Potential Mitigation of Spirotetramat-Induced Reproductive Toxicity by Tribulus terrestris in Domestic Pigeons (Columba livia domestica)
Potential Mitigation of Spirotetramat-Induced Reproductive Toxicity by Tribulus terrestris in Domestic Pigeons (Columba livia domestica)
Ali Bouzekri1,2*, Souheila Slimani1,2, Meryem Nassar1,2, Souad Zaaboub3 and
Nora Sakhraoui1
1Laboratory of Research in Biodiversity Interaction, Ecosystem and Biotechnology, University of 20 Auguest 1955, Skikda, Algeria
2Departement of Biology, University of 20 Auguest 1955, Skikda, Algeria
3Laboratory of Anatomopathology, Public Health Enterprise of Skikda, Algeria
ABSTRACT
Tribulus terrestris is traditionally used to treat various diseases. The current research aimed to study the protective effects of methanolic extract of Tribulus terrestris (TT) against Spirotetramat (SPT) induced reproductive toxicity in adult male domestic pigeons (Columba livia domestica). For ten consecutive weeks and under an artificial photoperiod (19L: 5D), thirty male pigeons weighing approximately 309,20 ± 14,41g were divided equally into six groups as follows: CT served as the control, SPT group orally given with 15 mg/kg BW/day, TT100 and TT50 groups orally administrated with 100 and 50 mg/kg BW/day of TT respectively, and SPT+ TT100 and SPT + TT50 groups. Testicular volume and body weight were measured each two weeks. Whereas histopathological profile and luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (T), total cholesterol (TC), LDL-C, HDL-C, and triglyceride (TG) were evaluated at week 10. The obtained data reveal that under a long photoperiod of 19L:5D, sexual activity lasted only 06 weeks in the control and TT groups, with a significant increase in testicular volume followed by spontaneous gonadal regression up to week 10. But testicular weights were superior in TT pigeons compared to the control during all experiments. However, the administration of SPT has suppressed gonadal growth and delayed photo-refractoriness. Sex hormones levels revealed a significant increase in LH and FSH levels in all groups compared to controls. However, a significant depletion in testosterone levels. Nevertheless, there was a substantial increase in TC, HDL-C, and LDL-C levels. Furthermore, co-administration of TT with SPT restored the lowered testicular volume, relative testicular weight, and T level but decreased the TC, HDL-C, and LDL-C levels. Finally, the histopathological investigation revealed degenerative changes in testes and gonad damage in the SPT group. However, TT reduced the damage induced by SPT. In conclusion, TT could be beneficial in preventing SPT reproductive toxicity and improving sex hormone synthesis.
Article Information
Received 20 April 2022
Revised 15 November 2022
Accepted 29 December 2022
Available online 08 March 2023
(early access)
Published 02 May 2024
Authors’ Contribution
AB, SS and MN contributed in the study design, experimental work, writing the manuscript. AB performed in statistical analysis. SZ contributed in the histopathological examination. AB and SS analyzed the sera and tissue samples. SN identified the plant. All authors interpreted the data and approved the final version.
Key words
Pigeons, Spirotetramat, Toxicity, Tribulus terrestris, Photoperiod, Seasonal reproduction
DOI: https://dx.doi.org/10.17582/journal.pjz/20220420130439
* Corresponding author: [email protected]
0030-9923/2024/0003-1403 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Exposure to environmental or xenobiotic substances may induce reproductive toxicity in living species (Oladele et al., 2016). Pesticides may also affect reproductive functions, including congenital abnormalities, reduced fecundity, infertility, and altered development (Collotta et al., 2013). SPT is a new tetramic acid-based insecticide that belongs to the keto-enol pesticide family. It has a unique mode of action that interacts with lipid synthesis (Gong et al., 2016b). Most infertile males have low sperm quality, as shown by reduced sperm counts, aberrant sperm geomorphology, and lower sperm motility (Haghmorad et al., 2019). Male infertility is caused by sperm abnormalities in the pre-testicular, testicular, and post-testicular phases (Dimitriadis et al., 2017). Many researchers have assessed SPT’s environmental and non-target species impacts. According to the results of their study, Huiming et al. (2012) concluded that SPT is absorbed and metabolised differently in different organs and tissues. In addition, Gutbrod et al. (2020) found that acetyl-CoA carboxylase activity was reduced by SPT exposure. Furthermore, Liu et al. (2011) report that rats were given 100 mg/kg/d SPT lost weight and had liver and genital damage after seven days. According to other research, SPT treatment can induce toxicity in zebrafish and oxidative stress in zebrafish ovaries. SPT exposure can affect FSH-r and LH-r gene expression and oocyte size and maturation, among other things (Liu et al., 2011). Apoptosis of cells and gonad histological inflammation showed that exposure to SPT caused gonad damage in zebrafish and that SPT altered the endocrine (Zhang et al., 2020b).
Medicinal plants have been traditionally employed for their health-improving characteristics for many years. The belief that natural medicines might improve general health and help people overcome chronic diseases has fueled a global interest in plant-based supplements (Neychev and Mitev, 2016). There is a strong interest in plants that enhance male fertility due to the widespread male fertility deficiency caused by multiple factors, including environmental pollutants. Therefore, herbal remedies are preferred for treating male infertility, because natural antioxidants found in plants to treat many diseases were shown to cure male infertility without adverse effects (Safarnavadeh and Rastegarpanah, 2011). Studies have shown that phytotherapy, also known as plant medicine can increase testosterone levels and improve male fertility (Bahmanpour et al., 2012; Zang et al., 2015; Fedail et al., 2016; Zhu et al., 2017). TT of the Zygophyllaceae family is a widely spread plant across the globe. It has antibacterial, anti-inflammatory, aphrodisiac, antioxidative, hepatoprotective, cardiotonic, anthelmintic, hypolipidemic, and diuretic properties (Chhatre et al., 2014; Singh et al., 2012; Hamidi et al., 2019; Tian et al., 2019). In addition, TT is commonly used in traditional Chinese medicine to treat various diseases, including coronary artery disease, post-stroke syndrome, cancer, hypertension, atherosclerosis, and myocardial infarction (Shahid et al., 2016). There are diverse active compounds in its various parts, including alkaloids, steroidal saponins, glycosides, flavonols, and flavonoids (Chhatre et al., 2014). TT influences spermatogenesis, as shown by changes in the testicular tubule, such as increased tubular volume, total tube length, and seminiferous epithelium height (Zhu et al., 2017). The TT extract increased the cytoplasmic, nuclear, and individual volume of Leydig cells in male Wistar rats (Neylanne et al., 2015). Several clinical examinations have shown that TT increases reproductive activity, including increased hormone levels such as estradiol, with testosterone being only slightly influenced and increasing reproductive activity, ovulation, and sexual desire (Gauthaman et al., 2002). In the testis, seminiferous tubules spermatogenesis is a multistep process highly regulated by hormones (Meri et al., 2013; Walker and Cheng, 2005). Furthermore, TT is a testosterone booster; TT saponins seem to bind with hypothalamus receptors that detect sex hormones, in part inhibiting the receptors directing to the hypothalamus, distorting the body’s sex hormone concentrations as lower than they certainly are (Sun et al., 2003). The hypothalamus signs of starting the synthesis of LH; When LH levels are augmented, the average production of testosterone also surges. The participation of TT in the enhancement of male sexual dysfunctions is well-recognized. TT has a protecting effect against cypermethrin (Poonam et al., 2013) and cadmium (Rajendar et al., 2011) induced testicular injury in the rat. According to (Haghmorad et al., 2019), treatment with TT and Anacyclus pyrethrum improved sex hormone levels and sexual indices. In addition, when Anacyclus pyrethrum and TT are used together, sexual parameters in Wistar rats, such as sex hormone concentrations, sperm quality, and male Wistar rat histoarchitecture, improve. Thus, the primary objective of this study is to evaluate the protective effect of TT against reproductive toxicity induced by SPT by assessing body weight, testicular volume, relative testicular weight, sex hormones (FSH, LH, and Testosterone) levels, and lipid parameters (total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglyceride) levels in the serum of pigeons.
Materials and Methods
Chemicals
The Spirotetramat formulation (Movento®, 150 OD. CAS No. 203313-25-1, purity 98.5%) was purchased from Bayer Crop Science (Germany) (Augsburg, Germany). A dose of 15 mg/kg/day was applied in this study, according to LD50 of Spirotetramat to birds is above 2000 mg/Kg (Maus, 2008).
Plant material and preparation of the extract
Aerial parts of Tribulus terrestris (1.5kg) were purchased from a herbal market (Setif-Algeria, January 2021). The plant sample was identified by a botanist (Dr Nora Sakhraoui, University of Skikda); 1 kg of the Tribulus powder was macerated in a hydro-methanolic solution (80%) for 24 h. Eventually, the extract was filtered using a paper filter. Finally, methanol was evaporated below the vacuum evaporator (Heating bath, cat No=18100984, RE-100 Pro) at 45oC to obtain a solid residue. Two doses of 50 and 100 mg/kg of the plant were applied to the animals at the treatment time.
Animals
Thirty adult males of domestic pigeons (Columba livia domestica) weighing 309.20±14.41g were obtained from Sikka (North-East of Algeria) at the end of January. At the arrived time, animals were housed in polypropylene cages measuring 90x90x90 cm (Animal House, Department of Biology, University of Skikda). For two weeks, they were acclimated in standard conditions 23±2°C, adequate aeration, and humidity of 50±10%). The pigeons were fed a standard chow diet (20% protein, 10% fat, and 70% carbohydrates) and given water ad libitum.
Experimental design
Thirty male pigeons were divided into six groups, each of five which they were orally treated for ten consecutive weeks as follows: (1) Group (CT): was used as a control and treated with distilled water. (2) Group (SPT) treated with Spirotetramat (15 mg/kg/day). (3) Group (TT100) treated with TT (100 mg/kg/day). (4) Group (TT50) treated with TT (50 mg/kg/day). (5) Group (SPT+TT100) treated with Spirotetramat (15 mg/kg/day) combined with TT (100 mg/kg/day). (6) Group (SPT+TT50) treated with Spirotetramat (15 mg/kg/day) combined with TT (50 mg/kg/day).
All animals were held below long artificial photoperiod (19L:5D) using an electrical clock.
Body weight and testicular volume
Gonadal status and body weight of animals were estimated each two weeks of the treatment. The testes were observed from a small incision between the last two ribs after a local anaesthetizing with viscous lidocain. The sizes of the left testis were assessed to the nearest 0.5 mm.
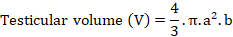
a is ½ the width, and b is ½ the length (long axis).
Samples collection
After ten weeks of treatment under a long photoperiod (19L:5D) pigeons were sacrificed. Blood samples were obtained and centrifuged at 4000 x g for 10 min. Testes were immediately collected, washed in distilled water, weighed, and fixed in 10% formol solution.
Analytical procedures
FSH, LH and testosterone (T) were assessed by Enzyme-Linked Fluorescent Assay (ELFA) using ELFA (VIDAS-BIOMÉRIEUX) automate.
Using a Beckman-Coulter Synchron LX20 PRO (Beckman-Coulter Inc, Fullerton, CA) and Syncron system reagents, total serum cholesterol (TC), high-density lipid-cholesterol (HDL-C), low-density lipid-cholesterol (LDL-C), and triglyceride (TG) levels in serum were determined in all groups.
Histopathology examination
Fixed tests were successively treated in ethanol, xylene, and paraffin designed for histological investigation. Testes tissues entrenched in paraffin wax and attached on glass slides were segmented into 4 μm thick sections, discoloured with hematoxylin and eosin, air-dried, and observed under a light microscope (Zeiss, 400X).
Statistical analysis
We used Graph pad prism version 9.2.0 (GraphPad Software, LLC, CA, USA) for statistical tests analysis of results. All values were expressed as mean±standard deviation (SD) of five animals. Data from different groups were assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for inter-group comparisons. All results were considered statistically significant when P<0.05.
Results
Body weight variations
The evolution of the bodyweight during the study under a long photoperiod (19L:5D) is shown in Figure 1A. In the beginning, the experimental pigeons had an average body weight of all pigeons at the commencement of the
treatment was 309.20±14.41g. Data obtained revealed a significant increase in body weight in the TT50 and TT100 groups compared to the control (P<0.001). However, starting the fourth week, the SPT group’s mean body weight was lower than the control group.
Testicular volume variation during the experiment
The variations in testis size evaluated during the current study are presented in Figure 1B. In the beginning, animals had a mean testicular volume of 648.5±85.12 mm3. Control pigeons kept under long artificial photoperiod (19L:5D) had completed their reproductive cycle. The gonadal development lasts six weeks with a higher volume of 1011.76 mm3. TT 100 mg and TT50 mg groups showed a higher significance (P < 0.01 and P < 0.001, respectively) in the testicular volume than the control. However, during a long photoperiod of (19L:5D), pigeons exposed to SPT revealed a lower testes volume. But, administration of combined TT+ SPT significantly increased the testicular volume (P < 0.001).
Testicular mass
The testes’ weight assessed at the end of this study is reported in Figure 2. Figure 2 demonstrated a significant increase (p < 0.001) in the testicular mass of animals treated by TT (TT100 and TT50) compared to the control group. The effect of SPT on the testes’ weight was significantly decreased (P < 0.001) compared to the control. However, treatment of TT (TT100 and TT50) combined with SPT caused a significant increase (P < 0.001) in testicular mass.
Sex hormones levels
FSH, LH, and testosterone levels in the serum of pigeons treated under a long photoperiod (19L: 5D) for ten (10) weeks consecutive are mentioned in Figure 3. This figure revealed that the FSH and LH concentrations were significantly (p < 0.001) increased in TT100, TT50 groups, and SPT groups (p < 0.05 and p < 0.001, respectively) compared to the control. While, serum testosterone levels were significantly (p < 0.001) decreased in the SPT exposed pigeons. However, administration of TT100 and TT50 mg/kg combined with SPT 15 mg/kg restored the lowered serum testosterone level provoked by SPT. The TT100 and TT50 mg/kg treated pigeons showed significant (p < 0.001) elevated serum testosterone levels.
Biochemical parameters levels
TC, HDL-C, LDL-C, and TG levels in the serum of pigeons treated for ten weeks under a long photoperiod (19L:5D) are shown in Figure 4. Results showed a significant increase (p < 0.001) in TG, LDL-C and (P < 0.05) in HDL-C serum levels in SPT exposed pigeons. All animals treated with TT combined with SPT significantly reduced the serum’s higher TC, HDL-C, LDL-C, and TG levels induced by SPT exposition.
Histopathology examination
Histological photomicrograph in the control group revealed a regular testis architecture (Fig. 5A1, A2). This group had generally formed normal seminiferous tubules with a consecutive stage of spermatogenesis. Normal epithelial tissue (thin, abundant spermatogenic cells and spermatozoa) was visible. Testes of the SPT group showed
degenerative phenomena represented by irregular and atrophy of seminiferous tubules with all consecutive phases of spermatogenesis, some abnormal spermatozoa in the tubular lumen, and a slight reduction in the interstitial spaces and a diminution in Sertoli cell numbers. Vascular congestion and fibrous thickening of the basal were also remarked (Figs. 5B1, B2). Pigeons treated with TT (100 and 50 mg/kg) showed normal testis histo-architecture, normal seminiferous tubules, and rich spermatozoa, Leydig cell, and spermatocytes were observed (Figs. 5C1, C2) and (Figs. 6A1, A2), while in SPT+TT (100 and 50 mg/kg) group showed testis tissue seems close to the control, spermatogenesis was preserved in most seminiferous tubules, and the lumen was most often occupied with spermatozoa (Figs. 6B1, B2, 6C1, C2). Furthermore, the TT extract has decreased the damage caused by SPT.
Discussion
The current study investigated the protective effects of TT methanolic extracts against SPT-induced reproductive toxicity in male pigeons. For this, two doses (50 and 100 mg/kg/day) of TT and 15 mg/kg /day of SPT
were applied for ten weeks to domestic male pigeons (Columba livia domestica) under a long photoperiod (19L:5D). Bodyweight, testicular volume, testes mass, sex steroid hormones (FSH, LH, and testosterone), cholesterol, HDL-C, LDL-C, TG levels in the serum, and testicular histopathology were evaluated to detect the testicular lesions.
Bodyweight is controlled by balancing food intake and energy expenditure (Simpson et al., 2008). In fatty acid biosynthesis, acetyl-CoA carboxylase converts acetyl-CoA to malonyl-CoA, the first step in fatty acid biosynthesis. Inhibition of this enzyme reduces fatty acid synthesis while
increasing fatty acid oxidation (Harwood, 2005). Our results suggest that the decrease in body weight following SPT administration in Columba livia pigeons may be due to the inhibition of acetyl-CoA carboxylase. Gong et al. (2016) revealed that SPT has substantially affected fertility in adults by inhibiting the acetyl-CoA carboxylase. In addition, a previous study demonstrated that acetyl-CoA carboxylase inhibitor therapy reduced adipose tissue and caused weight loss (Harwood, 2005). However, our findings showed a significant increase in body weight following TT administration. These results are accordant with Gautaman et al. (2003), Bashir et al. (2009), and Abadjieva et al. (2019), who found a significant rise in body weight in rats fed with different doses of TT extract. The obtained results suggest that the androgenic effect of TT causes an increase in appetite (Abadjieva et al., 2019).
Additionally, the present study revealed that under a long photoperiod (19L: 5D), domestic pigeons showed a fully reproductive cycle characterized by whole mature testes at the 6th week and followed by spontaneous gonadal regression. These results are accordant with Slimani et al. (2018). Moreover, Wingfield and Farner, (1993) found that for birds that increased photoperiod, an increase in gonadotrophin-releasing hormone (GnRH) secretion, which increases gonadotropin secretion and thus gonadal maturation hormones such as LH and FSH, which in turn induces gonad growth and steroid hormone production. However, the study showed that SPT treatment reduced testes size and disrupted the reproductive cycle. It is possible that SPT interfered with testicular function indirectly or directly by disrupting hypothalamic or pituitary gland activities (Recio et al., 2005; Mitra and Maitra, 2018). Furthermore, Zhang et al. (2020) have reported that SPT impaired gonad development and caused gonad injury. Damsgaard et al. (2016) indicate that Sertoli cell degeneration lowers the testicular volume. In this study, oral TT and SPT+TT co-treatment enhanced testicular volume and relative testicular weights. Other studies by Neylanne et al. (2015) and Bashir et al. (2009) found an increase in testes weight, total tube length, tubular volume, and seminiferous epithelium height in rats treated with TT extracts.
It is known that pesticides disrupt gonadal, adrenal, and thyroid function (Diamanti-Kandarakis et al., 2009; Slimani et al., 2011, 2014; Pandey et al., 2017). The present research showed a significant increase in FSH and LH levels. However, a decrease in testosterone levels following SPT exposure. Dandona and Rosenberg (2010) and Zhang et al. (2020) have reported a deficiency in testicular development, low serum testosterone and high LH and FSH concentrations. Moreover, Zhang et al. (2020) demonstrated that SPT exposure could reduce plasma estradiol-2, testosterone, 11-ketotestosterone, and numerous other genes, including hsd involved in testosterone production, which became inactive after SPT exposure. Testosterone biosynthesis is dependent on cholesterol availability; the rise in testosterone levels found in the present investigation following the SPT administration could be due to SPT’s indirect effect on testosterone biosynthesis. TT extract administration at two doses revealed a significant increase in testosterone, FSH, and LH levels compared to the control group. Also, combining TT with SPT significantly restored the high decrease in serum testosterone induced by SPT. These results are similar to Shalaby and Hammouda (2014), Pavin et al. (2018), Sanagoo et al. (2019), and Kamenov et al. (2017) findings. Our results suggest that TT extract contains antioxidant effects (Amin et al., 2006), and it also has an aphrodisiac effect that can increase testosterone production (Gauthaman et al., 2003). The capacity of TT to inhibit the generation of oxygen free radicals prevents testicular tissue peroxidation. We further proposed that TT may protect Sertoli cells by increasing testosterone synthesis in the testicles (Gauthaman and Ganesan, 2008). Furthermore, TT improves reproductive function in male rats by naturally boosting LH secretion, leading the body to produce excess testosterone (Adimoelja, 2000; Shalaby and Hammouda, 2014). Sharma et al. (2020) found that TT Methanolic extracts increased LH, enhancing Leydig cell number and function. Therefore, the saponin in TT leaves stimulates the pituitary gland to produce more LH. Because luteinizing hormone makes testosterone, it could help with sperm production, erectile dysfunction, and sexual satisfaction (YJ et al., 2001).
Several studies have shown that HDL-C cholesterol is the primary substrate for testosterone synthesis in rats (Charreau et al., 1981; Chu et al., 2003). Cholesterol is a precursor for steroid synthesis and is essential for male reproduction (Yokoyama, 2000; Parton and Hancock, 2004). Wise et al. (1993) found a positive correlation between testosterone and cholesterol in boar serum. But a recent study found that low testosterone causes severe hypercholesterolemia (Cai et al., 2015). The obtained results showed a significant decrease in serum T levels following SPT exposure. This suggests that SPT indirectly inhibits testosterone synthesis by inhibiting the enzyme acetyl-CoA carboxylase, which is involved in fatty acid synthesis and cholesterol accumulation. In our study, Only TT treatment did not affect TC, LDL-C, HDL-C, or TG levels. But when TT was combined with SPT, TG levels decreased significantly. Similar findings were reported by Chu et al. (2003), Altug et al. (2009), and Hussain et al. (2009), who found that TT could decrease serum TC and TG levels. In addition, previous studies showed that people with dyslipidaemia had reduced LDL-C and TC levels after using tribestan (Doncheva et al., 2006). Moreover, TT saponins have modulated lipid metabolism (Yang et al., 1999) and hyperglycemia (Li et al., 2002). Furthermore, Lirette et al. (1993) indicate that the saponins and flavonoids in TT inhibit enzyme processes that generate cholesterol. As a result, when SPT lowers TC, LDL-C, and HDL-C levels, TT becomes a very effective hypolipidemic compound.
Finally, the histological analysis revealed that SPT exposure caused irregularities and atrophy of seminiferous tubules, with aberrant spermatozoa in the tubular lumen. According to Zhang et al. (2020a), interstitial connective tissue hyperplasia and widening and the absence of seminiferous tubule walls were observed after SPT exposure, indicating that SPT exposure could cause lobule histology changes, which in turn could disrupt average sperm production. Additionally, our results showed a diminution in Sertoli cell numbers with vascular congestion and fibrous thickening. Many studies have reported that the seminiferous tubule and interstitial cells were injured, and the seminiferous epithelium was destroyed after SPT exposure Zhang et al. (2020a). However, co-treatment of TT with SPT restored regular testicular morphology and reduced SPT-induced damage. Our findings corroborate Bashir et al. (2009) findings of increased spermatogenetic cysts and late stages of spermatogenesis after TT treatment. Also, a study performed by Kamboj et al. (2011) and Kumar and Singh (2016) found that TT’s antioxidant properties can help decrease free radical damage.
Conclusion
Spirotetramat at 15 mg/kg/day for 75 days may be caused gonadal damage, endocrine system alteration and inhibition, and lipid metabolism disruption. On the other hand, treatment with Tribulus terrestris reduced testicular damage, improved sexual hormone (T, FSH, and LH) production, and regulated lipid metabolism. Therefore, Tribulus terrestris has higher antioxidant activity and could be considered a first-line treatment for male reproductive toxicity and other endocrine disorders.
Acknowledgement
The authors would like to thank all those who contributed to the completion of this work. Special thanks go to the laboratory of research in biodiversity interaction, ecosystem and biotechnology, where this research was conducted.
Funding
The study received no external funds.
IRB approval
The study was approved by the ethics committee of University of 20 August 1955 - Skikda, Algeria.
Ethical statement
All procedures applied in this study, including animal housing and experimentation, were in accord with the conformist guidelines of the Animal Ethics Committee of Skikda University.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abadjieva, D., Grigorova, S., Gjorgovska, N. and Kistanova, E., 2019. Dose-dependent effect of Tribulus Terrestris dry extract on reproductive organs of growing male rabbits. Maced. J. Anim. Sci., 9: 19–23. https://doi.org/10.54865/mjas1991019a
Adimoelja, A., 2000. Phytochemicals and the breakthrough of traditional herbs in the management of sexual dysfunctions. Int. J. Androl. Suppl., 23: 82–84. https://doi.org/10.1046/j.1365-2605.2000.00020.x
Altug Tuncer, M., Yaymaci, B., Sati, L., Cayli, S., Acar, G., Altug, T. and Demir, R., 2009. Influence of Tribulus terrestris extract on lipid profile and endothelial structure in developing atherosclerotic lesions in the aorta of rabbits on a high-cholesterol diet. Acta Histochem., 111: 488–500. https://doi.org/10.1016/j.acthis.2008.06.004
Amin, A., Lotfy, M., Shafiullah, M. and Adeghate, E., 2006. The protective effect of Tribulus terrestris in diabetes. Annls N. Y. Acad. Sci., 1084: 391–401. https://doi.org/10.1196/annals.1372.005
Bahmanpour, S., Vojdani, Z., Panjehshahin, M.R., Hoballah, H. and Kassas, H., 2012. Effects of carthamus tinctorius on semen quality and gonadal hormone levels in partially sterile male rats. Korean J. Urol., 53: 705–710. https://doi.org/10.4111/kju.2012.53.10.705
Bashir, A., Tahir, M., Samee, W. and Munir, B., 2009. Effects of Tribulus Terrestris on testicular developement of immature albino rats. Biomedica, 25: 63–68.
Cai, Z., Xi, H., Pan, Y., Jiang, X., Chen, L., Cai, Y., Zhu, K., Chen, C., Xu, X. and Chen, M., 2015. Effect of testosterone deficiency on cholesterol metabolism in pigs fed a high-fat and high-cholesterol diet. Lipids Hlth. Dis., 14: 1–10. https://doi.org/10.1186/s12944-015-0014-5
Charreau, E.H., Calvo, J.C., Nozu, K., Pignataro, O., Catt, K.J. and Dufau, M.L., 1981. Hormonal modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in gonadotropin-stimulated and desensitized testicular Leydig cells. J. Biol. Chem., 256: 12719–12724. https://doi.org/10.1016/S0021-9258(18)42954-7
Chhatre, S., Nesari, T., Somani, G., Kanchan, D. and Sathaye, S., 2014. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev., 8: 45–51 https://doi.org/10.4103/0973-7847.125530.
Chu, S., Qu, W., Pang, X., Sun, B. and Huang, X., 2003. Effect of saponin from Tribulus terrestris on hyperlipidemia. Zhong Yao Cai = Zhongyaocai J. Chin. Med. Mater., 26: 341–344.
Collotta, M., Bertazzi, P.A. and Bollati, V., 2013. Epigenetics and pesticides. Toxicology, 307:35-41. https://doi.org/10.1016/j.tox.2013.01.017
Damsgaard, J., Joensen, U.N., Carlsen, E., Erenpreiss, J., Blomberg, J.M., Matulevicius, V., Zilaitiene, B., Olesen, I.A., Perheentupa, A., Punab, M., Salzbrunn, A., Toppari, J., Virtanen, H.E., Juul, A., Skakkebæk, N.E. and Jørgensen, N., 2016. Varicocele is associated with impaired semen quality and reproductive hormone levels: A study of 7035 healthy young men from six European countries. Eur. Urol., 70: 1019–1029. https://doi.org/10.1016/j.eururo.2016.06.044
Dandona, P. and Rosenberg, M.T., 2010. A practical guide to male hypogonadism in the primary care setting. Int. J. clin. Pract., 64: 682–696. https://doi.org/10.1111/j.1742-1241.2010.02355.x
Diamanti-Kandarakis, E., Bourguignon, J.P., Giudice, L.C., Hauser, R., Prins, G.S., Soto, A.M., Zoeller, R.T. and Gore, A.C., 2009. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev., 30: 293–342. https://doi.org/10.1210/er.2009-0002
Dimitriadis, F., Adonakis, G., Kaponis, A., Mamoulakis, C., Takenaka, A. and Sofikitis, N., 2017. Pre-testicular, testicular, and post-testicular causes of male infertility. https://doi.org/10.1007/978-3-319-44441-3_33
Doncheva, N., Protich, M., Sheinkova, G., Kalinov, K. and Dobreva, D., 2006. Effect oftribestan on lipid metabolism and hormonal status in men in andropause. Bulg. Med., 13: 8–11.
Fedail, J.S., Ahmed, A.A., Musa, H.H., Ismail, E., Sifaldin, A.Z. and Musa, T.H., 2016. Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats. Asian Pac. J. Reprod., 5: 434–441. https://doi.org/10.1016/j.apjr.2016.07.014
Fraites, M.J.P., Cooper, R.L., Buckalew, A., Jayaraman, S., Mills, L. and Laws, S.C., 2009. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicol. Sci., 112: 88–99. https://doi.org/10.1093/toxsci/kfp194
Gauthaman, K., Adaikan, P.G. and Prasad, R.N.V., 2002. Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci., 71: 1385–1396. https://doi.org/10.1016/S0024-3205(02)01858-1
Gauthaman, Kalamegam, P.A., Ganesan, and Prasad, R.N., 2003. Extract (Protodioscin): An evaluation using a rat model. J. Altern. Complement. Med., 9: 257–265. https://doi.org/10.1089/10755530360623374
Gauthaman, K. and Ganesan, A.P., 2008. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction–an evaluation using primates, rabbit and rat. Phytomedicine, 15: 44-54. https://doi.org/10.1016/j.phymed.2007.11.011
Gong, Y., Shi, X., Desneux, N. and Gao, X., 2016. Effects of spirotetramat treatments on fecundity and carboxylesterase expression of Aphis gossypii Glover. Ecotoxicology, 25: 655–663. https://doi.org/10.1007/s10646-016-1624-z
Gutbrod, P., Gutbrod, K., Nauen, R. and Elashry, A., 2020. Inhibition of acetyl - CoA carboxylase by spirotetramat causes growth arrest and lipid depletion in nematodes. Sci. Rep., 10: 1-11. https://doi.org/10.1038/s41598-020-69624-5
Haghmorad, D., Mahmoudi, M.B., Haghighi, P. and Alidadiani, P., 2019. Improvement of fertility parameters with Tribulus terrestris and Anacyclus pyrethrum treatment in male rats. Int. Braz. J. Urol., 45: 1043–1054. https://doi.org/10.1590/s1677-5538.ibju.2018.0843
Hahn, T.P. and Macdougall-shackleton, S.A., 2008. Adaptive specialization, conditional plasticity and phylogenetic history in the reproductive cue response systems of birds. https://doi.org/10.1098/rstb.2007.2139
Hamidi, A., Ehsan, M., Yazdi, T., Amiri, M.S., Hosseini, H.A. and Darroudi, M., 2019. Terrestris L. extract and evaluation of their photocatalyst. Res. Chem. Intermed., 45: 2915–2925. https://doi.org/10.1007/s11164-019-03770-y
Harwood Jr., H.J., 2005. Treating the meytabolic syndrome: Acetyl-CoA carboxylase inhibition. Expert Opin. Ther. Targets, 9: 267–281. https://doi.org/10.1517/14728222.9.2.267
Huiming, W., Fanglin, W., Guonian, Z. and Yonggen, L., 2012. Study on distribution and metabolism of spirotetramat in rat. Chin. J. Pestic. Sci., 14: 417–422.
Hussain, A.A., Mohammed, A.A., Ibrahim, H.H. and Abbas, A.H., 2009. Study the biological activities of tribulus terrestris extracts. World Acad. Sci. Eng. Technol., 57: 433–435.
Kamboj, P., Aggarwal, M., Puri, S. and Singla, S.K., 2011. Effect of aqueous extract of Tribulus terrestris on oxalate- induced oxidative stress in rats. Indian J. Nephrol., 21: 154–159. https://doi.org/10.4103/0971-4065.83727
Kamenov, Z., Fileva, S., Kalinov, K. and Jannini, E.A., 2017. Evaluation of the efficacy and safety of tribulus terrestris in male sexual dysfunction. A prospective, randomized, double-blind, placebo-controlled clinical trial. Maturitas (An International Journal of Midlife Health and Byond) https://doi.org/10.1016/j.maturitas.2017.01.011
Kumar, P. and Singh, P., 2016. Tribulus terrestris ameliorates aluminium chloride induced alterations in oxidative status and functional markers in the liver, kidney, brain, and testis of the laboratory mouse. Indian J. Biochem. Biophys., 53: 179–186.
Li, M., Qu, W., Wang, Y., Wan, H. and Tian, C., 2002. Hypoglycemic effect of saponin from Tribulus terrestris. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater., 25: 420–422.
Lirette, A., Robinson, A., Lawson, P., Firth, N.L., Crober, D.C., Lawson, P.D., Firth, N.L. and Robinson, A.R., 1993. Effect of oat bran, cotton seed hulls and guar gum on chicken egg and blood lipids during the early laying period. Can. J. Anim. Sci., 73: 673–677. https://doi.org/10.4141/cjas93-074
Liu, S., Zhou, Q. and Wang, Y., 2011. Responses of the earthworm Eisenia fetida exposed to soil contaminated with HHCB. Chemosphere, 83: 1080–1086. https://doi.org/10.1016/j.chemosphere.2011.01.046
Maus, C., 2008. Ecotoxicological profile of the insecticide spirotetramat. Bayer Crop Sci. J., 61: 159–180.
Meri, Z.B., Irshid, I.B., Migdadi, M., Irshid, A.B., Mhanna, S.A. and Mhanna, S.A., 2013. Does cigarette smoking affect seminal fluid parameters? A comparative study. Oman med. J., 28: 12–15. https://doi.org/10.5001/omj.2013.03
Mitra, A. and Maitra, S.K., 2018. Reproductive toxicity of organophosphate pesticides. Annls clin. Toxicol., 1: 1–9.
Neychev, V. and Mitev, V., 2016. Pro-sexual and androgen enhancing effects of Tribulus terrestris L.: Fact or fiction. J. Ethnopharmacol., 179: 345–355. https://doi.org/10.1016/j.jep.2015.12.055
Neylanne, N., Muniz, P., Augusto, M., Félix, R., Costa, T., Pereira, S., Gustavo, L., Rocha, P., Miranda, J.R., Gilberto, M., Eduardo, J., Pereira, B., Kelly, S. and Bertolucci, V., 2015. Sperm quality and testicular histomorphometry of wistar rats supplemented with extract and fractions of fruit of Tribulus terrestris L. Braz. Arch. Biol. Technol., 58: 891–897. https://doi.org/10.1590/S1516-89132015060278
Oladele, A., Opeyemi, O., Eseigbe, I., Kehinde, A. and Abraham, O., 2016. Neuro-endocrine effects of aqueous extract of Amaranthus viridis (Linn.) leaf in male Wistar rat model of cyclophosphamide-induced reproductive toxicity. Toxicol. Rep., 3: 608–619. https://doi.org/10.1016/j.toxrep.2016.07.007
Pandey, S.P., Tsutsui, K. and Mohanty, B., 2017. Endocrine disrupting pesticides impair the neuroendocrine regulation of reproductive behaviors and secondary sexual characters of red munia (Amandava amandava). Physiol. Behav., 173: 15–22. https://doi.org/10.1016/j.physbeh.2017.01.030
Parton, R.G. and Hancock, J.F., 2004. Lipid rafts and plasma membrane microorganization: Insights from Ras. Trends Cell Biol., 14: 141–147. https://doi.org/10.1016/j.tcb.2004.02.001
Pavin, N.F., Izaguirry, A.P., Soares, M.B., Spiazzi, C.C., Sebastian, A., Mendez, L., Leivas, F.G., Brum, S., Weber, F. and Cibin, S., 2018. Tribulus terrestris protects against male reproductive damage induced by cyclophosphamide in mice. Oxidat. Med. Cell. Longevity, 2018: 9. https://doi.org/10.1155/2018/5758191
Poonam, S., UI-Huq, A. and Singh, R., 2013. Cypermethrin induced reproductive toxicity in male Wistar rats: Protective role of Tribulus. J. environ. Biol., 34: 857–862.
Rajendar, B., Bharavi, K., Rao, G.S., Kishore, P.V.S., Kumar, P.R., Kumar, C.S.V.S. and Patel, T.P., 2011. Protective effect of an aphrodisiac herb Tribulus terrestris Linn on cadmium-induced testicular damage. Indian J. Pharmacol., 43: 568–574. https://doi.org/10.4103/0253-7613.84974
Recio, R., Ocampo-Gómez, G., Morán-Martínez, J., Borja-Aburto, V., López-Cervantes, M., Uribe, M., Torres-Sánchez, L. and Cebrián, M.E., 2005. Pesticide exposure alters follicle-stimulating hormone levels in Mexican agricultural workers. Environ. Hlth. Perspect., 113: 1160–1163. https://doi.org/10.1289/ehp.7374
Safarnavadeh, T. and Rastegarpanah, M., 2011. Antioxidants and infertility treatment, the role of Satureja khuzestanica: A mini-systematic review. Iran. J. Reprod. Med., 9: 61–70.
Sanagoo, S., Sadeghzadeh, O.B., Gassab, A.N., Salehi-Pourmehr, H., Hazhir, N. and Farshbaf-Khalili, A., 2019. Effect of Tribulus terrestris L. on sperm parameters in men with idiopathic infertility: A systematic review. In: Complementary therapies in medicine. Churchill Livingstone. 42: 95–103. https://doi.org/10.1016/j.ctim.2018.09.015
Shahid, M., Riaz, M., Talpur, M.M. and Pirzada, T., 2016. Phytopharmacology of Tribulus terrestris. J. Biol. Regul. Homeost. Agents, 30: 785–788.
Shalaby, M.A. and Hammouda, A.A.E.K., 2014. Assessment of protective and anti- oxidant properties of Tribulus terrestris fruits against testicular toxicity in rats. J. Int. Ethnopharmacol., 3: 113–118. https://doi.org/10.5455/jice.20140627123443
Sharma, M., Arya, D., Bhagour, K. and Gupta, R.S., 2020. Androgenic and spermatogenic potential of methanolic extracts of Tribulus terrestris in reproductively disrupted male albino rats. Pl. Arch., 20: 1743–1747.
Simpson, K.A., Martin, N.M. and Bloom, S.R., 2008. Hypothalamic regulation of appetite. Expert Rev. Endocrinol. Metab., 3: 577–592. https://doi.org/10.1586/17446651.3.5.577
Singh, S., Nair, V. and Gupta, Y.K., 2012. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J. Pharmacol. Pharmacother., 3: 43–47. https://doi.org/10.4103/0976-500X.92512
Slimani, S., Boulakoud, M.S. and Abdennour, C., 2011. Pesticide exposure and reproductive biomarkers among male farmers from north-east Algeria. Annls biol. Res., 2: 290–297.
Slimani, S., Boulakoud, M.S., Abdennour, C. and Gueddah, D., 2014. Antracol administration has disturbed the reproductive cycle of domestic pigeon Columba livia domestica. Adv. environ. Biol., 8: 82–91.
Slimani, S., Hamouda, S., Souadi, C., Silini, S., Abdennour, C. and Delimi, L., 2018. The fungicide thiram may disrupt reproductive cycle of domestic male pigeon (Columba livia domestica) subjected to a long photoperiod. Pakistan J. Zool., 50: 1693–1701. https://doi.org/10.17582/journal.pjz/2018.50.5.1693.1701
Sun, B., Qu, W. and Bai, Z., 2003. The inhibitory effect of saponins from Tribulus terrestris on Bcap-37 breast cancer cell line in vitro. J. Chin. med. Mater., 26: 104–106.
Tian, C., Chang, Y., Zhang, Z., Wang, H., Xiao, S. and Cui, C., 2019. Heliyon extraction technology, component analysis, antioxidant, antibacterial, analgesic and anti-inflammatory activities of flavonoids fraction from Tribulus terrestris L. leaves. Heliyon, 5: e02234. https://doi.org/10.1016/j.heliyon.2019.e02234
Walker, W.H. and Cheng, J., 2005. FSH and testosterone signaling in Sertoli cells. Reproduction, 130: 15–28. https://doi.org/10.1530/rep.1.00358
Wingfield, J. and Farner, D., 1993. Endocrinology of reproduction in wild species. In: Avian biology (eds. D.S. Farner, J.R. King and K.C. Parkes). Academic Press, New York, pp. 163–327.
Wise, T., Young, L.D. and Pond, G., 1993. Reproductive, endocrine, and organ weight differences of swine selected for high or low serum cholestero. J. Anim. Sci., 71: 2732–2738. https://doi.org/10.2527/1993.71102732x
Yang, Y., Wu, T., He, K. and Fu, Z. G., 1999. Efffect of aerobic exercise and ginsenosides on lipid metabolism in diet-induced hyperlipidemia mice. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin., 20: 563–565.
Yokoyama, S., 2000. Release of cellular cholesterol: Molecular mechanism for cholesterol homeostasis in cells and in the body. Biochim. biophys. Acta Mol. Cell Biol. Lipids, 1529: 231–244. https://doi.org/10.1016/S1388-1981(00)00152-9
Zang, Z.J., Ji, S.Y., Dong, W., Zhang, Y.N. and Zhang, E.H., 2015. A herbal medicine, saikokaryukotsuboreito, improves serum testosterone levels and affects sexual behavior in old male mice. pp. 5538. https://doi.org/10.3109/13685538.2014.963042
Zhang, J., Qian, L., Wang, C., Teng, M., Duan, M., Zhou, Y., Chen, X., Bo, R., Wang, C. and Li, X., 2020a. Dysregulation of endocrine disruption, apoptosis and the transgenerational toxicity induced by spirotetramat. Chemosphere, 240: 124900. https://doi.org/10.1016/j.chemosphere.2019.124900
Zhu, W., Du, Y., Meng, H., Dong, Y. and Li, L., 2017. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. pp. 1–16. https://doi.org/10.1186/s13065-017-0289-x
To share on other social networks, click on any share button. What are these?









