overview of the nutrient content of a material. MC was determined using the gravimetric method. CP was analyzed using the Kjeldahl method, while CF was measured using the Soxhlet method with hexane as the solvent.
Rice Bran Oil Extraction
The rice bran oil extraction was performed to evaluate the free fatty acid levels using the Soxhlet method, in accordance with AOAC (1980) standards. Approximately 215 ± 5 grams of rice bran were placed in a Soxhlet apparatus, with hexane used as the solvent. The extraction process continued for 5 days, or until all the rice bran oil had been extracted.
Determination of Free Fatty Acid (FFA) Content of Rice Bran
FFA is commonly used as an indicator of material stability during storage. The FFA content was measured according to AOCS (1997) guidelines. Five grams of rice bran oil were dissolved in 50 ml of warm neutralized alcohol with 2 ml of phenolphthalein indicator. Titration with 0.1 N sodium hydroxide (NaOH) was conducted until a pale pink color appeared. The FFA content was expressed as oleic acid, using the formula:
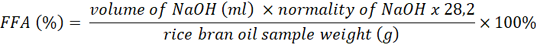
Statistical Analysis
This study employed a Randomized Complete Block Design with 10 treatments and four groups based on rice bran collection timing, resulting in a total of 40 rice bran samples. Data were analyzed using analysis of variance at a 95% confidence level (p < 0.05), followed by Duncan Multiple Range Test (DMRT) to identify significant differences. Data analysis was conducted using IBM SPSS Statistics version 25.
RESULTS AND DISCUSSIONS
Total Phenolic Content (TPC) and Antioxidant Activity of Moringa oleifera Leaf
The TPC of MOLE used in this study was 2970 mg Gallic Acid Equivalent (GAE) per 100g, with a DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of 93.11%. The TPC is similar to that reported by Selim et al. (2021), who found a TPC of 2089 mg GAE per 100 g. TPC represents the total amount of secondary metabolites in plants that are phenolic or possess hydroxyl groups. Phenolic compounds possess hydroxyl (-OH) groups capable of donating hydrogen atoms to free radicals, thereby stabilizing them. A positive relationship between phenolic compound and antioxidant activity has been demonstrated by Saji et al. (2020). The DPPH radical scavenging activity of the MOLE in this study was higher than the 85.2%-89.67% reported by Torres-Castillo et al. (2013). Variations in TPC and radical scavenging activity of MOLE across studies can be attributed to several factors, including the age of the Moringa leaves used and the extraction solvent (Nobossé et al., 2018), plant variety and environmental conditions (Sanli and Karadogan, 2017), and extraction methods (Susanty et al., 2019).
Moisture Content (MC) and Water Activity (Aw) of Rice Bran
The MC of rice bran across all treatments varied, ranging from 8.57% to 10.2% (Figure 1). Heated-0 and Heated-30 had the lowest MC (P < 0.05), specifically 8.57% and 9.38%, respectively. This indicates that the heating method is the most effective stabilization technique for reducing the MC of rice bran. A lower MC leads to a reduction in Aw or free water content, making it more difficult for spoilage microbes to proliferate and decreasing the likelihood of chemical reactions that affect nutrients (Siswanti et al., 2019). In this study, the MC of rice bran remained below the safe threshold of 13% before and after 30 days of storage, as established by the Indonesian National Standard (SNI, 2013).
There is a very strong correlation (P < 0.01) between MC and Aw (Table 1), where a decrease in MC is accompanied by a decrease in Aw, and vice versa. This finding is also supported by the study of Sharma et al. (2014). Similar fluctuations in MC and Aw of rice bran were observed between the initial measurement prior to storage and after 30 days of storage (Figures 1 and 2). These fluctuations are attributed to the environmental conditions resulting from aerobic storage. Troller and Christian (1978) described the relationship between Aw and humidity, noting that relative humidity is equivalent to Aw multiplied by 100 under equilibrium conditions. The average Aw of rice bran across all stabilization methods became more stable after 30 days of storage, compared to the greater variability observed before storage (Figure 2). This stabilization is attributed to the rice bran’s moisture adjusting to the room humidity of 58.6%, reaching equilibrium. This finding is consistent with the research of Moreno et al. (2021), who demonstrated the relationship between Aw and moisture absorption and desorption in achieving equilibrium.
Table 1: Pearson correlation coefficient relationship between rice bran quality variables.
|
Moisture content |
Water activity |
Compacted bulk density |
Crude protein |
Crude fat |
Free fatty acid |
|
|
Moisture content |
1 |
0.85** |
0.16 |
-1 |
-0.53** |
-0.14 |
|
Water activity |
1 |
0.3 |
0.15 |
-0.34* |
0.04 |
|
|
Compacted bulk density |
1 |
0.78** |
-0.24 |
0.88** |
||
|
Crude protein |
1 |
-0.2 |
0.77** |
|||
|
Crude fat |
1 |
-0.09 |
||||
|
Free fatty acid |
1 |
** correlation is significant at the 0.01 level; * correlation is significant at the 0.05 level.
The Aw of rice bran across all treatment varied, ranging from 0.529 to 0.618 (Figure 2), which was higher than the value of 0.5 reported by Sharma et al. (2014). Heated-0 exhibited the lowest Aw in this study, followed by MOLE-30 and BHT-30 (P < 0.05), specifically 0.529, 0.587, and 0.588, respectively. This indicates that the addition of MOLE and BHT successfully reduced its Aw of rice bran to the lowest levels after 30 days of storage (MOLE-30 and BHT-30). The result suggest that MOLE is as effective as BHT in maintaining and reducing the Aw of rice bran after 30 days of storage. MOLE contains phenolic compounds with antibacterial properties, which may inhibit microbial growth in rice bran by lowering the Aw, or free water content, necessary for microbial proliferation, thereby preserving rice bran quality. The antibacterial activity of phenolic compounds is attributed to their lipophilic properties, which increase bacterial cell membrane permeability, disrupt cellular homeostasis, and result in cell death through ion loss and cell denaturation (Waqas et al., 2023).
Compacted Bulk Density (CBD) dan Crude Protein (CP) Content of Rice Bran
The range of CBD values in this study, 439 to 504 g l-1 (Figure 3), is consistent with that reported by Ridla et al.(2023), which ranged from 407.35 to 507.82 g l-1. Figure 3 also shows that Heated-0 had the lowest CBD, while MOLE-30 and BHT-30 exhibited the highest CBD (P < 0.05), specifically 438.5 g/l, 503.6 g/l and 503.4 g/l, respectively. Higher CBD values indicate better nutrient quality, as high-quality nutrients have higher molecular weights. The addition of MOLE was found to be as effective as BHT in maintaining and improving nutrient quality, as reflected by the high CBD vales after 30 days of storage. This effect is likely due to the polyphenols in MOLE, which have a high affinity for binding with proteins, thereby increasing the molecular weight (Ozdal et al., 2013). In contrast, the low CBD value in Heated-0 can be attributed to the structural degradation of the rice bran caused by heating, which increases its porosity (Irakli et al., 2021).
The CP content of the rice bran used in this study ranged from 9.49% to 10.34% (Figure 4), meeting the grade III classification according to the Indonesian National Standard, which requires a minimum CP content of 8% (SNI, 2013). Control-0 and Heated-0 had the lowest CP content, while BHT-30 exhibited the highest CP content (P < 0.05), specifically 9.49%, 9.65%, and 10.34%, respectively. The low CP content in Control-0 and Heated-0 indicates that all stabilization methods used in this study were able to maintain the CP content at levels not lower than the control treatment before storage. After 30 days of storage, an increase in the CP content was observed across all stabilization methods. This increase is attributed to the MC and crude fat content in the rice bran. The BHT-30 resulted in the highest CP content after 30 days of storage, possibly due to higher polyphenol levels in BHT compared to MOLE, which lead to a greater increase in CP. Nevertheless, the addition of MOLE and MOLP can help preserve the CP content in rice bran during 30 days of storage due to the presence of phenolic compounds that bind to free radicals, thereby protecting proteins, lipids, sugars, and DNA from oxidative damage (Giannenas et al., 2020; Balasundram et al., 2006).
Additionally, heating treatment at 110°C for 20 minutes did not significantly reduce the CP content nor differ from that of the control. Oven heat stabilization can further improve protein structure, due to interaction between polyphenols and proteins (Ertürk and Meral, 2019). Further support comes from Sari et al.(2023), who found that heating rice bran at temperatures between 105°C and 140°C for 20-30 minutes is optimal for increasing the phenolic content, thereby enhancing polyphenol-protein interactions.
There is a strong correlation between CBD and crude protein content (P < 0.01) (Table 1). This correlation suggests that CBD is a reliable physical testing method for predicting the nutrient quality of a material. The correlation is further supported by the simultaneous increases in CBD and CP values in rice bran after 30 days of storage across all stabilization treatments (Figures 3 and 4).
Crude Fat (CF) and free fatty acid (FFA) content of Rice Bran
The CF content of rice bran before and after 30 days of storage ranged from 11.09% to 12.18% (Figure 5). MOLP-30 exhibited the lowest CF content, while Heated-0 and BHT-30 had the highest CF content (P < 0.05), specifically 11.09%, 12.18% and 11.89%, respectively. The high CF content in Heated-0 and Heated-30 is influenced by the low MC resulting from the heating treatment. The relationship between moisture and CF content is further evidenced by a moderate negative correlation (Table 1); as MC increases, CF content decreases, and vice versa. The addition of MOLP to rice bran was the most effective in producing the lowest CF content after 30 days of storage (MOLP-30). MOLP itself has a low CF content of 6.96% (Selim et al., 2021), which contributing to the reduction of CF content in rice bran. While a high lipid content in rice bran is desirable as an energy source in feed, it also increases the risk of deterioration. Rice bran is particularly sensitive to rancidity during storage due to its high levels of unsaturated lipids and the presence of hydrolytic and oxidative enzymes, such as lipase and lipoxygenase (Thanonkaew et al., 2012). Therefore, reducing lipid content is preferred to maintain the quality and stability of rice bran over a longer period.
FFA content is commonly used as an indicator of fat hydrolysis by lipase, which occurs due to the reaction between fat and water. An increase in FFA leads to the saturation of fat structures and can also cause rancidity and unpleasant odors (Irakli et al., 2018). Therefore, FFA content can serves as a key indicator of fat stability. As shown in Figure 6, the FFA content of rice bran before storage ranged from 8.28% to 9.48%, but after 30 days of storage, the FFA content increased significantly, ranging from 34.04% to 35.92%. Heated-0 had the lowest FFA content (8.28%), and after 30 days of storage, Heated-30 had lower FFA content than the other treatments (34.04%) (P < 0.05). The heating treatment resulted in a statistically lower FFA content in Heated-0, and although the increase in FFA levels was similar to other stabilization methods, Heated-30 still maintained significantly lower FFA levels after 30 days. This study demonstrates that heating is the most effective stabilization method for reducing FFA content in rice bran. The lower MC in the heated rice bran contributed to lower FFA levels. Heating is crucial for deactivating enzyme activity and preventing further degradation of rice bran (Yilmaz et al., 2014). Additionally, this study found that natural antioxidants (MOLP and MOLE) and synthetic antioxidant (BHT) were not as effective as heating in reducing FFA content in rice bran. The findings of this study contradict the hypothesis that natural antioxidants would reduce the FFA content in rice bran. Previous studies indicate that phenolic compounds in natural antioxidants exhibit anticholesterol effects, which may inhibit the activity of enzymes associated with lipid metabolism (Waqas et al., 2023). This inhibition could contribute to the prevention of lipid degradation. The interaction between phenolic compounds and lipids is likely due to the lipophilic nature of phenolic compounds, enabling them to dissolve in fats (Waqas et al., 2023). The differing outcomes observed in this study may be due to insufficient penetration of antioxidants into the more fibrous rice bran, preventing complete interaction with the antioxidants (Mujahid et al., 2005).
The average FFA content in rice bran after 30 days of storage increased significantly from the levels recorded at day 0. The FFA range before storage in this study was higher than that reported by Arora et al.(2015), who found FFA content in crude rice bran oil to be 6%-8%. The increase in FFA content over storage time aligns with the findings of Mujahid et al.(2005) and Yilmaz (2016), which showed that the highest increase in FFA occurs within the first 30 days of storage. Under appropriate conditions, FFA content can increase by 1%-7% per day (Arora et al., 2015). After 30 days, the FFA range in this study is consistent with the findings of Mujahid et al.(2005), which reported an average of 30 ± 10.6%. Yilmaz (2016) reported even higher FFA levels after 30 days, at 50%-65%. Variations in FFA content and its increase across different studies are attributed to several factors, including storage environmental conditions and storage media used. Pongrat and Songsermpong (2019) also noted that not only temperature but also the MC of the sample plays a crucial role in inactivating lipoxygenase.
CONCLUSIONS AND RECOMMENDATIONS
Given its high total phenolic content and antioxidant activity, Moringa oleifera leaves are a viable alternative to synthetic antioxidants for stabilizing rice bran quality after 30 days of storage. The 600 ppm extract demonstrated superior efficacy in preserving rice bran quality compared to its powdered form. However, this study does not examine the effects on rice bran oxidation levels or account for potential variations in effectiveness across different rice bran varieties. Future research could address these factors. Additionally, further investigation with extended and more detailed storage periods is recommended to monitor quality changes over time. Field-scale testing is also essential to assess the practical effectiveness of these findings.
ACKNOWLEDGEMENTS
This research was funded by the Education Fund Management Institution, Ministry of Finance, Republic of Indonesia (LPDP Kemenkeu RI), and awarded to the primary author (Number : KEP-2389/LPDP/LPDP.3/2022).
NOVELTY STATEMENT
The novelty of this article lies in the field of animal feed processing technology, specifically techniques for preserving the quality of rice bran, which is prone to rancidity during storage, by utilizing a natural material - Moringa leaves. The article also reinforces existing studies, demonstrating that natural antioxidants are equally effective and can serve as viable alternatives to synthetic antioxidants. Furthermore, it provides valuable insights into the significant benefits of Moringa leaves and other techniques for maintaining the quality of rice bran.
AUTHOR’S CONTRIBUTIONS
MTPS: Develop concept and methodology, conduct research and data analysis, write and compile articles, and revise articles.
MR: Serve as the article correspondent, develop concept and methodology, and perform supervision and validation of research results.
HAS: Develop concept and methodology, and perform supervision and validation of research results.
Conflict of Interest
The authors declare that there is no conflict of interest.
REFERENCES
Aditama RS, Sukria HA, Mutia R (2021). Evaluation of nutrient and antioxidant activity on steam bleaching of Moringa oleifera leaves. In E3S Web Conf., 306: 1-6. https://doi.org/10.1051/e3sconf/202130604016
AOAC International (1980). Association of official analytical chemists of the official methods of analysis. Association of Official Analytical Chemists, Washington DC.
AOCS (1997). Sampling and analysis of commercial fats and oils: official method. Association of Oil Chemists Society, Champaign IL.
Arora R, Toor AP, Wanchoo RK (2015). Esterification of high free fatty acid rice bran oil: Parametric and kinetic study. Chem. Biochem. Eng. Q., 29(4): 617-623. https://doi.org/10.15255/CABEQ.2014.2117
ASAE (1983). Method of determining and expressing fineness of feed materials by sieving. American Society of Agricultural Engineers Standards S 3192. Yearbook of standards, American Society of Agricultural Engineers Standards, St. Joseph, MO.
Balasundram N, Sundram K, Samman S (2006). Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurance, and potential uses. Food Chem., 99: 191-203. https://doi.org/10.1016/j.foodchem.2005.07.042
BPS (2023). Harvest area and rice production in Indonesia 2022. Badan Pusat Statistik, Jakarta
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol., 28: 25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
Carocho M, Ferreira IC (2013). A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspective. Food Chem. Toxicol., 51: 15-25. https://doi.org/10.1016/j.fct.2012.09.021
Ditjen Farmalkes (2011). Indonesian herbal pharmacopeia. Supplement II. First Edition. Kementerian Kesehatan, Jakarta.
Ertürk B, Meral R (2019). The impact of stabilization on functional, molecular and thermal properties of rice bran. J. Cereal Sci., 88: 71-78. https://doi.org/10.1016/j.jcs.2019.05.011
Giannenas I, Sidiropoulou E, Bonos E, Christaki E, Florou-Paneri P (2020). The history of herbs, medicinal and aromatic plants, and their extracts: Past, Current Situation and Future Perspectives. In: Florou-Paneri, P, Christaki, E, Giannenas, I (Editors). Feed Additives. Academic Press, Cambridge, MA (USA). https://doi.org/10.1016/B978-0-12-814700-9.00001-7
Irakli M, Kleisiaris F, Mygdalia A, Katsantonis D (2018). Stabilization of rice bran and its effect on bioactive compounds content, antioxidant activity and storage stability during infrared radiation heating. J. Cereal Sci., 80: 135-142. https://doi.org/10.1016/j.jcs.2018.02.005
Irakli M, Lazaridou A, Biliaderis C (2021). Comparative evaluation of the nutritional, antinutritional, functional, and bioactivity attributes of rice bran stabilized by different heat treatments. Foods, 10. https://doi.org/10.3390/foods10010057
Jennings B, Akoh C (2019). Effectiveness of natural versus synthetic antioxidants in a rice bran oil-based structured lipid. Food Chem., 114(4): 1456-1461. https://doi.org/10.1016/j.foodchem.2008.11.031
Masih LP, Sing S, Elamathi S, Anandhi P, Abraham T (2019). Moringa: A multipurpose potential crop - A review. Proc. Indian National Sci. Acad., 589-601. https://doi.org/10.16943/ptinsa/2019/49653
Meral R (2021). Determination of thermal, molecular changes, and functional properties in stabilized rice bran. Int. J. Food Eng., 17(4): 247-256. https://doi.org/10.1515/ijfe-2020-0168
Moo-Huchin V, Canto-Pinto J, Cuevas-Glory L, Sauri-Duch E, Perez-Pacheco E, Betancur-Ancona D (2019). Effect of extraction solvent on the phenolic compounds content and antioxidant activity of Ramon nut (Brosimum alicastrum). Chemical Papers [Preprint], 73: 1647-1657. https://doi.org/10.1007/s11696-019-00716-x
Moreno J, Ratphitagsanti W, Suwannaporn P, Kamonpatana P (2021). Stabilization of rice bran using ohmic heating or ultra-superheated steam. Agriculture and Natural Resources, 55(5): 816-825. https://doi.org/10.34044/j.anres.2021.55.5.12
Mujahid A, Haq I, Asif M, Gilani A (2005). Effect of various processing techniques and different levels of antioxidant on stability of rice bran during storage. J. Sci. Food Agric., 85(5): 847-852. https://doi.org/10.1002/jsfa.2026
Nobossé P, Fombang E, Mbofung C (2018). Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr., 6(8): 2188-2198. https://doi.org/10.1002/fsn3.783
Ozdal T, Capanoglu E, Altay F (2013). A review on protein - phenolic interactions and associated changes. Food Res. Int., 51(2): 954-970. https://doi.org/10.1016/j.foodres.2013.02.009
Pongrat P, Songsermpong S (2019). Stabilization of rice bran using a continuous microwave oven. Agric. Nat. Res., 53(4): 373-377.
Ridla M, Adjie RHN, Ansor S, Jayanegara A, Martin RSH (2023). Correlation of physical characteristics and nutrient content of rice bran. J. Anim. Sci., 20(1): 1-8. https://doi.org/10.24014/jupet.v20i1.18374
Rivai ATO (2020). Compounds identification in Moringa oleifera extract. Indones. J. Fundam. Sci., 6(2): 63-70.
Sadiah I, Indiarto R, Cahyana Y (2022). Characteristic and phenolic compounds of Moringa leaf extract microcapsules (Moringa oleifera) with a combination of maltodextrin and whey protein isolate. J. Agric. Ind. Technol., 32(3): 273-282.
Saji N, Schwarz L, Santhakumar A, Blanchard C (2020). Stabilization treatment of rice bran alters phenolic content and antioxidant activity. Cereal Chem., 97(2): 281-292. https://doi.org/10.1002/cche.10243
Sanli A, Karadogan T (2017). Geographical impact on essential oil composition of endemic Kundmannia anatolica Hub.-Mor. (Apiaceae). Afr. J. Tradit. Complement. Altern. Med., 14(1): 131-137. https://doi.org/10.21010/ajtcam.v14i1.14
Sari MTP, Ridla M, Sukria HA (2023). The optimal condition of dry-heat stabilization using oven on phenolic content and antioxidant activity of rice bran: A meta-analysis. Bulletin of Animal Science, 47(4): 248-254. https://doi.org/10.21059/buletinpeternak.v47i4.84810
Selim S, Seleiman M, Hassan M, Saleh A, Mousa M (2021). Impact of dietary supplementation with moringa oleifera leaves on performance, meat characteristics, oxidative stability, and fatty acid profile in growing rabbits. Animals, 11(2): 1-16. https://doi.org/10.3390/ani11020248
Sharif MK, Butt MS, Anjum FM, Khan SH (2014). Rice Bran: A novel functional ingredient’, critical reviews in food science and nutrition. Crit. Rev. Food Sci. Nutr., 54(6): 807-816. https://doi.org/10.1080/10408398.2011.608586
Sharma S, Kaur S, Dar B, Singh B (2014). Storage stability and quality assessment of processed cereal brans. J. Food Sci. Technol., 51(3): 583-588. https://doi.org/10.1007/s13197-011-0537-3
Siswanti, Anandito RBK, Nurhartadi E, Iskandar BD (2019). Effect of various heat treatment on physical and chemical characteristics of red rice bran (Oryza nivara L.) Rojolele. IOP Conference Series: Mater. Sci. Eng., 633(1): 012046. https://doi.org/10.1088/1757-899X/633/1/012046
SNI (2013). Quality Standard of Rice Bran 3178:2013. Badan Standarisasi Nasional, Jakarta.
Sultana B, Anwar F, Asi M, Chatha S (2008). Antioxidant potential of extracts from different agro wastes: Stabilization of corn oil. Grasas y Aceites, 59(3): 205-217. https://doi.org/10.3989/gya.2008.v59.i3.510
Sultana S (2020). Research progress on nutrition, function and application of Moringa oleifera leaves. Metab. Open, 8; 100061.
Susanty, Yudistirani S, Islam M (2019). Extraction method for obtaining the highest flavonoid content from Moringa leaf extract (Moringa oleifera Lam). Convertion J., 8(2): 31-36.
Thanonkaew A, Wongyai S, McClements D, Decker E (2012). Effect of stabilization of rice bran by domestic heating on mechanical extraction yield, quality, and antioxidant properties of cold-pressed rice bran oil (Oryza saltiva L.). LWT-Food Sci. Technol., 48(2): 231-236. https://doi.org/10.1016/j.lwt.2012.03.018
The United States Pharmacopeial Convention (2014). Uniformity of Dosage Units. Stage 6 Harmonization.
Torres-Castillo J, Sinagawa-Garcia S, Martinez-Avila G, Lopez-Flores A, Sanchez-Gonzalez E, Aguirre-Arzola V, Torres-Acosta R, Olivarez-Saenz E, Osorio-Hernandez E, Gutierrez-Diez A (2013). Moringa oleifera: Phytochemical detection, antioxidants, enzymes and antifungal properties. Int. J. Exp. Bot., 82: 193-202.
Troller JA, Christian JHB (1978). Water activity and food. food science and technology: a series of monograph. Academic Press Inc, New York
Waqas M, Salman M, Sahrif M (2023). Application of polyphenolic compounds in animal nutrition and their promising effects. J. Anim. Feed Sci., 32(3): 233-256. https://doi.org/10.22358/jafs/159718/2023
Yilmaz N (2016). Middle infrared stabilization of individual rice bran milling fractions. Food Chem., 190: 179-185. https://doi.org/10.1016/j.foodchem.2015.05.094
Yilmaz N, Tuncel N, Kocabiyik H (2014). Infrared stabilization of rice bran and its effects on γ-oryzanol content, tocopherols and fatty acid composition. J. Sci. Food Agric., 94(8): 1568-1576. https://doi.org/10.1002/jsfa.6459
Yuliyana T, Rimbawan R, Damayanthi E, Palupi E (2023). Antihypertensive activity of Moringa oleifera leaves: A preliminary meta-analysis. Malays. J. Med. Health Sci., 19: 1-2.
Zhang YC, Luo M, Fang XY, Zhang FQ, Cao MH (2020). Energy value of rice , broken rice , and rice bran for broiler chickens by the regression method. Poult. Sci., 100(4): 100972. https://doi.org/10.1016/j.psj.2020.12.069
To share on other social networks, click on any share button. What are these?






