Toxicity and Behavioral Outcomes of the Ethanolic Extract of Schinus Terebinthifolius Raddi fruits in Wistar Rats
Research Article
Toxicity and Behavioral Outcomes of the Ethanolic Extract of Schinus Terebinthifolius Raddi fruits in Wistar Rats
Soumia Ed-Day1*, Meryam Laanaya1, Chaimae El Kourchi3, Radia Elgui1, Fatima Ezzahra Kacimi2, Latifa Didou1, Samira Boulbaroud2, Hicham Harhar3, Fatima-Zahra Azzaoui1
1Unit of Neuroscience, Neuroimmunology and Behavior, Laboratory of Biology and Health, Department of Biology, Faculty of Science, Ibn Tofail University, Kenitra, Morocco; 2Biotechnology and Sustainable Development of Natural Resources Unit, multidisciplinary faculty, Sultan Moulay Slimane University, Beni Mellal, Morocco; 3Laboratory of Nanotechnology Materials and Environment, Faculty of Science, Mohamed V University, Rabat, Morocco.
Abstract | Background: The Brazilian pepper tree, Schinus terebinthifolius Raddi, is an evergreen shrub in the Anacardiaceae family. The berries or fruits of Schinus terebinthifolius Raddi are highly regarded for their aromatic flavor and have drawn interest due to their possible therapeutic applications in illness prevention and treatment. This study aims to determine the acute oral toxicity of the ethanolic extract obtained from Schinus terebinthifolius Raddi fruits and assess the impact of the berries extract on behavioral patterns in Wistar rats. Methods: 48 adult female Wistar rats were divided into six groups (n=8) and administered orally (by gavage) the following doses: 2.5, 5, 25, 50, and 250 mg/kg of the ethanolic extract, while the control group received distilled water. Biochemical, histological, and behavioral assessments were conducted at the end of the experiment. Results: In the acute oral toxicity test, the extract did not produce any signs of toxicity or mortality underscoring the safety profile of the diverse doses administered in this study. Biochemical analyses of blood indicated changes in lipid profile including total cholesterol and triglyceride. Behavioral evaluations revealed noticeable enhancement in memory outcomes, particularly at doses of 2.5, 25, and 250 mg/kg. Histopathological examinations substantiated the absence of toxic effects on the liver and kidneys. Conclusion: The acute administration of the ethanolic extract of Schinus terebinthifolius Raddi fruits did not elicit any toxic effects in Wistar rats, thus contributing valuable insights into this plant’s safety and physiological impact.
Keywords | Acute toxicity, Schinus terebinthifolius Raddi, Glycemia, Lipid profile, Behavioral assessments, Wistar rats
Received | July 25, 2024; Accepted | August 29, 2024; Published | October 23, 2024
*Correspondence | Ed-Day Soumia, Unit of Neuroscience, Neuroimmunology and Behavior, Laboratory of Biology and Health, Department of Biology, Faculty of Science, Ibn Tofail University, Kenitra, Morocco; Email: soumia.edday@uit.ac.ma
Citation | Ed-Day S, Laanaya M, El Kourchi C, Elgui R, Kacimi FE, Didou L, Boulbaroud S, Harhar H, Azzaoui FZ (2024). Toxicity and behavioral outcomes of the ethanolic extract of Schinus Terebinthifolius Raddi fruits in wistar rats. Adv. Anim. Vet. Sci. 12(12): 2315-2325.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.12.2315.2325
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The use of medicinal plants has been a key part of traditional practices across various cultures for centuries, based on the idea that nature provides bioactive compounds capable of influencing physiological processes and offering therapeutic benefits (Fabricant and Farnsworth, 2001). These compounds, including terpenes, alkaloids, flavonoids, and phenolics, have been shown to possess anti-inflammatory, antioxidant, and antimicrobial properties (Misra et al., 2024; Balkrishna et al., 2024; Moreira et al., 2014). In Morocco, with its rich plant diversity, medicinal plants have long been used for health and healing (Kachmar et al., 2021). However, scientific validation of their safety and efficacy is crucial, as recent studies have focused on their therapeutic potential, toxicity, and mechanisms of action, since toxicity studies are necessary to identify potential adverse effects, such as liver and kidney damage, allergic reactions, which can be life-threatening (Balkrishna et al., 2024; Moreira et al., 2014).
Plants belonging to the Anacardiaceae family, such as Schinus terebinthifolius Raddi, are known to be a rich source of diverse and potentially useful compounds (Feriani et al., 2021). Schinus terebinthifolius Raddi, commonly known as Aroeira or the Brazilian pepper tree, is native to South and Central America and parts of Africa. In Morocco, it is often grown as an ornamental tree because of its nice leaves and tasty fruits, and it has a long history of versatile applications, including use in dyeing, tanning, decorative purposes, and as a spice (Glen, 2002).
Previous studies have demonstrated that Schinus terebinthifolius Raddi does not exhibit toxic effects in various experimental models (Todirascu-Ciornea et al., 2019; Patočka and de Almeida, 2017; Lima et al., 2009). Various parts of the Aroeira plant, including leaves, flowers, bark, fruits or berries, and seeds, have traditionally been used in folk medicine to treat diverse illnesses and troubles (Feriani et al., 2021). These include uterine inflammation, urinary tract and respiratory tract infections (Pinto et al., 2020), skin wounds and ulcers (Todirascu-Ciornea et al., 2019), mucous membrane injuries (Paula et al., 2019), In addition to sexually transmitted diseases, infections of genitourinary systems (Rocha et al., 2017), rheumatism, diarrhea, arthritis, gingivitis, tumors, gout, pain, fever and, ulcers and infections of the digestive systems (Dos Santos et al., 2015; Arq et al., 2013; Affonso et al., 2012; El-Massry, 2009; Lima et al., 2009; Cavalher-Machado, 2008).
Despite the extensive traditional use of Schinus terebinthifolius Raddi fruits, there remains a notable paucity of research exploring the potential behavioral effects and detailed toxicity profiles associated with this plant. Based on its known antioxidant and anti-inflammatory activities, this plant could have beneficial effects on behavior. Therefore, the present study aims to assess the acute oral toxicity of the ethanolic extract derived from Schinus terebinthifolius Raddi fruits cultivated in Morocco and to evaluate the impact of this extract on the behavioral profiles of Wistar rats.
MATERIALS AND METHODS
Schinus Terebinthifolius Raddi Extraction
The berries of Schinus terebinthifolius Raddi were collected from Kenitra city, which is situated in western Morocco at coordinates 34° 15’ 39.64” N, -6° 34’ 48.72” W. The species was identified, and a sample was preserved in the herbarium of the Biology and Health laboratory at the Faculty of Science, Ibn Tofail University, Kenitra, Morocco, under number: ST-007/2021. The chosen berries were subjected to a drying process in an oven at 30 °C overnight and then ground. The obtained powder was first extracted with n-hexane in a Soxhlet apparatus. 20 grams of the powder was extracted with 250 mL of n-hexane at 50 °C for 6 hours in a Soxhlet apparatus. After extraction, the suspension was filtered using filter paper, and the n-hexane was removed by evaporation using a rotary evaporator at 55 °C. The resulting oil was stored at 4 °C until further use. The same plant material was subjected to a second ethanol extraction (20 g of the same plant material with 250 mL of ethanol) following the same protocol (Hu et al., 2021; El Kourchi et al., 2024).
Animals and Housing
Adults female Wistar rats (weighing 214.56±0.36g), aged 3 months old, were obtained from the breeding center of the Faculty of Science, Ibn Tofail University (ITU). The rats were housed in specific cages under regulated circumstances, with a constant temperature of 22 ± 2ºC and a 12-hour light/12-hour dark cycle (with lights on at 6 am). They were provided with free access to food and water (standard diet). The experimental design used a balanced approach to treatment administration and behavioral observation, ensuring that order and timing effects were avoided. All animals were blinded to the examiner and behavioral tests were conducted in blinded and randomized conditions. All experimental procedures followed the guidelines outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The animal ethics committee at ITU authorized the protocols used in this study.
Experimental Design
The acute oral toxicity test was conducted following the OECD guideline 425 for testing of chemicals, using female Wistar rats as recommended by the guideline. A total of 48 adult animals were divided into 6 groups of 8 animals each. The control group received distilled water orally at a dose of 1ml/100g body weight. The remaining groups received a single oral administration of the ethanolic extract of Schinus terebinthifolius Raddi (EEST) berries at the following doses: 2.5, 5, 25, 50, and 250mg/kg.
Following the administration of the extract, each rat was carefully observed individually for the first 4 hours and then daily for 14 days. This observation aimed to identify the general toxicity signs such as death, agitation, decreased activity, somnolence, breathing, and salivation. On day 14 of the study, the animals were subjected to a series of
behavioral tests to evaluate their general locomotor activity, anxiety levels, and memory performance. Then on day 21, all animals were anesthetized and sacrificed, and blood and organ samples were collected for further biochemical and histological analysis. The detailed experimental design is shown in Figure 1.
Behavioral Testing
To minimize stress for the animals, behavioral tests were conducted in the morning on separate days, as detailed below. Before testing, rats underwent a 24-hour acclimation period in the testing environment to reduce stress and familiarize them with the setup. Efforts were made to further minimize stress by maintaining consistent handling procedures, uniform techniques for transporting and placing the animals, and ensuring that the testing environment was quiet and free from disturbances.
Locomotor Activity Testing in the Open Field (OF) Test
To assess general locomotor activity levels an open field test was used. The assessment took place in a white enclosure of dimensions: 100x100x40cm; whose floor is divided into 25 equal squares (16 peripherals and 9 central) the slide was illuminated by a 60W lamp and controlled by a computer-linked camera. Each rat was positioned in the center, and their behavior was recorded for 7 minutes. The locomotor activity is estimated by the number of total cells crossed (Azzaoui et al., 2009).
Anxiety Testing
To assess rats’ anxiety levels, two tests were used: the elevated plus maze (EPM) and the light-dark box (LDB) tests.
Elevated plus maze test (EPM): To evaluate anxiety-like behavior, we used EPM which is an ethological model of anxiety. The Elevated Plus Maze apparatus consists of a “+” shaped maze elevated from the ground (50 cm) with two open arms (50 x 10 cm) and two arms with walls or closed arms (50 x 10 cm), connected by a platform (10 x 10 cm). A 60 W lamp illuminated the central platform, giving the rat an aversive spatial condition. Each animal was positioned on the platform facing the open arm and its activity was recorded for 5 minutes. An entry is defined as all four paws of the rat entering an arm. In between testing, the apparatus was cleaned using ethanol. The duration spent in each arm and the frequency of entries in the closed and open arms were noted from a video clip. The level of anxiety is determined by the number of entries into and the time spent in the open arms (N’Go et al., 2013).
Light-dark box (LDB) test: The LDB test was used to assess anxiety. The test is based on the conflict of the natural tendencies of rats to avoid lighted and open areas.
The apparatus contains two chambers (25×25×25 cm), one is dark whereas the other is highly illuminated (Bourin and Hascoët, 2003). The rats were individually positioned in the dark box and their activity was recorded for 6 minutes using a camera. The relative number of dark-light transitions indicates the anxiety level of the rat.
Memory Testing
To evaluate learning and memory performance, the novel object recognition and Y-maze tests were used.
Novel object recognition (NOR) test: To assess the recognition memory, a NOR test was used. The apparatus consisted of a black cubic box measuring 50 x 50 x 50 cm that was lit from above by a lamp. The NOR test was conducted over three days. On the first day, each rat had 5 minutes to freely explore the empty device. On the second day, the animal was exposed to an apparatus holding two identical objects (A and A). After a 2-hour interval, one of the familiar objects was replaced with a novel object (A and B) to test short-term memory. Each rat was given to freely explore the objects for 5 minutes, and the time spent investigating each object was recorded. After 24 hours, the rats were again allowed to explore the apparatus, which now included two objects, one familiar (A) and one unfamiliar (C). This phase was designed to evaluate long-term memory. The time spent investigating the objects was recorded for 5 minutes (Ed-Day et al., 2021; N’Go et al., 2013; Azzaoui et al., 2009; Ennaceur, 1988).
Y-Maze test: To evaluate spatial working memory, a Y-maze test was conducted. The apparatus consists of three identical arms. Once placed in one of the three arms, the rat spontaneously tries to explore the other arms he is not familiar with. For 5 minutes, each rat was assessed individually, and the percent of spontaneous alternation was determined using the formula provided by Sierksma et al. (2014), as follows:
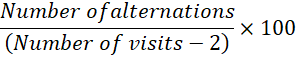
Sample Collection
Following the behavioral testing, on day 21 all animals were weighed and profoundly sedated with a 7% chloral solution (0.5 mL/100 g body weight). They were then decapitated, and their blood and organs were quickly collected.
Biochemical Blood Parameters and Plasma Collection
The blood samples were centrifuged for 20 minutes at 1000 ×g and then the plasma was utilized to measure parameters such as glycemia, lipid profile, as well as transaminases ASAT (Aspartate aminotransferase) and ALAT (Alanine aminotransferase).
Table 1: Measurement of body weight variation (g) among the control group and treated groups with the ethanolic extract of Schinus terebinthifolius fruits during the acute oral toxicity study.
|
Body weight (g) |
||
|
Day 1 |
Day 14 |
|
|
Control group |
214,92±0,13 |
242,10±23,82 |
|
2.5 mg/kg |
214,96±29,79 |
266,13±71,05 |
|
5 mg/kg |
214,56±26,56 |
242,20±34,61 |
|
25 mg/kg |
214,89±22,29 |
238,84±23,26 |
|
50 mg/kg |
214,65±22,061 |
225,32±15,738 |
|
250 mg/kg |
214,59±27,86 |
221,80±27,78 |
Data are displayed as Mean ± standard deviation. The significance level is 0.05.
Histological Analysis
The histological study consists of five key steps, including fixation, processing, embedding, sectioning, and staining. After the decapitation of rats, organs (liver and kidneys) were fixed in 10% formalin, then processed in the circulation automaton to dehydrated tissues. The dehydrated tissues were then embedded in paraffin, and then 3 μm thick slices were cut, the morphological structure was elaborated using Hematoxylin and Eosin staining, and observations were made under a microscope.
Data Analysis
The data were presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using statistical software. One-way ANOVA was used to determine differences between groups. Tukey’s post hoc test was used for multiple comparisons to find group differences. Statistical significance was determined at p < 0.05.
RESULTS AND DISCUSSION
The extraction yield in % of Schinus Terebinthifolius Raddi
In the present study, the hexanic extract yielded an average of 13.05% with a standard variation of 0.25%, indicating a relatively consistent extraction yield. Similarly, the ethanolic extract yielded 27.98% on average with a standard deviation of 0.52%.
Acute Oral Toxicity Study
In the acute toxicity study, the administration of EEST fruits to the rats did not result in any observable signs of toxicity, behavioral abnormalities, or mortality, suggesting the safety profile of the doses used. The lack of any adverse effects or death indicates that the median lethal dose likely exceeds 250 mg/kg, the highest dose evaluated in this study.
Effect of Single Oral Administration of the Ethanolic Extract of Schinus Terebinthifolius Raddi Fruits on the Variation of Body Weight and Food and Water Consumption
In the present study, no significant alterations in body weight were observed at the end of the experiment among control and treated groups (Table 1). Moreover, no changes in food and water intake were observed among all the rats. Both the treated and control rats consumed food and water normally throughout the experiment.
Effect of Single Oral Administration of the Ethanolic Extract of Schinus Terebinthifolius Raddi Fruits on Relative Organ Weight
At the end of the experiment, there were no statistically significant differences between the treated and control groups regarding relative organ weight (Table 2).
Effect of Single Oral Administration of the Ethanolic Extract of Schinus Terebinthifolius Raddi Fruits on Behavioral Performance
Locomotor activity in open field test: After 14 days, the results showed that rats receiving doses of 2.5, 25, and 250 mg/kg of the extract displayed an elevation in the total number of squares traversed compared to the control rats. However, these increases in locomotor activity were not statistically significant (F5, 42 = 1.490, p= 0.2137) (Figure 2).
Table 2: Relative organ weight values (g) of control rats and rats treated with ethanolic extract of S. Terebinthifolius berries were recorded at the end of the study.
|
0.0906 ±0.01 |
0.0924 ±0.002 |
0.0914 ±0.01 |
0.0925 ±0.01 |
0.092 ±0.006 |
0.092 ±0.008 |
|
|
Kidney |
1.8978 ±0.13 |
1.846 ±0.12 |
1.8298 ±0.07 |
1.8376 ±0.16 |
1.8374 ±0.05 |
1.857 ±0.09 |
|
Heart |
0.8764 ±0.02 |
0.8724 ±0.09 |
0.8658 ±0.11 |
0.8632 ±0.07 |
0.8546 ±0.12 |
0.8764 ±0.12 |
|
Stomach |
4.0822 ±0.87 |
4.1462 ±0.81 |
4.5988 ±0.41 |
4.357 ±0.41 |
4.1234 ±0.34 |
4.2474 ±0.53 |
|
Spleen |
0.7694 ±0.14 |
0.7412 ±0.10 |
0.748 ±0.18 |
0.7478 ±0.14 |
0.7488 ±0.01 |
0.7536 ±0.03 |
|
Lung |
1.9426 ±0.16 |
1.9222 ±0.04 |
1.9532 ±0.39 |
1.944 ±010 |
1.9544 ±0.52 |
1.974 ±0.07 |
|
Liver |
8.6174 ±0.77 |
8,1616 ±0.39 |
8.553 ±0.48 |
8.64 ±0.46 |
8.5912 ±0.60 |
8.584 ±0.47 |
|
Brain |
1.7926 ±0.14 |
1.7404 ±0.08 |
1.7832 ±0.13 |
1.7870 ±0.24 |
1.7996 ±0.15 |
1.7850 ±0.07 |
|
Control group |
2.5 mg/kg |
5 mg/kg |
25 mg/kg |
50 mg/kg |
250 mg/kg |
Data are displayed as Mean ± standard deviation. The significance level is 0.05.
Measures of Anxiety
Elevated Plus Maze (EPM) Test: In the EPM test, the treated groups receiving doses of 2.5, 5, 25, and 250 mg/kg of the EEST, visited the open arms less frequently compared to the control group. However, this decrease in open-arm entries was not statistically significant (F5, 42 = 0.5795, p = 0.7154) (Figure 3A). Furthermore, rats that received 50mg/kg of the EEST exhibited an elevation in the number of entries in the open arms compared to all other groups. Likewise, as observed in Figure 3B, except for the group that received the highest dose of 250 mg/kg, all other treated groups exhibited a reduction in the time spent exploring the open arms. Notably, the group that received the 5 mg/kg dose showed a statistically significant decrease (p < 0.01) in the time spent in the open arms compared to the control group. However, the reduction in open arm time among the other treated groups did not reach statistical significance (F5, 42 = 4.864, p = 0.0013).
Light-dark box test: During the DLB test, the groups treated with the lower doses of 2.5 and 5 mg/kg of the EEST spent less time in the dark chamber of the DLB compared to the control group and the groups that received the other tested doses. However, this reduction in time spent in the dark chamber was not statistically significant (F5, 42 = 0.2568, p = 0.9340) (Figure 4A). Conversely, rats given the lower dose of 2.5 and 5 mg/kg of the EEST spent a longer time in the white chamber of the test compared to the control group and the other treated groups, but this increase in time spent in the light chamber was not statistically significant (F5, 42 = 0.2568, p = 0.9340) (Figure 4B). Regarding the number of entries into the white chamber of the DLB test (Figure 4C), all treated groups displayed a decrease in this parameter compared to the control group. This decrease in white chamber entries was insignificant (F5, 42 = 1.015, p = 0.4208).
Memory Performance
Y-maze test: In the Y-maze test (Figure 5), the groups treated with the lower doses of 2.5, 5, and 25 mg/kg of the EEST exhibited a reduction in spontaneous alternation behavior compared to the control and other treated groups. However, this decrease in spontaneous alternation was not statistically significant (F5, 42 = 2.420, p = 0.0514).
Novel object recognition test: In the NOR test, the recognition index of short-term memory (Figure 6A) was elevated slightly in the groups treated with 2.5 and 25 mg/kg of the EEST compared to the control group. However, this increase was not statistically significant (F5, 42 = 0.5502, p = 0.7372). Furthermore, the recognition index of long-term memory (Figure 6B) increased in the groups treated with 25 and 250 mg/kg of the EEST compared to the control group. Nevertheless, there were no statistically significant differences in the long-term memory recognition index (F5, 42 = 0.2043, p = 0.9589).
The administration of the extract at varying doses elicited modest alterations in several biochemical parameters. Notably, a subtle reduction in blood glucose levels was observed in the groups that received 5, 25, and 50 mg/kg of the extract. Additionally, a slight decrease in ASAT and ALAT activities was detected in the group treated with the lowest dose of 2.5 mg/kg. Furthermore, a marginal decrease in total cholesterol, HDL cholesterol, and LDL cholesterol levels was across all groups that received the extract, compared to the control group. Interestingly, a modest increase in triglyceride levels was observed in the groups administered 25, 50, and 250 mg/kg of the extract compared to the control group. However, these alterations did not reach statistical significance when compared to the control group (Table 3).
Histopathological Study
Microscopic examination of the liver (Figure 7) and kidney (Figure 8) from animals given the ethanolic extract
Table 3: Effects of the ethanolic extract of Schinus terebinthifolius Raddi fruits on biochemical blood parameters.
|
Parameters |
Groups |
|||||
|
Control group |
2.5 mg/kg |
5 mg/kg |
25 mg/kg |
50 mg/kg |
250 mg/kg |
|
|
Gly (g/l) |
1.25±0,22 |
1.25±0.20 |
1.17±0.06 |
1.19±0.05 |
1.17±0.03 |
1.22±0.13 |
|
ASAT (U/l) |
75.6±39.34 |
63.38±31.06 |
74.82±5.14 |
75.622±27.27 |
76.16±27.84 |
73.52±25.87 |
|
ALAT (U/l) |
43.42±12.51 |
41.3±8.41 |
45.66±7.30 |
45.82±7.17 |
44.9±11.26 |
46.22±10.28 |
|
Total Chol (g/l) |
0.62±0.12 |
0.56±0.12 |
0.52±0.09 |
0.52±0.06 |
0.52±0.03 |
0.564±0.05 |
|
HDL-Chol (g/l) |
0.33 ± 12.51 |
0.32 ±0.09 |
0.30±0.02 |
0.32±0.01 |
0.31±0.01 |
0.33±0.03 |
|
LDL-Chol (g/l) |
0.21±0.06 |
0.20±0.02 |
0.19±0.11 |
0.20±0.09 |
0.20±0.07 |
0.19±0.02 |
|
Tri (g/l) |
0.39±0.08 |
0.38±0.23 |
0.38±0.08 |
0.34±0.02 |
0.36±0.03 |
0.35±0.07 |
Data are displayed as Mean ± standard deviation; n: 5; the significance level is 0.05; Gly: Glycemia; ASAT: Aspartate aminotransferase; ALAT: Alanine aminotransferase; Chol: Cholesterol; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; Tri: Triglyceride.
of Schinus terebinthifolius Raddi fruits at doses of 2.5mg/kg, 5mg/kg, 25mg/kg, 50mg/kg, and 250mg/kg revealed no notable alterations in color or texture when compared to the control group. Furthermore, histological analysis of the examined organs revealed no abnormalities.
The present study evaluated the acute oral toxicity of the ethanolic extract of Schinus terebinthifolius Raddi fruits and the potential behavioral effects of the doses tested. The extract was administered orally to Wistar rats at various doses, ranging from 2.5 to 250 mg/kg. The results indicated no toxic signs or mortality were observed during the experimental period. This suggests that the tested doses of the extract may be considered safe when administered orally, highlighting its potential as a therapeutic agent and warranting further exploration of its safety and efficacy. Previous studies conducted on albino mice, zebrafish, and female and male Wistar rats using extracts from the bark and fruits of ST have yielded similar results (Alqathama et al., 2023; Todirascu-Ciornea et al., 2019; Affonso et al., 2012; Lima et al., 2009; Pires et al., 2004; Kennedy et al., 1986). In addition, the present findings showed no alterations in body weight variation and relative organ weight (Tables 1 and 2), which may stem from the unchanged food and water intake during the 14-day study period. Rats consumed the same amount of food and water before and after receiving the ethanolic extract. In agreement with the findings of the present study, Lima et al. (2009) and Pires et al. (2004) also noted no significant differences in the evolution of physiological parameters, such as water and feed intake and body weight, among the different treatment groups. Additionally, Lima et al. (2009), found that the absolute and relative tissue weights of both female and male Wistar rats were not significantly altered by treatment with Schinus terebinthifolius bark extract. This suggests that the extract does not induce substantial physiological changes in tissue morphology or mass between sexes. However, their study also reported that the acute and subacute toxicity of ST bark did not exhibit any sex-dependent effects, indicating a uniform response across genders. Despite these findings, the potential for sex differences in the toxicity profile of the fruit extract remains an important area for further investigation to ensure accurate safety assessments and therapeutic applications. Therefore, additional studies focusing on sex-specific responses to the ethanolic extract of ST fruits are warranted to fully elucidate its safety and efficacy.
Regarding the effects of the EEST on cognitive functions. Numerous scientific reports have highlighted the prominent role of total phenols and flavonoids as the main bioactive components extracted from the leaves and barks of ST and their reported neuroprotective effects on cognitive functions (da Silva Nascimento et al., 2023; Lima et al., 2009).
In the present study, it was observed that the administered doses did not significantly alter the general locomotor activity of the tested rats, suggesting that the extract did not exert a notable influence on the locomotor activity of rats. Despite the slight elevations observed in the total number of squares visited among groups treated with doses of 2.5, 25, and 250mg/kg of the extract, these elevations can be attributed to the animals’ increased apprehension when exposed to a new environment. Our observations are in alignment with the findings reported by a previous study conducted by Scheid et al. (2018), which likewise found that Schinus terebinthifolius did not induce any changes in locomotor activity in the OF test.
When evaluating anxiety-related behaviors, the findings indicate that rats administered dose of 5 mg/kg of the EEST displayed more anxious behaviors in the EPM test, suggesting that at these doses, the extract may have an anxiogenic effect. These results are contrary to a different study by Scheid et al. (2018), which showed that the methanol fraction from ST leaves did not alter anxiety levels in the EPM test. The lack of anxiogenic effects at the 2.5 and 250 mg/kg dose may be due to differences in the chemical composition or pharmacokinetics of the EEST compared to the methanol fraction used in the study of Scheid et al. (2018).
Regarding the effects of the extract of ST on memory performance. In the Y-maze test, rats administered doses of 50 and 250 mg/kg of the EEST exhibited similar results to those observed in the control group. However, the findings from the NOR test revealed a slight increase in the recognition index of both short-term and long-term memory in the 2.5, 25, 50, and 250 mg/kg groups of rats administered the EEST. This memory-enhancing effect is consistent with previous research indicating that S. terebinthifolius may promote neuroprotective actions mediated by its antioxidant activity. Notably, this neuroprotective potential of S. terebinthifolius has been observed in both a rotenone-induced rat model of Parkinson’s disease and a zebrafish model of scopolamine-induced memory deficits (Sereniki et al., 2016; Todirascu-Ciornea et al., 2019).
The presence of active compounds such as β-phellandrene, α-pinene, terpinen-4-ol, and α-phellandrene, as well as other components like β-pinene, dl-limonene, c-terpinene, α-terpineol, and α-terpinene, may contribute to the observed improvement in memory deficits. The synergistic effects of these compounds could also play a crucial role in the neuroprotective effects of ST. Lee et al. (2017), demonstrated that α-pinene has neuroprotective effects against scopolamine-induced memory impairment in C57BL/6 mice by increasing the mRNA expression of choline acetyltransferase in the cortex and the activity of antioxidant enzymes in the hippocampus via activation of NF-E2-related factor 2. This suggests that the neuroprotective effects of ST may be mediated by the activation of similar pathways.
The biochemical blood analysis revealed a normal glycemia average of approximately between 1.17 and 1.26 g/L, which is within the expected physiological range in rats. However, the doses of 5, 25, and 50 mg/kg of the tested extract showed a slight decrease in blood glucose. These findings are consistent with previous studies that have reported the antidiabetic properties of ST extract, which can help regulate blood glucose homeostasis (Iwanaga et al., 2019). The chemical constituents of ST, such as gallic acid, gallotannins, and glycosylated flavonols (Lima et al., 2009), may be responsible for these effects.
Furthermore, the activity of the hepatic enzymes ASAT and ALAT was not significantly altered by the ST extract, except for a slight decrease observed at the lower dose of 2.5 mg/kg. These findings align with a study by Lima et al. (2009), which found that the ST extract did not affect the activities of ASAT and ALAT, indicating that the extract did not induce any overt toxicity or damage to the liver or kidneys. This was further confirmed by the histological examination of these organs, which showed the absence of any toxic effects.
The lipid profile analysis revealed a decrease in total cholesterol, HDL cholesterol, LDL cholesterol levels, suggesting that the ST extract may have a beneficial effect on these parameters. This is consistent with a study conducted by Feriani et al. (2021), which found that ST extract lowered high lipid biomarkers levels.
The cholesterol-lowering function of ST extract may be due to the inhibitory activity of HMG-CoA reductase, the key enzyme in the metabolic pathway of cholesterol synthesis. The lower cholesterol may be linked to the increased activity of lipoprotein lipase, which has been considered a carrier of lipids to peripheral tissues for utilization or storage.
While the current study shows promising results, future research should address chronic toxicity, identify active compounds, and conduct additional behavioral assessments to further evaluate the extract’s safety and efficacy.
CONCLUSIONS AND RECOMMENDATIONS
The ethanolic extract of Schinus terebinthifolius Raddi fruit, when administered at the tested doses in the present study, did not induce any observable toxic effects in Wistar rats, even at the highest dose of 250 mg/kg. However, the lack of significant findings in behavioral tests, as well as the limited biochemical and histological data, indicate that, while the extract appears to be safe at the tested doses, the evidence is insufficient to fully establish its safety profile. The observed trends in biochemical parameters and behavioral performance point to possible benefits, but they also highlight the need for additional research to fully examine the extract’s safety and usefulness. This study provides preliminary insights, and future investigations should address these limitations and explore the extract’s effects in more detail.
Limitations
Our study has some limitations. Firstly, it examined the impact of a single oral administration of the extract on short-term behavioral outcomes. Future research should involve extended follow-up periods, such as 30 days or more, to evaluate the extract’s long-term safety and efficacy, as well as to define dose-response relationships. Extended observations may reveal delayed toxic effects or benefits not apparent in short-term research. Additionally, future investigations should explore the extract’s effects on neuroinflammation, particularly markers of inflammation and oxidative stress, to better understand its impact on neuronal health. Studies could also investigate whether the extract contributes to neuronal injury or regeneration, potentially revealing neuroprotective or neurotoxic mechanisms.
ACKNOWLEDGEMENTS
We express our sincere gratitude to the Neurosciences, Neuroimmunology, and Behavioral Unit for their invaluable technical support.
NOVELTY STATEMENT
This study aims to investigate the oral acute toxicity and cognitive effects of ethanolic extract from Schinus terebinthifolius Raddi fruit in Wistar rats. Despite its traditional use in medicine, there has been limited focus on its toxicity and behavioral effects. Our findings indicate a lack of toxicity across a wide dose range, along with promising trends in lipid profile improvement and enhanced memory potential at lower doses.
Abbreviations
ALAT: Alanine aminotransferase
ASAT: Aspartate aminotransferase
EEST: Ethanolic extract of Schinus terebinthifolius Raddi
EPM: Elevated Plus Maze
HandE: Hematoxylin and eosin
ITU: Ibn Tofail University
LDB: Light-Dark Box
NIH: National Institutes of Health
NOR: Novel Object Recognition Test
OECD: Organization for Economic Co-operation and Development
ST: Schinus terebinthifolius
AUTHOR’S CONTRIBUTIONS
All authors contributed equally to the manuscript.
Conflicts of Interest
There are no conflicts of interest in this study.
REFERENCES
Affonso CRG, Fernandes RM, De Oliveira JMG, De Carvalho E, Martins MDC, De Lima SG, De Sousa Júnior GR, Maria MZ, Zanini SF (2012). Effects of the essential oil from fruits of schinus terebinthifolius raddi (Anacardiaceae) on reproductive functions in male rats. J. Braz. Chem. Soc., 23(1): 180-185. https://doi.org/10.1590/S0103-50532012000100025
Alqathama A, Abdelhady MIS, Al-Omar MS, Barghash MF, Shallan AI (2023). Antioxidant, Anti-inflammatory and Cytotoxic Activity of Schinus terebinthifolia Fruit and Isolation of a New Immunomodulatory Polyphenolic Compound. Pharm. Mag., 19(1): 13-22. https://doi.org/10.1177/09731296221138632
Arq A, Cir B, Jos O, Malafaia O, Ribas-filho JM, Czeczko NG, Haquim R, Santos P, Abud R, Santos P (2013). Influence of Schinus terebinthifolius Raddi (aroeira) and Carapa guianensis Aublet (andiroba) in the healing process of gastrorraphies. Arq. Bras. Cir. Dig., 26(2): 84-91. https://doi.org/10.1590/S0102-67202013000200003
Azzaoui F-Z, Ahami AO, Khadmaoui A (2009). Impact of lead sub-chronic toxicity on recognition memory and motor activity of Wistar rat. Pak. J. Biol. Sci., 12(2): 173-177. https://doi.org/10.3923/pjbs.2009.173.177
Balkrishna A, Sharma N, Srivastava D, Kukreti A, Srivastava S, Arya V (2024). Exploring the Safety, Efficacy, and Bioactivity of Herbal Medicines: Bridging Traditional Wisdom and Modern Science in Healthcare. Future Integr. Med., 3(1): 35-49. https://doi.org/10.14218/FIM.2023.00086
Bourin M, Hascoët M (2003). The mouse light/dark box test. Eur. J. Pharmacol., 463(1-3): 55-65. https://doi.org/10.1016/S0014-2999(03)01274-3
Cavalher-Machado SC, Rosas EC, Brito FDA, Heringe AP, De Oliveira RR, Coelho Kaplan MA, Figueiredo MR, Henriques MDGMDO (2008). The antiallergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int. Immunopharmacol., 8(11): 1552-1560. https://doi.org/10.1016/j.intimp.2008.06.012
Da Silva Nascimento M, Dos Santos PH, de Abreu FF, Shan AYKV, Amaral RG, Andrade LN, Souto EB, Santos MIS, de Souza Graça A, Souza JB, Raimundo E, Silva JP, Tavares JF, de Oliveira E, Silva AM, Correa CB, Montalvão MM, Piacente S, Pizza C, Camargo EA, Dos Santos Estevam C (2023). Schinus terebinthifolius Raddi (Brazilian pepper) leaves extract: in vitro and in vivo evidence of anti-inflammatory and antioxidant properties. Inflammopharmacology, 31(5): 2505-2519. https://doi.org/10.1007/s10787-023-01316-8
Dos Santos MRG, Da Silva JHS, Caxito MLDC (2015). Brief review on the medicinal uses and antimicrobial activity of different parts of Schinus terebinthifolius Raddi. Int. J. Pharm. Pharm. Sci., 7(12): 1-7.
Ed-Day S, Boulbaroud S, Didou L, Elgui R, Ahami A, Azzaoui FZ (2021). Neuroprotective effects of Lepidium sativum L. on memory impairments in Wistar rat: Behavioral and neurochemical study. E3S Web Conf., 319: 1-4. https://doi.org/10.1051/e3sconf/202131901015
El Kourchi C, Belhoussaıne O, Elhrech H, Harhar H, Ullah R, Bari A, Maggi F, Caprioli G, Bouyahya A, Tabyaouı M (2024). Antidiabetic, antioxidant, and phytochemical profile of Pennisetum glaucum cultivated in central-southern Morocco and imported from India. J. Agric. Food Res., 101197. https://doi.org/10.1016/j.jafr.2024.101197
El-Massry KF, El-Ghorab AH, Shaaban HA, Shibamoto T (2009). Chemical compositions and antioxidant/antimicrobial activities of various samples prepared from Schinus terebinthifolius leaves cultivated in Egypt. J. Agric. Food Chem., 57(12): 5265-5270. https://doi.org/10.1021/jf900638c
Ennaceur A, Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1. Behavioral data. BehavBrainRes, 31: 47-59. https://doi.org/10.1016/0166-4328(88)90157-X
Fabricant DS, Farnsworth NR (2001). The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect., 109(1): 69-75. https://doi.org/10.1289/ehp.01109s169
Feriani A, Tir M, Arafah M, Gómez-Caravaca AM, Contreras M del M, Nahdi S, Taamalli A, Allagui MS, Alwasel S, Segura-Carretero A, Harrath AH, Tlili N (2021). Schinus terebinthifolius fruits intake ameliorates metabolic disorders, inflammation, oxidative stress, and related vascular dysfunction, in atherogenic diet-induced obese rats. Insight of their chemical characterization using HPLC-ESI-QTOF-MS/MS. J. Ethnopharmacol., 269. https://doi.org/10.1016/j.jep.2020.113701
Glen HF (2002). Cultivated Plants of Southern Africa: Botanical Names, Common Names, Origins, Literature. Jacana in association with the National Botanical Institute, 428.
Hu B, Xi X, Li H, Qin Y, Li C, Zhang Z, Liu Y, Zhang Q, Liu A, Liu S, Luo Q (2021). A comparison of extraction yield, quality and thermal properties from Sapindus mukorossi seed oil between microwave assisted extraction and Soxhlet extraction. Ind. Crops Prod., 161: 113185. https://doi.org/10.1016/j.indcrop.2020.113185
Iwanaga CC, Ferreira LDAO, Bernuci KZ, Fernandez CMM, Lorenzetti FB, Sehaber CC, Vieira Frez FC, Bernardes SS, Panizzon GP, Linde GA, Vieira MDC, Zanoni JN, Cortez DAG (2019). In vitro antioxidant potential and in vivo effects of Schinus terebinthifolia Raddi leaf extract in diabetic rats and determination of chemical composition by HPLC-ESI-MS/MS. Nat. Prod. Res., 33(11): 1655-1658. https://doi.org/10.1080/14786419.2018.1425848
Kachmar MR, Naceiri Mrabti H, Bellahmar M, Ouahbi A, Haloui Z, El Badaoui K, Bouyahya A, Chakir S (2021). Traditional Knowledge of Medicinal Plants Used in the Northeastern Part of Morocco. Evid Based Complement Alternat Med, 6002949. doi: 10.1155/2021/6002949. PMID: 34512779; PMCID: PMC8426073. https://doi.org/10.1155/2021/6002949
Kennedy GL, Ferenz RL, Burgess BA (1986). Estimation of acute oral toxicity in rats by determination of the approximate lethal dose rather than the LD50. J. Appl. Toxicol., 6: 145-148. https://doi.org/10.1002/jat.2550060302
Lee GY, Lee C, Park, GH, Jang JH (2017). Amelioration of scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evidence-Based Complementary and Alternative Medicine, 2017:4926815. https://doi.org/10.1155/2017/4926815
Lima LB, Vasconcelos CFB, Maranhão HML, Leite VR, Ferreira PA, Andrade BA, Araújo EL, Xavier HS, Lafayette SSL, Wanderley AG (2009). Acute and subacute toxicity of Schinus terebinthifolius bark extract. J. Ethnopharmacol., 126(3): 468-473. https://doi.org/10.1016/j.jep.2009.09.013
Misra RC, Thimmappa R, Bonfill M (2024). Editorial: Advances in discoveries of plant phytochemicals. Front. Plant Sci., 1-4 .https://doi.org/10.3389/fpls.2024.1414150
Moreira D de L, Teixeira SS, Monteiro MHD, De-Oliveira ACAX, Paumgartten FJR (2014). Traditional use and safety of herbal medicines. Rev. Bras. Farmacognosia, 24(2): 248-257. https://doi.org/10.1016/j.bjp.2014.03.006
N’Go PK, Azzaoui F-Z, Soro PR, Samih M, Ahami AOT, Najimi M, Chigr F (2013). Developmental Effects of Malathion Exposure on Recognition Memory and Spatial Learning in Males Wistar Rats. J. Behav. Brain Sci., 03(03): 331-340. https://doi.org/10.4236/jbbs.2013.33033
Paula A, Boleti DA, Vieira C, Alexandre C, Brentan D, Miranda L (2019). Comparative Biochemistry and Physiology , Part C Microbiological quality , chemical pro fi le as well as antioxidant and antidiabetic activities of Schinus terebinthifolius Raddi. Comp. Biochem. Physiol., Part C, 220: 36-46. https://doi.org/10.1016/j.cbpc.2019.02.007
Patočka J, de Almeida JD (2017). Brazilian Pepper Tree: Review of Pharmacology. Mil. Med. Sci. Lett., 86(1): 32-41. https://doi.org/10.31482/mmsl.2017.005
Pinto IC, Seibert JB, Pinto LS, Santos VR, Sousa RF De, Sousa LRD, Amparo TR, Santos VMR (2020). Preparation of glass - ionomer cement containing ethanolic Brazilian pepper extract (Schinus terebinthifolius Raddi) fruits : chemical and biological assays. Sci. Rep., 10(1): 22312. https://doi.org/10.1038/s41598-020-79257-3
Pires OC, Taquemasa AVC, Akisue G, Oliveira F, Araújo CEP (2004). Análise preliminar da toxicidade aguda e dose letal mediana (DL50) comparativa entre frutos de pimenta-do-reino do Brasil (Schinus terebinthifolius Raddi) e pimenta do reino (Piper nigrum L.). Lat. Am. J. Pharm., 23: 176-182.
Rocha PDSD, Campos JF, Nunes-Souza V, Vieira MDC, Boleti APA, Rabelo LA, Dos Santos EL, de Picoli Souza K (2017). Antioxidant and Protective Effects of Schinus terebinthifolius Raddi Against Doxorubicin-Induced Toxicity. Appl. Biochem. Biotechnol., 184(3): 869-88. https://doi.org/10.1007/s12010-017-2589-y
Scheid T, Moraes MS, Henriques TP, Riffel APK, Belló-Klein A, Poser GLV, Ethur EM, Partata WA (2018). Effects of Methanol Fraction from Leaves of Schinus terebinthifolius Raddi on Nociception and Spinal-Cord Oxidative Biomarkers in Rats with Neuropathic Pain. Evid. Based Complement. Altern. Med., 2018: 5783412. https://doi.org/10.1155/2018/5783412
Sereniki A, Linard-Medeiros CFB, Silva SN, Silva JBR, Peixoto Sobrinho TJS, da Silva JR, Alvesa LDS, Smailic SS, Wanderley AG, Lafayette SSL (2016). Schinus terebinthifolius administration prevented behavioral and biochemical alterations in a rotenone model of Parkinson’s disease. Rev. Bras. Farmacognosia, 26(2): 240-245. https://doi.org/10.1016/j.bjp.2015.11.005
Sierksma ASR, van den Hove DLA, Pfau F, Philippens M, Bruno O, Fedele E, Ricciarelli R, Steinbusch HWM, Vanmierlo T, Prickaerts J (2014). Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology, 77: 120-130. https://doi.org/10.1016/j.neuropharm.2013.09.015
Todirascu-Ciornea E, El-Nashar HAS, Mostafa NM, Eldahshan OA, Boiangiu RS, Dumitru G, Hritcu L, Singab ANB (2019). Schinus terebinthifolius Essential Oil Attenuates Scopolamine-Induced Memory Deficits via Cholinergic Modulation and Antioxidant Properties in a Zebrafish Model. Evid. Based Complemen. Altern. Med., 5256781. https://doi.org/10.1155/2019/5256781
To share on other social networks, click on any share button. What are these?






