Comparison of Photochemical Tissue Bonding Using Rose Bengal Dye and Conventional Suturing for Closure of Incisional Cutaneous Wounds in Canine Model
Research Article
Comparison of Photochemical Tissue Bonding Using Rose Bengal Dye and Conventional Suturing for Closure of Incisional Cutaneous Wounds in Canine Model
Saad Ahmad1, Shahbaz ul Haq1, Shujaat Hussain2, Khurram Ashfaq1, Shahrood Ahmed Siddiqui3,4, Arsalan Khan5*, Abubakar Yameen1, Muhammad Wasim Usmani6, Rafiq Ullah7 and Muhammad Arslan Aslam1*
1Department of Clinical Medicine and Surgery, Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan; 2Faculty of Veterinary and Animal Sciences, PMAS Arid Agriculture University, Rawalpindi, Pakistan; 3Vaccine Production Unit, Tandojam, Sindh, Pakistan; 4Livestock and Fisheries Department, Government of Sindh, Pakistan; 5Veterinary Research and Disease Investigation Center, Dera Ismail Khan, Pakistan; 6Department of Veterinary Pathology, Faculty of Veterinary and Animal Sciences, Ziauddin University, Karachi, Pakistan; 7Department of Zoology, Abdul Wali Khan University, Mardan, Pakistan.
Abstract | The healing of cutaneous wounds is a fundamental aspect of surgical procedures, with traditional suturing being the most common method of closure. However, novel techniques such as Photochemical Tissue Bonding (PTB) have emerged, offering potential improvements in healing outcomes. This study aimed to compare the efficacy of PTB, utilizing Rose Bengal dye and monochromatic green light, with that of conventional suturing in a canine model. Twenty mongrel canines were divided into two groups: Group A underwent PTB, and Group B received conventional suturing for closure of ventral midline cutaneous incisions. Parameters assessed included healing score, healing time, tensile strength of the healed tissue, and histological characteristics. The PTB group demonstrated superior outcomes in several parameters. Tensile strength was significantly higher in the PTB group (79.06 ± 2.3) compared to the suturing group (72.22 ± 1.5) (p<0.05). Epidermis and dermis thicknesses were also significantly greater in the PTB group (135.2 ± 3.7 µm and 1532 ± 146 µm, respectively) than in the suturing group (p<0.05). Additionally, the PTB group showed a higher collagen content percent (85.8 ± 1.8%) and an improved healing score (2.9 ± 0.07) compared to the suturing group. Most notably, the healing time was significantly shorter in the PTB group (3.2 ± 0.86 days) versus the suturing group (14.3 ±1.91 days) (p<0.05). The findings suggest that PTB is a superior method for cutaneous wound closure in canines compared to conventional suturing, evidenced by enhanced tissue integrity, faster healing times, and improved histological outcomes.
Received | February 04, 2024; Accepted | July 31, 2024; Published | January 03, 2025
*Correspondence | Arsalan Khan; Muhammad Arslan Aslam, Department of Clinical Medicine and Surgery, Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan; Email: drarsalankhandvm@gmail.com, drmarslan3@gmail.com
Citation | Ahmad, S., S.U. Haq, S. Hussain, K. Ashfaq, S.A. Siddiqui, A. Khan, A. Yameen, M.W. Usmani, R. Ullah and M.A. Aslam. 2025. Comparison of photochemical tissue bonding using rose Bengal dye and conventional suturing for closure of incisional cutaneous wounds in canine model. Sarhad Journal of Agriculture, 41(1): 22-31.
DOI | https://dx.doi.org/10.17582/journal.sja/2025/41.1.22.31
Keywords | Conventional suturing, Dogs, Photochemical tissue bonding, Rose Bengal dye, Wound healing
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
In routine pathology, incisions present a formidable obstacle to resolving clinical issues. Recurrent morbidity and mortality are observed as a result of complications in these lesions, whether they occur early or late (Alonso et al., 1996; Natarajan et al., 2000). To facilitate healing, the margins of a wound are traditionally sutured together. Suturing is presumed to have higher initial costs due to the necessity of qualified healthcare practitioners and medical materials (including sutures, needles, and instruments) (Rose and Tuma, 2023). Considerable endeavor has been dedicated to the reduction of wound burden and the comprehension of the physiology of healing. Furthermore, it highlights the continuous advancements in technology and novel therapeutic approaches aimed at effectively managing chronic and grievous wounds (Ongarora et al., 2022; Tatarusanu et al., 2023).
The various processes involved in acute tissue restoration, which are initiated by tissue injury, can be categorized into four distinct time-based phases: (i) homeostasis and coagulation come into effect immediately after a lesion; (ii) inflammation develops shortly thereafter; (iii) Proliferation, which involves the majority of healing processes and commences days after the lesion, and (iv) Wound remodeling, which may persist for a year or longer and includes the formation of scar tissue (Broughton et al., 2006). The appropriate approach to wound management should efficiently facilitate the healing process and can substantially impact the final clinical outcome (Kolimi et al., 2022).
Closure of surgical incisions through straightforward, expeditious, and non-inflammatory approach is the goal of cutaneous surgery; however, achieving the intended outcomes in wound healing has consistently proven to be a formidable challenge (Niederstätter et al., 2021). This could be the result of numerous adverse effects, including suture marks along the suture track caused by epidermal growth if the suture knots are held firmly or remain in place for an extended period of time. One minor challenge is the comparatively laborious nature of suture placement, especially when dealing with lengthy wounds. Additionally, sutures and staples lack aesthetic appeal and can induce complications including foreign-body reactions, scarring, and stenosis (Kamegaya et al., 2005).
Furthermore, non-surgical procedures (e.g., wound dressings) and advanced wound closure methods (e.g., tissue adhesives, staples, photochemical tissue bonding) may be less invasive and have reduced initial costs. These techniques function on the premise that non-surgical closure of wounds can promote various healing modalities, reduce the incidence of infections, and enhance aesthetic outcomes (Hu et al., 2023). Nonetheless, their efficacy may be influenced by the incision’s shape, size, location, and type. Suture-free techniques for the management of surgical incisions and excisions consist of adhesives, sealants, lesion healing strips, and staples. Similar to fibrin-based bindings, organic sealants establish long-lasting connections that are both biocompatible and hemostatic. However, these products are difficult to operate, costly, and may contribute to the transmission of disease (Currie et al., 2001). In both emergency rooms and residences, enhanced cyanoacrylate adhesives are now widely available (Singer et al., 2004). The application of these substances to close surgical wounds in high-pressure areas, the face, and other visible sites is limited as a result of the risk of compromised cosmetics (Bernard et al., 2001).
Photochemical tissue bonding, in contrast to the aforementioned methods, is an auspicious endeavor that holds potential for implementation across various surgical domains (Mulroy et al., 2000). It is a technique that employs photosensitizing dye-stained photochemical reactions that take place at the in close proximity to the tissue interface. The dye absorbs photons of visible light radiation, resulting in the formation of robust covalent bonds between molecules on the approximated surfaces (Chan et al., 2002). Reactive species generated upon light stimulation of the dye are capable of interacting with active electron donors and acceptors, such as cysteine, tyrosine, and tryptophan, which are amino acids found in proteins (Andrés et al., 2022).
Photochemical Tissue Bonding involves the application of a photosensitizing dye, like Rose Bengal, to a wound site followed by exposure to specific wavelengths of light, typically green light. Upon light absorption, the dye interacts with molecular oxygen in the tissue, generating reactive oxygen species (ROS) (Alqerban, 2021; Silva et al., 2023). These ROS initiate a photochemical reaction, leading to the formation of covalent bonds between collagen molecules and other structural proteins in the tissue, strengthening the tissue matrix and promoting wound closure. PTB enhances tissue adhesion, tensile strength, and healing processes while reducing inflammation and scarring compared to traditional methods (Khorsandi et al., 2022; La Monika et al., 2024).
Given the current absence of animal studies employing photochemical tissue bonding (PTB) for skin closure, the purpose of this investigation was to assess the efficacy and potential of PTB in wound repair while also contrasting its performance with that of conventional suturing.
Materials and Methods
Materials
A Sigma-Aldrich Rose Bengal dye was acquired from the marketplace. A monochromatic green light source was also procured.
Experimental animals
Twenty healthy adult mongrel canines, one of each sexes, were procured from the approved Government contractor and animals’ supplier of University of Agriculture, Faisalabad, and chosen for this objective. All animals were consistently and indoorly managed in cages of size 10 x 6 x 3 feet dimensions, throughout the duration of the research. Every individual animal was regarded as a distinct experimental model. The animals were classified into two groups, having ten animals in each group. One of the groups was treated with PTB and the other group served as control group in which the canines were treated using conventional suturing technique.
The canines involved in the study were provided with a balanced diet comprising commercial feed formulated to meet their nutritional requirements, with feeding occurring twice daily to ensure consistent nutrient intake and prevent hunger or excessive food consumption. Clean and fresh water was available at all times to ensure proper hydration, and the canines were housed in well-lit indoor facilities with access to natural light during the day, following a regular day-night cycle. Indoor temperatures were maintained at comfortable levels using appropriate temperature control measures. The animals were provided with comfortable bedding materials such as straw or padded mats to create a cozy resting area, and regular cleaning of housing facilities and feeding areas was conducted to maintain hygiene and prevent the spread of diseases. Waste materials were promptly removed, and surfaces were sanitized using alpha guard disinfectants to ensure a clean environment for the animals throughout the study period.
Appropriate laboratory and clinical examinations were conducted throughout the duration of the study to assess the health status of every animal. Throughout the research period, animals exhibiting any indication of illness or deviating from the fundamental parameters of health, namely temperature, pulse, and respiration rate, were systematically excluded from the study.
Preparation of the operative site
Surgical site and adjacent liberal area was thoroughly washed with antiseptic to conduct aseptic surgery.
Infliction of surgical wounds
Before undergoing surgery, the animals were kept off feed for approximately 12 hours without food. The animals were administered ketamine hydrochloride (Ketarol®, Global Pharmaceuticals, Pakistan) at a dosage of 20 mg/kg body weight and acepromazin at a dosage of 0.02 mg/kg body weight via intramuscular anaesthetic injection. They were then placed in a dorsal recumbent position. For delineating incision sites, permanent markers were employed. On each animal, a ventral midline cutaneous incision was performed on belly after proper shaving the area. The scalpel was used to make the incision of 3-8 cm. The healing process was assessed on a daily basis through measurements and observations. After surgical procedure, the animals were transferred to the cage.
After surgery, the animals received immediate postoperative care, including monitoring vital signs and administering pain management as needed. For the Photochemical Tissue Bonding (PTB) group, after exposure to monochromatic green light following Rose Bengal dye application, the surgical site has been inspected. Both PTB and suturing groups received wound care, ensuring cleanliness and monitoring for signs of inflammation or infection. Additionally, they were provided with postoperative analgesia and housed in a quiet, comfortable environment to promote recovery. Approximately 02 weeks were given to complete wound healing.
Treatment protocol
The following treatment protocol was implemented
Table 1: Treatment protocols of the groups under study.
|
Groups |
No of animals |
Treatment protocol |
No of wound per Animal |
|
Group A |
( n =10) |
PTB ( application of RB dye* + exposure of 532nm monochmatic green light) |
01 |
|
Group B |
( n = 10 ) |
Suturing with silk by |
01 |
|
Interrupted |
|||
|
horizontal mattress |
|||
|
suture pattern |
* Freshly prepared 0.1% solution of RB dye in phosphate buffer saline (PBS).
for both groups:
Group A: Wounds were exposed to monochromatic green light (532 nm) for a duration of 200 seconds subsequent to the application of dye (RB) on the incision plane (Yao et al., 2010).
Group B: Wound closure was achieved using an interrupted horizontal mattress suture pattern with 2/0 silk suture material (Table 1).
To evaluate wound healing efficacy of PTB as compared to sutures following parameters were recorded.
Healing score: was characterized as: excellent, good and fair.
- Excellent- minimum inflammation, no exudation, no dehiscence, gradual decreasing of width of cutting edges.
- Good- minimum inflammation with little exudation, no dehiscence, gradual decreasing of thickness of cutting edge.
- Fair- marked inflammation, presence of infection and exudation (Islam et al., 2014).
Healing time: Refers to time (days) duration from wound creation to the absolute healing and epithelization. It was approximated by addition of daily interpretations till scar is fallen off (Kumar et al., 2008).
Tensile strength
The tensile strength of a substance denotes the magnitude of tension it can endure prior to structural or geometric deformation occurs. The degree of integrity and repair is indicated by the tensile strength of the repaired tissue. During absolute repair, the tensile strength of the repaired tissue was evaluated utilizing Tensometer, a tensile calculation device available in the Department of Fiber and Textile Technology. The breaking force was calculated using the formulas that followed the increase in tensile strength.
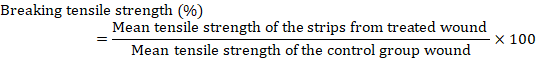
Tissue samples of abdominal skin on which incisional wounds were made and fixed in 10 % neutral buffered formalin, dehydrated through graded alcohols, cleared in chloroform and xylene and were impregnated with paraffin wax. Specimens were stained with hematoxylin and eosin and were observed using light microscope (Bernard et al., 2001)
Tissue samples for histological evaluation were obtained from the abdominal skin where the incisional wounds were made. After the wounds were created, the tissue samples were fixed in 10% neutral buffered formalin to preserve their structure. Subsequently, the samples were dehydrated through graded alcohols, cleared in chloroform and xylene to remove water and any remaining fixative, and finally impregnated with paraffin wax to provide support for thin sectioning. Once embedded in paraffin wax blocks, thin sections were cut using a microtome and mounted on glass slides. These slides were then stained with hematoxylin and eosin (H&E. The stained slides were observed under a light microscope, specifically using a Nikon Opt iPhoto 2 microscope at 10X magnification. Photomicrographs were captured for each sample slide to document the tissue characteristics. Parameters such as collagen percentage, keratinization, thickness of dermis and epidermis layers in micrometers, and connective tissue type were evaluated for each photomicrograph. Additionally, collagen content was quantified as a percentage using an automated image analysis system, specifically Image J(R). This involved comparing the treated wound samples to control group wound samples treated using the suturing method, allowing for an objective assessment of collagen content and distribution in the tissue samples.
Histometrical methods
At 10X using Nikon Opt iPhoto 2 microscope, all the sample slides were subjected to photomicrography. Collagen%, keratinization, thickness of dermis and epidermis layers in µm and connective tissue type were the characteristics which were considered for each photomicrograph.
Additionally, the collagen content was measured as a percentage in tissue samples using an automated image analysis system. Tissue samples were stained with H&E, observed under a light microscope, and analyzed for collagen fibers’ presence and distribution. The percentage of collagen content was then calculated by comparing treated wound samples to the control group wound samples treated using suturing method.
It was achieved by using Image J(R), a fully automated image analysis system.
Statistical analysis
Data thus generated were analyzed by ANOVA and Chi-square tests using SPSS software version 24.0 and p-value of 0.05 or less was deemed significant (Steel and Torrie, 2004).
Results
Photochemical Tissue Bonding offers a multifaceted approach to improving wound healing. Firstly, PTB stimulates cellular processes crucial for tissue repair. PTB also facilitates increased collagen deposition within the wound bed and by stimulating fibroblasts, the cells responsible for collagen synthesis, PTB enhances the production and organization of collagen fibers. This results in a stronger and more organized extracellular matrix, contributing to improved tensile strength and tissue integrity. PTB is also associated with a reduced inflammatory response compared to conventional suturing. Furthermore, PTB preserves the natural architecture of the tissue by avoiding thermal damage, which can occur with other tissue bonding methods.
Tensile strength
As far as the tensile strength of the healed tissues is concerned, a statistically significant difference was observed among the treatment groups where the wounds treated with photochemical tissue bonding attained higher tensile strength (79.06 ± 2.3) compared to suturing group (72.22 ± 1.5).
Epidermis thickness
Regarding thickness of epidermis, it was measured in micrometers. The overall epidermis thickness observed in animals subjected to photochemical tissue bonding was 135.2 ± 3.7. Whereas, the corresponding values for dogs which underwent suturing was 121.82 ± 2.6 (Figure 1 (A,), (B)).
Figure 1 (A), Histopathological evaluation of wound healed with PTB. The photomicrograph showed maximum leucocytic infiltration. More prominent and regular re-epithelization was seen. Prominent keratinization was seen, thickness of epidermis and dermis was more. H & E; 10X. (B) Histopathological evaluation of wound healed with silk. The silk treated photomicrograph showed less thickness of epithelium as compared to PTB. Thickness of epidermis and dermis was less. No inflammatory cells were seen. H & E; 10X.
Dermis thickness
The overall thickness of dermis observed in dogs of group A which were subjected to photochemical tissue bonding was 1532 ± 146 µm. Whereas, thickness of dermis for the animals in group B which underwent suturing was 1277 ± 66 µm. There was a statistically significant difference in terms of thickness of
Table 2: Comparison of different parameters under study.
|
Parameter |
Group |
Mean ± SE |
p-value |
|
Tensile strength (%) |
Photochemical tissue bonding |
79.06 ± 2.3 |
0.002* |
|
Suturing |
72.22 ± 1.5 |
||
|
Dermis thickness (µm) |
Photochemical tissue bonding |
153.2 ± 14 |
0.005* |
|
Suturing |
127.7 ± 6.6 |
||
|
Collagen content deposition (%) |
Photochemical tissue bonding |
85.8 ± 1.8 |
0.026* |
|
Suturing |
80.3 ± 1.1 |
||
|
Healing time (Days) |
Photochemical tissue bonding |
3.2 ± 0.86 |
0.001* |
|
Suturing |
14.3 ± 1.91 |
||
|
Healing score (1-3) |
Photochemical tissue bonding |
2.9 ± 0.07 |
0.007* |
|
Suturing |
1.7 ± 0 .21 |
dermis between the treatments where thickness of dermis was statistically more in PTB as compared to conventional suturing Figure 1 (A, B).
Collagen content percent
The overall collagen content percent for the animals subjected to photochemical tissue bonding was 85.8 ± 1.8 %. Whereas, collagen content percent for the animals of the group B which underwent suturing was 80.3 ±1.1 (Table 2). There was a statistically considerable difference between the two methods for wound closure where collagen percent of the animals of PTB group was higher than the group of conventional suturing Figure 2 (A, B).
Figure 2 (A), Histopathological evaluation of wound healed with PTB. The photomicrograph showed loose connective tissue containing thick collagen fiber deposition and more space was seen between the collagen fibers. H & E; 10X. (B): Histopathological evaluation of wound healed with silk. The photomicrograph showed dense connective tissues containing thin collagen fibers. There was less space between collagen fibers. H & E; 10X
Regarding healing score, it was graded on a scale ranging from 1 to 3. The overall healing score observed in dogs of group A, which were subjected to photochemical tissue bonding was 2.9 ± 0.07 whereas the corresponding values for dogs which underwent suturing was 1.7 ± 0.21. There was a statistically considerable distinction among the two methods for wound closure.
In Group A (PTB), out of 10 animals, 70% had excellent healing, 20% had good healing, and 10% had fair healing. In Group B (Suturing), out of 10 animals, 10% had excellent healing, 40% had good healing, and 50% had fair healing.
Healing time
Healing time was measured in days. The overall healing time for the animals subjected to photochemical tissue bonding was 3.2 ±0.86 days, whereas healing time for animals of group B which underwent suturing was 14.3 ±1.91 days. There was a statistically considerable distinction in terms of healing time between the treatments where healing time of PTB was statistically lower as compared to conventional suturing.
Discussion
In both animals and humans, wounds typically involve the separation of delicate tissue. Wound healing is an intricate process involving numerous interrelated mechanisms that precede a variety of cellular-level procedures that are chemically and hormonally linked (Chan et al., 2008).
A combination of chemical energy and light is utilised in a healing process known as photochemical tissue bonding. One inherent benefit of employing photochemical tissue bonding (PTB) as opposed to thermal mechanisms is that PTB is not temperature-dependent. As a result, the thermal mechanisms of tissue repair fail to preserve the denaturation and structural association of the tissues in their natural state. The binding of photosensors to separated or wounded tissue components aids in tissue repair, which ultimately heals the wound. Electromagnetic energy is employed to achieve the objective of tissue sealing (Kochevar et al., 2008).
The dogs in group A, which underwent photochemical tissue bonding, achieved an overall healing score of 2.9 ± 0.07. In contrast, the dogs in group B, which underwent suturing, achieved the same value of 1.7 ± 0.21. A statistically significant distinction existed between the two wound closure methods. The results of the experiment are consistent with those reported by Kamegaya et al. (2005), who observed a higher histological healing score in PTB as opposed to suturing.
The healing period for animals in group B that underwent suturing was 14.3 ±1.91 days, whereas the healing period for animals subjected to photochemical tissue bonding was 3.2 ±0.86 days. A notable disparity in healing time was observed between the therapies, with PTB exhibiting a significantly shorter healing time than conventional suturing. The experimental results are consistent with those reported by Capon et al. (2001), which demonstrated that laser treatment male hairless rats led to a four-fold reduction in the time required for healing when compared to traditional suturing. This can be attributed to the diminished inflammatory response demonstrated by laser healing as opposed to conventional suturing, which induces a conspicuous inflammatory reaction.
The photochemical tissue bonding-induced animals exhibited a collagen content percentage of 85.8 ± 1.8%. In contrast, the percentage of collagen present in group B animals that were sutured was 80.3 ±1.1. A statistically significant disparity was observed in the percentage of collagen present in the animals of the PTB group compared to the group that underwent conventional suturing for wound closure. The results of the experiment are consistent with those reported by Pugliese et al. (2003), who found that laser-healed collagen fibres (in rats) had a greater thickness and greater collagen content after 2, 3, 5, and 7 days compared to conventional suturing.
The epidermis thickness of animals in group A, which underwent photochemical tissue bonding, was measured to be 135.2 ± 3.7. In contrast, the corresponding values for canines that were sutured were 121.82 ± 2.6. A total dermal thickness of 153.2 ± 14 µm was observed in canines belonging to group A that underwent photochemical tissue bonding.
In contrast, the dermal thickness of the suturing-treated animals in group B was measured to be 127.7 ± 6.6 µm. Simhon et al. (2004) observed a statistically significant disparity between the two treatments regarding the thickness of the epidermis and dermis in animals. Specifically, the PTB group exhibited a significantly greater thickness of the epidermis in comparison to the conventional suturing group. This difference can be attributed to the infiltration of granulation tissues, which produce a coagulum that functions as a protective coating subsequent to tissue healing. With regard to the tensile strength of the repaired tissues, a notable disparity was identified between the treatment groups: lesions subjected to photochemical tissue bonding achieved a significantly higher tensile strength (79.06 ± 2.3) than those treated with suturing (72.22 ± 1.5). The experimental results are consistent with those reported by Capon et al. (2001), who observed that tissues repaired using laser technology exhibited greater tensile strength than those repaired using suturing. The increased thickness of collagen fibers and collagen content in the healed tissue, which may be the consequence of a reduced inflammatory response, could account for this.
Photochemical Tissue Bonding (PTB) enhances the infiltration of granulation tissue by stimulating cellular processes crucial for tissue repair, promoting increased collagen deposition, inducing angiogenesis, and reducing inflammation. These effects create a favorable microenvironment for the formation of granulation tissue, essential for wound healing (Su et al., 2019).
Throughout history, Rose Bengal has been utilized for its safe properties as both a topical ophthalmic and systemic hepatic diagnostic agent (Delpart et al., 1924). The combined application of 532 nm laser radiation and Rose Bengal had not previously resulted in cutaneous toxicity or phototoxicity in rabbit skin, with the exception of instances where high irradiances induced thermal injury (Wachter et al., 2003). Our research findings corroborate the aforementioned observation regarding toxicity, as the application of PTB did not induce erythema and did not demonstrate any indication of delayed harm when the sites were cooled throughout the irradiation process. Under certain clinical circumstances, PTB has demonstrated superiority to alternative sutures. PTB distinguishes itself from alternative sutures by consistently adhering to tissue surfaces at the molecular level, whereas other sutures bind to the epidermis at various points. Furthermore, PTB prevents foreign body reactions and tissue injuries during the healing process of skin tissues, whereas sutures increase the likelihood of such events and tissue injuries due to the constant passage of the needle and tying of the knot. Scarring of the skin is an adverse consequence of suture use, as it disrupts the passage of blood through capillaries and increases the risk of infection.
In our study, PTB demonstrated superior collagen deposition compared to suturing, likely due to its stimulation of fibroblast activity and migration, induction of angiogenesis, reduction of inflammation, and preservation of tissue architecture. These factors collectively promote collagen synthesis and deposition at the wound site, leading to enhanced wound healing outcomes with PTB.
Conclusions and Recommendations
Based on the findings, it can be concluded that PTB is a more effective alternative to suturing for wound healing. PTB results in a shorter healing time, an excellent healing score, and increased collagen deposition in the underlying tissues, increased thickness of the epidermis and dermis, and greater breaking vigor in the healed tissue. No wound complications such as dehiscence, inflammation, or infection were observed in animals treated with PTB, and no adverse effects were observed on their health. Therefore, PTB is considered to be a more effective technique in comparison to conventional suturing. Prior to clinical application, additional trials should be devised to account for tissue toxicity of the dyes, temperature fluctuations in the tissues, and damage to adjacent tissues.
Acknowledgements
The authors are highly grateful to the Directorate of Graduate Studies and Office of Research, University of Agriculture, Faisalabad, for approval of the study.
Novelty Statement
This manuscript presents a novel comparison between Photochemical Tissue Bonding (PTB) using Rose Bengal dye and conventional suturing for closing incisional cutaneous wounds in a canine model. The study demonstrates that PTB offers significant advantages over traditional suturing, including enhanced tensile strength, greater epidermal and dermal thickness, higher collagen content, and improved healing scores. Notably, PTB significantly reduces healing time compared to conventional suturing. These findings suggest that PTB provides superior wound closure outcomes, making it a promising alternative to traditional suturing methods in veterinary surgical procedures.
Author’s Contribution
Saad Ahmad: Conceptualized the research framework and led the study design.
Shahbaz ul Haq: Developed the experimental methodology and data collection techniques.
Shujaat Hussain: Offered critical insights and refined the research questions.
Khurram Ashfaq: Supervised and conceptualized the study.
Shahrood Ahmed Siddiqui: Compiled and analyzed the data.
Arsalan Khan: Responsible for the experimental design, writing the original draft, and subsequent revisions.
Abubakar Yameen: Provided technical support and expertise in data analysis tools.
Muhammad Wasim Usmani: Conducted the investigation.
Rafiq Ullah: Assisted with methodology and data collection.
Muhammad Arslan Aslam: Contributed to the research design and methodology.
Ethical pproval
This study was approved by Directorate of Graduate Studies and Office of Research, University of Agriculture, Faisalabad. It met all set criteria of animal ethics.
Conflict of Interest
The authors declared no conflicts of interest
References
Alonso, J.E., J. Lee and A.R. Burgess and B.D. Browner. 1996. The management of complex orthopedic injuries. Thorac. Surg. Clin., 76(4): 879-903. https://doi.org/10.1016/S0039-6109(05)70486-2
Alqerban, A. 2021. Effectiveness of riboflavin and rose Bengal photosensitizer modified adhesive resin for orthodontic bonding. Pharmaceuticals. 14(1):48. https://doi.org/10.3390/ph14010048
Andrés, C.M.C., J.M.P.D.L. Lastra, A.C. Juan, F.J. Plou and E. Pérez-Lebeña. 2022. Impact of reactive species on amino acids—biological relevance in proteins and induced pathologies. Int. J. Mol. Sci., 23(22): 14049. https://doi.org/10.3390/ijms232214049
Attinger, C.E., J.E. Janis and J. Steinberg, J. Schwartz, A. Al-Attar and C. Kara. 2006. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg., 117(7S):72S-109S. https://doi.org/10.1097/01.prs.0000225470.42514.8f
Bancroft, J.D. and M. Gamble. 2008. Theory and practice of histological techniques. Elsevier Heal Sci.,
Bernard, L., J. Doyle and S.F. Friedlander, N.F. Gibbs and B.B. Cunningham. 2001. A prospective comparison of octyl cyanoacrylate tissue adhesive (dermabond) and suture for the closure of excisional wounds in children and adolescents. Arch. Dermatol., 137(9): 1177-80. https://doi.org/10.1001/archderm.137.9.1177
George, Broughton I.I., J.E. Janis and C.E. Attinger. 2006. The basic science of wound healing. Plast Reconstr. Surg., 117(7S):12S-34S. https://doi.org/10.1097/01.prs.0000225430.42531.c2
Capon, A., E. Souil and B. Gauthier, C. Sumian, M. Bachelet, B. Buys, B.S. Polla and S. Mordon. 2001. Laser assisted skin closure (LASC) by using a 815-nm diode-laser system accelerates and improves wound healing. Lasers in Surgery and Medicine: Lasers Surg. Med., 28(2): 168-75. https://doi.org/10.1002/lsm.1035
Chan, E.W., Y.Y. Lim and L.F. Wong, F.S. Lianto, S.K. Wong and K.K. Lim. 2008. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem., 109(3): 477-83. https://doi.org/10.1016/j.foodchem.2008.02.016
Chan, B.P., I.E. Kochevar and R.W. Redmond. 2002. Enhancement of porcine skin graft adherence using a light-activated process. J. Surg. Res., 108(1): 77-84. https://doi.org/10.1006/jsre.2002.6516
Currie, L.J., J.R. Sharpe and R. Martin. 2001. The use of fibrin glue in skin grafts and tissue-engineered skin replacements. Plast. Reconstr. Surg., 108:1713-26. https://doi.org/10.1097/00006534-200111000-00045
Degreef, H.J. 1998. How to heal a wound fast. Dermatol. Clin., 16(2):365-75. https://doi.org/10.1016/S0733-8635(05)70019-X
Delprat, G.D., N.N. Epstein and W.J. Kerr. A new liver function test: The elimination of rose bengal when injected into the circulation of human subjects. Arch Intern Med 1924; 34(4):533-41. https://doi.org/10.1001/archinte.1924.00120040119011
Hunt, T.K., H. Hopf and Z. Hussain 2000. Physiology of wound healing. Adv. Skin Wound Care, 13:6.
Hu, X and M.W. Grinstaff. 2023. Advances in hydrogel adhesives for gastrointestinal wound closure and repair. Gels., 9(4):282. https://doi.org/10.3390/gels9040282
Islam, M.A., N.S. Juyena and R.N. Ferdousy and M.A.A. Mamun. 2014. Effects of different suture patterns and materials on healing of incised skin wounds in cattle. J. Adv. Vet. Anim. Res., 31(1):27-37. https://doi.org/10.3329/bvet.v31i1.22840
Kamegaya, Y., W.A. Farinelli and A.V. Vila Echague, H. Akita, J. Gallagher, T.J. Flotte, R.R. Anderson, R.W. Redmond and I.E. Kochevar. 2005. Evaluation of photochemical tissue bonding for closure of skin incisions and excisions. Lasers Surg. Med., 37(4): 264-70. https://doi.org/10.1002/lsm.20221
Khorsandi, K., R. Hosseinzadeh, H. Esfahani, K. Zandsalimi, F.K. Shahidi and H. Abrahamse . 2022 Oct. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm Regen. 4;42(1):40. doi: 10.1186/s41232-022-00226-6. https://doi.org/10.1186/s41232-022-00226-6
Kochevar, I.E., C.R. Taylor and J. Krutmann . 2008. Fundamentals of cutaneous photobiology and photoimmunology. Fitzpatrick’s dermatology in general medicine. 1:1031-48.
Kolimi, P., S. Narala, D. Nyavanandi, A.A.A. Youssef and N. Dudhipala . 2022. Innovative treatment strategies to accelerate wound healing: Trajectory and recent advancements. Cells., 11(15): 2439. https://doi.org/10.3390/cells11152439 https://doi.org/10.3390/cells11152439
Kumar, M.S., S. Kirubanandan and R. Sripriya and P.K. Sehgal. 2008. Triphala promotes healing of infected full-thickness dermal wound. J. Surg. Res., 144(1): 94-101. https://doi.org/10.1016/j.jss.2007.02.049
La Monica, F, S. Campora and G. Ghersi . 2024. Collagen-based scaffolds for chronic skin wound treatment. Gels., 10(2): 137. https://doi.org/10.3390/gels10020137 https://doi.org/10.3390/gels10020137
Mulroy, L., J. Kim and I. Wu, P., Scharper, S.A. Melki, D.T. Azar, R.W. Redmond and I.E. Kochevar. 2000. Photochemical keratodesmos for repair of lamellar corneal incisions. Invest Ophthalmol Vis Sci., 41(11):3335-40.
Natarajan, S., D. Williamson and A.J. Stiltz and K. Harding. 2000. Advances in wound care and healing technology. Am J Clin Dermatol., 1(5):269-75. https://doi.org/10.2165/00128071-200001050-00002
Niederstätter, I.M., J.L. Schiefer and P.C. Fuchs. 2021. Surgical strategies to promote cutaneous healing. Med Sci., (Basel). 16;9(2):45. https://doi.org/10.3390/medsci9020045
Ongarora, B.G. 2022. May. Recent technological advances in the management of chronic wounds: A literature review. Health Sci. Rep., 19;5(3): e641. https://doi.org/10.1002/hsr2.641
Pugliese, L.S., A.P. Medrado and S.R. Reis and Z.A. Andrade. 2003. The influence of low-level laser therapy on biomodulation of collagen and elastic fibers. Pesqui Odontol Bras; 17(4): 307-13. https://doi.org/10.1590/S1517-74912003000400003
Rose, J. and Tuma F. Sutures And Needles. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539891/
Silva, T., A.J.L. Lunardi, Barros A.C.S.M., Mandetta ARH, Grudzien E, San-Martín M, Horliana ACRT, Bussadori SK and Motta LJ. 2023. Application of Photodynamic Therapy in Pediatric Dentistry: Literature Review. Pharmaceutics. Sep 18;15(9):2335. doi: 10.3390/pharmaceutics15092335. https://doi.org/10.3390/pharmaceutics15092335
Simhon, D, T. Brosh, M. Halpern, A. Ravid, T. Vasilyev, N. Kariv, A. Katzir and Z. Nevo. 2004. Closure of skin incisions in rabbits by laser soldering: I: Wound healing pattern. Lasers Surg Med; 35(1):1-1. https://doi.org/10.1002/lsm.20074
Singer, A.J. and Thode Jr, H.C. 2004. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg; 187(2):238-48.
Steel, R.G. and Torrie, J.H. Principles and Procedures of Statistics McGraw-Hill Book Co. New York. 1960:16-8.
Su, L., Zheng, J., Wang Y., Zhang, W. and Hu, D. 2019. Emerging progress on the mechanism and technology in wound repair. Biomed Pharmacother. ; 117:109191. https://doi.org/10.1016/j.biopha.2019.109191
Tatarusanu, S.M., F.G., Lupascu, B.S., Profire, A., Szilagyi, I., Gardikiotis, A.T., Iacob, I., Caluian, L., Herciu, T.C., Giscă, M.C. and Baican. 2023. Modern approaches in wounds management. Polymers. 15(17):3648. https://doi.org/10.3390/polym15173648
Wachter, E., C. Dees, J. Harkins, T. Scott, M. Petersen, R.E. Rush and A. Cada. 2003. Topical rose Bengal: Pre-clinical evaluation of pharmacokinetics and safety. Lasers Surg. Med., 32(2):101-10. https://doi.org/10.1002/lsm.10138
Yao, M., Yaroslavsky, A. and F.P. Henry, R.W. Redmond and I.E. Kochevar. 2010.Phototoxicity is not associated with photochemical tissue bonding of skin. Lasers Surg Med; 42(2):123-31. https://doi.org/10.1002/lsm.20869
To share on other social networks, click on any share button. What are these?








