Population Dynamics and Damage Threshold of Meloidogyne incognita to the Dinsire Hot Pepper Variety
Population Dynamics and Damage Threshold of Meloidogyne incognita to the Dinsire Hot Pepper Variety
Shiferaw Demissie Tola1, Diriba Muleta2, Fassil Assefa2 and Beira Hailu Meressa1*
1College of Agriculture and Veterinary Medicine, Jimma University P.O. Box 307, Jimma, Ethiopia; 2Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia.
Abstract | Predicting the damage caused by certain nematode population densities is crucial in deciding whether or not to cultivate pepper and selecting the most suitable management strategies. To understand the relationship between the initial nematode density (Pi) and the final nematode population (Pf), and the damage potential of Meloidogyne incognita to var. Dinsire, a study was conducted under greenhouse conditions. A geometric series of 13 initial densities (0, 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128 J2 (g soil)-1) of M. incognita was subjected to Dinsire. The treatments were arranged in a randomized complete design with four replications and terminated after 120 days of nematode inoculation. This study showed that the final nematode population, plant growth, and yields decreased as initial nematode inoculums increased. Maximum suppression fresh weight of shoot (43.8%) and fruit (52%) was recorded at Pi ≥ 8 J2 (g soil)-1. The analysis of Seinhorst’s yield loss model indicated the highest tolerance limit (T=0. 64 egg + J2 (g soil)-1) recorded for leaf number, while the relative minimum yield (m) of 0.91 and 0. 86 were the highest m values for root length and shoot height, respectively. Furthermore, the maximum multiplication rate (a) and population densities (M) were estimated as 8813.2 and 3420.1 (eggs and J2 (g soil)–1), respectively. Therefore, evaluating hot pepper varieties for resistance using a wide range of Pi could generate more reliable information on the host status of pepper varieties.
Received | August 23, 2023; Accepted | October 27, 2023; Published | November 13, 2023
*Correspondence | S.D. Tola and B.H. Meressa, College of Agriculture and Veterinary Medicine, Jimma University P.O. Box 307, Jimma, Ethiopia; Email: shiferaw.demissie@ju.edu.et, beira.hailu@ju.edu.et
Citation | Tola, S.D., Muleta, D., Assefa, F. and Meressa, B.H., 2023. Population dynamics and damage threshold of Meloidogyne incognita to the dinsire hot pepper variety. Pakistan Journal of Nematology, 41(2): 108-117.
DOI | https://dx.doi.org/10.17582/journal.pjn/2023/41.2.108.117
Keywords | Inoculums, Multiplication rate, Minimum relative yield, Pi, Tolerance limit
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Hot pepper cultivation is widespread in Ethiopia in dry and rainy seasons. However, the yield obtained is lower than the potential production of the country. From 2010/11 to 2021/22, pepper production decreased by 50% in Ethiopia (CSA, 2010-2022). This decline may be primarily due to soil-borne pathogens such as root-knot nematode (RKN) (Demissie et al., 2022). Several Meloidogyne spp., including M. incognita, M. arenaria, M. javanica, and M. hapla, are major threats to pepper production worldwide (Thies and Fery, 2000). Mandefro and Mekete (2002) highlighted the challenges of pepper production under the threat of RKN in Ethiopia.
Pepper production faces a significant challenge globally due to the emergence of virulent RKN, especially M. incognita races (Hajihassani et al., 2019; Putri et al., 2020). Bucki et al. (2017) have explained how virulent races of M. incognita populations infect pepper genotypes that carry the Me and N genes (R-genes), while Bommalinga et al. (2013) have determined that M. incognita infection can cause a reduction in pepper yield of more than 15%. Pepper plants infected by nematodes often display stunted growth due to the parasite’s utilization of water and nutrients from the plant. This results in poor root function that affects the plant’s ability to absorb minerals and water from the soil and significantly reducing in pepper yield. This is particularly true when the initial nematode population is high (Requena et al., 2011; Hu et al., 2020).
Predicting the damage caused to plant growth and yield is possible by examining the soil’s nematode density before planting crops (Hussain et al., 2011). The plant’s damage level usually depends on the host status, pathogen virulence, and environmental suitability (Fourie et al., 2010; Moosavi, 2015). Hence, the host’s response is the major driving force behind the nematode population’s dynamicity (Norton et al., 1989).
Population dynamics refers to changes in nematode densities over a period regulated by biotic and abiotic factors (Blasco, 2016; Daramola et al., 2021). When there is sufficient food and favorable conditions, the initial (Pi) and final (Pf) nematode populations have a direct proportionate relationship with the presence of susceptible or tolerant hosts (Seinhorst, 1968; Blasco, 2016). However, when Pi becomes too high and reaches the maximum carrying capacity of the root system, both Pf and the maximum multiplication rate (a) decline due to scarcity of food caused by intraspecific competition among the nematode population (Seinhorst, 1968).
The relationship between Pi and Pf was modeled to estimate the maximum multiplication rate (a) at a lower Pi value and the maximum population density (M) at a higher Pi value of Meloidogyne spp. on crops using Seinhorst’s population dynamics model (Seinhorst, 1968; Teklu et al., 2014). Generally, the information obtained from the population dynamics of M. incognita and the yield loss during modeling helps design effective nematode management strategies and extrapolate to other nematode species and vegetables.
Ahmed et al. (2013) demonstrated that the population densities of M. incognita increased from 250-8000 J2 per plant, resulting in an increase in dry weight reduction in chili (C. annuum) from 1.6 -43.9%. Similarly, Di Vito et al. (1992) found that resistant cultivars of sweet peppers were reduced by 50% at the Pi of M. incognita ≥ 32 J2 (g soil)-1. Studying nematode population dynamics has multiple benefits, including reducing unnecessary agrochemical inputs due to pre-plant information about nematode density in soil and determining the exact level of host resistance to their respective pathogens and damage threshold. Therefore, this study aims to determine i) the responses of var. Dinsire to a series of M. incognita initial inoculums, and ii) the population dynamics of M. incognita on var. Dinsire and damage threshold.
Materials and Methods
Preparation of hot pepper seedlings and nematode inoculums
The pepper variety ‘Dinsire’ seeds, which performed well against RKN in the previous experiment, were obtained from the Bako Agriculture Research Centre. The pepper seeds were planted in germination trays filled with sterilized sand soil and kept under greenhouse conditions with a minimum and maximum temperature of 14.1 and 39.6oC, respectively, with relative humidity (%) between 33.8 and 99.9 (TESTO 445, Ace Instruments, Germany). The plants had a 12-hour light and dark period and were watered with 10 ml of tap water every morning.
Meloidogyne incognita was previously isolated from the major pepper-growing areas of the Jimma Zone and identified as a significant pest of pepper (Demissie et al., 2022). It was multiplied on a susceptible hot pepper variety rootstock (Bako local) under greenhouse conditions and used as a source of inoculum.
Transplanting and inoculation of pepper seedlings
Seedlings were transplanted at the stages of four true leaves into 1l plastic pots filled with oven-sterilized sandy soil, a total of 13 treatments involved with four replications for each treatment and arranged in a completely randomized design (CRD) on raised benches in the greenhouse. A day before inoculation, the nematode suspension was left at room temperature, and the volume of the stock suspension was adjusted to the highest density required. A log series of 2x (i.e. x an integer ranging from -4 to 7) or (0, 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128 J2 (g soil)-1) was prepared by making a serial dilution from the stock suspensions, accordingly. Then, a 10 ml suspension containing the respective nematode’s initial inoculum densities was inoculated into a three-day post-transplanted pepper plant by pipetting it into four holes made of a rod metal around the stem of each plant. Control plants without M. incognita received a similar volume of tap water (Moosavi, 2015). A net fibre was placed over the pot’s top surface to prevent water loss, as described by Ehwaeti et al. (1998). The plants were watered with 60-70 ml of tap water. Each plant was fertilized with 0.3 g of NPSB monthly for three months. The experiment was terminated after 120 days of nematode inoculation.
Measurements of plant parameters and modeling
After 120 days of nematode inoculation, the plants were harvested, and the following measurements were taken: plant height (cm), fruit, shoot and root length, and fruit diameter. The number of leaves, branches, flowers, and fruits per plant were also recorded. The fresh weight (g) of fruit, shoot, and root was weighed. Additionally, the impact of M. incognita initial population on plant growth parameters was modeled. For every nematode density, before fitting the Seinhorst yield loss model to the data of plant growth and yield components, each measurement was averaged over the replicates of each treatment and computed using the Seinhorst Equation (Seinhorst, 1998). Furthermore, standard errors were calculated for each parameter, and for every measurement, the goodness of fit was estimated using the coefficient of determination (R2).
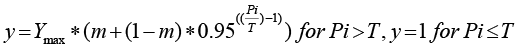
Where: y = plant height (cm), fresh weight (g), plant parts (in number). Ymax = yield when Pi ~ 0; m = relative minimum yield when Pi ~ ∞.
T = Tolerance limit, the nematode density above which yield starts to decline.
Nematode population parameters and modeling of population dynamics
At harvest, the pepper plant was carefully uprooted from each pot, shaken gently to remove rhizosphere soil, and slowly washed using tap water to avoid the soil attached to the roots. After cutting the roots into 2-3 cm, root galls were counted under a stereo-microscope, and the final nematode population was estimated from the entire root system and soil in the pot at harvest. Eggs and J2 were extracted from the root using a 0.51% NaOCl solution. Whereas the modified Baermann tray method was used to extract vermiform nematode from aliquots of 100 ml soil (Hooper et al., 2005). After concentrating the extracted nematodes in a 100 ml graduated cylinder, the densities of nematodes per plant were assessed from repeated 1 ml aliquots under a light compound microscope (100×) (A. KRUSS OPTRONIC, Germany). The reproduction factor was determined at all initial inoculum levels (Greco and Di Vito, 2009). The equation below was used to model the relation between Pi and Pf and estimate the maximum multiplication rate (a) as well as the maximum population density (M) of M. incognita on hot pepper (Teklu et al., 2014).
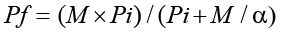
Statistical analysis and modeling
The data of plant measurements, such as leaf, branch, flower, fruit number, root, shoot length, and fresh weight, along with nematode parameters, including the number of galls, Pf, and RF, were analyzed using SAS version 9.3 through analysis of variance (ANOVA). Additionally, R version 4.2.1 and R studio 2022.07.0+548 were used for fitting the model to the data.
Results and Discussion
Impacts of M. incognita initial population on pepper growth and yield component
Pepper plants were established and maintained for evaluation up to harvesting. After 30 days of nematode inoculation, the plants exhibited poor growth performance with above-ground symptoms such as yellowing of leaves, stunted growth, and delayed development of reproductive structures, particularly in plants inoculated with higher inoculum densities (Figure 1). However, var. Dinsire previously had vigorous growth when inoculated with 2000 J2 of M. incognita (unpublished data), which could be attributed to the influence of J2 infectivity and environmental factors (Thies, 2011). Pepper plants inoculated with a low initial nematode population showed even greater growth and yield than the uninfected control plants (Figures 1 and 2). This has been explained that plants produce auxin-like substances during mild nematode infections thereby increasing growth (Greco and Di Vito, 2009).
An increase in nematode Pi, resulted in a significant reduction of plant growth and nematode multiplication, particularly when Pi ≥ 8 J2 (g soil)-1 in which the number of branches, shoot length, shoot fresh weight, fruit number, fruit length, and fruit fresh weight were reduced by 63%, 28%, 44%, 54%, 24%, and 52%, respectively (Table 1). Similarly, Moosavi (2015) indicated the high suppression above-ground part of bell pepper at Pi of M. javanica ≥ 8 eggs and J2 (g soil) -1. Aguiar et al. (2014) reported a 40 and 55% reduction in the fruit weight of resistance (cv. Charleston Belle) and susceptible bell pepper (cv. sweet mini pepper), respectively, when infected with 1.2 J2 (g soil)-1 of M. incognita. The delay in flowering might be responsible for the reduction in fruit weight in this study. Moosavi (2015) has also found both a bell pepper’s growth and yield were suppressed by 50% when the value of Pi was equal to 7 and 9.3 eggs and J2 (g soil)-1 of M. javanica, correspondingly. Similarly, a growth suppression of M. incognita on susceptible sweet peppers (cv. Yolo Wonder) by 84% and 50% on the resistant genotypes at the Pi ≥ 32 J2 (g soil)-1 was reported (Di Vito et al., 1992).
Tolerance limit (T) and relative minimum yield (m)
The mean of each plant growth parameter and yield components plotted against the Pi and Seinhorst model fitted to their mean values that Pi and plant growth components were negatively correlated (Figure 3). Nevertheless, the flower number appeared to increase due to the nematodes’ initial population density, causing the flowering time delay (Figure 3E). Estimated model parameter values for plant growth parameters and yield components are displayed in Table 2. The yield components, such as fruit diameter, weight, number, and length exhibited a tolerance limit of 0.232, 0.183, 0.181, and 0.139 J2 (g soil)-1, respectively, whereas from growth components the lowest tolerance limit registered for root (T = 0.074) and shoot (T = 0. 111) (Figure 3 and Table 2). On the other hand, at the highest inoculums level of M. incognita (Pi = 128 J2(g soil)-1), the lowest relative minimum yield was recorded for fresh fruit weight (0. 59, i.e. ~ 41% fruit yield loss) and branch number (0.61, ~ 39% branch number loss). Presently observed fruit yield loss in var. Dinsire was less than the former report on var. Melka Awaze (54% yield loss) due to M. incognita infection (Abegaz, 2020), also Moosavi (2015) illustrated M. javanica caused the loss of bell pepper yield by 84%.This indicate that Dinsire can tolerate higher inoculum of nematode. The root length was less affected; its damage threshold was detected only when the Pi exceeded 0.074 J2 (g soil)-1.
Using the value of Ymax, the influence of nematode on pepper growth parameters and yield component were compared, of which leaf number (LN) was less affected in the presence of the lowest initial inoculum densities of nematodes in that 98.5, 88.8, 52.1, and 45.1 as maximum (Ymax) leaf numbers, total fresh weight (g), shoot height (cm) and weight (g) were predicted, subsequently, nevertheless 1.2 (cm) and 1.4 as measured as the lowest Ymax of fruit diameter and flower number, respectively (Table 2). This implied that the pressure of Pi is more pronounced on yield components of crops.
Table 2: Parameter values for Seinhorst equation-1 for the relation between initial population density (Pi) of M. incognita and pepper growth and yield. Pi and T are expressed in M.incognita (g dry soil)-1, but y and m are proportions, and Ymax is expressed as a respective S.I. unit.
|
Plant parameters |
Seinhorst variable (Equation), Y= m+(1-m) zPi-T, Pi >T, y = 1, Pi ≤T |
|||||||
|
T |
m |
Ymax |
SET |
SEm |
SEymax |
R2 |
DF |
|
|
Fresh fruit weight |
0.183 |
0.591 |
18.15 |
0.092 |
0.072 |
1.09 |
0.723 |
10 |
|
Fresh shoot weight |
0.195 |
0.7267 |
45.08 |
0.113 |
0.065 |
2.25 |
0.608 |
10 |
|
Total fresh weight |
0.267 |
0.671 |
88.78 |
0.137 |
0.066 |
4.29 |
0.691 |
10 |
|
Flower number |
0.427 |
4.84 |
1.43 |
0.244 |
3.04 |
0.872 |
0.667 |
9 |
|
Fruit number |
0.181 |
0.765 |
13.35 |
0.204 |
0.151 |
1.60 |
0.071 |
9 |
|
Branch number |
0.066 |
0.612 |
17.71 |
0.106 |
0.104 |
2.71 |
0.492 |
9 |
|
Leaf number |
0.64 |
0.714 |
98.493 |
0.324 |
0.046 |
2.81 |
0.828 |
9 |
|
Fruit diameter |
0.232 |
0.695 |
1.19 |
0.128 |
0.069 |
0.07 |
0.642 |
9 |
|
Fruit length |
0.139 |
0.809 |
3.03 |
0.145 |
0.068 |
0.18 |
0.405 |
9 |
|
Shoot height |
0.111 |
0.864 |
52.11 |
0.092 |
0.05 |
2.24 |
0.395 |
9 |
|
Root length |
0.074 |
0.905 |
21.51 |
0.111 |
0.047 |
0.955 |
0.245 |
9 |
T, tolerance limit; m, relative minimum; Ymax, the yield at densities lower than T; SET, standard error of tolerance limit; SEm, standard error of minimum yield; SEymax, standard error of SEymax; R2, coefficient of determination; DF., Degree of Freedom.
The T value of Dinsire to M. incognita for shoot height (T= 0.11, m= 0.86), fruit length (T= 0.14, m= 0.81), fruit number (T= 0.18, m= 0.77) and fruit weight (T= 0.18, m=0.59) were less than the report of Abegaz (2020) on var. Melka Awaze was considered resistant to this nematode, while Dinsire recorded higher m values for shoot height, fruit length, fruit length, and fruit weight at the highest initial population density of the nematode. Di Vito et al. (1985) report showed a T of 0.17 J2 (g soil)-1 to M. incognita as the registered tolerance limit for sweet pepper fruit weight. Similarly, Di Vito et al. (1992) reported 0.3 J2 (g soil)-1 of M. incognita as the tolerance limit of yield for susceptible sweet pepper cultivars. The m value of a sweet pepper resistance cultivar (Di Vito et al., 1992) and ‘Dinsire’ in this study were similar for fruit yields. Ferris et al. (1986) also recorded a tolerance limit and minimum yield of chili pepper for yield (T= 0. 039; m = 0.001) to the M. incognita.
The presence of variations in tolerance limits and relative minimum yields could be attributed to genotypes, level of virulence among nematode races and populations, the way inoculum preparation, infestation techniques, time, soil type, and environmental conditions (Di Vito et al., 1986; Ravichandra, 2014; Moosavi, 2015). Thus, determining the host responses to its major pathogen in a given area is an input for choosing the appropriate management option. The presented high minimum yield at the highest Pi of M. incognita in all growth parameters and yield components in Dinsire, as well as the establishment and demonstrated good growth performance up to a Pi of less than 8 J2 (g soil)-1 was obtained. This is greater than the highest mean Meloidogyne sp. density (6 J2 (g soil)-1) recorded from major pepper-producing fields around the Jimma area (Demissie et al., 2022). Therefore, var. Dinsire can be cultivated with minimum damage in areas where the mean density of M. incognita is less than 8 J2 g soil.
Population dynamics
Using the population dynamics model, Pi fitted to the mean values of Pf and the var. Dinsire is shown to be a suitable host for M. incognita. The direct relationship between the initial and final nematode population and gall numbers was only depicted up to Pi ≤ 4 (Table 1 and Figure 4). An increase in Pi was inversely related to its reproduction factor (RF), in which 9288 (i.e. able to be multiplied by 9288- fold from its initial population densities) was recorded as the highest reproductive factor at Pi =0.125 J2 (g soil)-1, and the lowest RF of 5.2 at the highest Pi (128 J2 (g soil)-1) (Table 1 and Figure 4). Furthermore, 8813.2 and 3420.1 (eggs + J2 (g soil)-1) were estimated as the maximum reproduction rate (a) and maximum population density (M) of M. incognita on var. Dinsire, respectively (sea1=8432.1; R2 = 0.76; df= 10). The gall number increased on Dinsire, as Pi raised from 0.0625 to 4 J2 (g soil)-1. The lowest gall number recorded at Pi = 32 J2 (g soil)-1 was 562 in contrast to 1590 determined at Pi = 0.5 J2 (g soil)-1 (Figure 4). For M. javanica, a reproductive factor value of 496 was recorded at a Pi =0.125 J2 (g soil)-1 on bell pepper (Moosavi, 2015).
Dinsire supported the multiplication of M. incognita at all Pi and its population dynamics curve lies above the equilibrium line (Figure 4) that the plant was able to satisfy the demand by the nematode for its reproduction. Previous studies indicated pepper cultivars at primary screening identified as resistant using single inoculum density of M. incognita (0.67 J2/ g of soil) became susceptible (1 J2 (g soil)-1) when subjected to various initial inoculum levels of M.incognita (Thies, 2011; Abegaz, 2020). Thus, exposing the host plant to various inoculum levels of nematodes is the best alternative for determining the host status before handover to growers. Only a few pepper varieties have been reported to be resistant to M. incognita (Djian-Caporalino, 2011; Agaba and Fawole, 2015).
A decrease in the final nematode population, reproduction rate, maximum population densities, and gall formation was revealed with an increase in Pi. Lindsey and Clayshulte (1982) remarked on the reduction of M. incognita reproduction at higher inoculum levels of nematodes attributed to more damage to the host root. As a result, the plant becomes stunted, reducing the nematode reproduction rate due to limitations in food and space supplies (Di Vito et al., 1986, 1992).
The maximum multiplication rate (8813.2) obtained in this study is greater than previously reported values of M. incognita on pepper varieties (Di Vito et al., 1986, 1992; Ferris et al., 1986; Abegaz, 2020) which might be due to difference in the host, pathogenicity, method of nematode extraction and duration or extracted time (Teklu et al., 2016).
Conclusions and Recommendations
The var. Dinsire supported the multiplication of M. incognita along a series of all initial inoculum levels. Thus, evaluating pepper genotype to various levels of initial nematode density is more appropriate than single inoculum density to determine the host status before being recommended for end users. The considerable minimum yield in all plant growth parameters and components revealed that Dinsire may be cultivated in pepper fields infested with M. incognita or as part of IPM components.
Acknowledgments
This work was financially supported by the Ministry of Education of Ethiopia and Jimma University. We also thank Bako Agricultural Research Center for providing pepper seeds and Dr Misghina Teklu for collaborating on the model fitting.
Novelty Statement
The study evaluated pepper resistance to M. incognita and its impact on plant growth and yield, which found Dinsire, can tolerate high initial nematode density. The results can also be extrapolated to other nematode species and crops, making it useful for pepper growers and researchers.
Author’s Contribution
All authors have contributed to the work. SDT and BHM designed the study. SDT conducted the experiment, collected and analysed the data, and prepared the manuscript draft. All authors reviewed the manuscript and approved its final version.
Funding
This work was supported by the Ethiopian Ministry of Education and Jimma University.
Availability of data and material
Raw data were generated at Jimma University and are available upon request from the corresponding authors.
Conflict of interest
The authors have declared no conflict of interest.
References
Abegaz, B., 2020. Management of Meloidogyne incognita and M. javanica populations using host resistance, botanicals, and organic amendments on hot pepper (Capsicum annum and C. Frutescens) genotypes in Ethiopia. PhD dissertation, Haramaya University, Haramaya, Ethiopia.
Agaba, T.A. and Fawole, B., 2015. Screening of some pepper cultivars for resistance to Meloidogyne incognita (Chitwood). Int. J. Food Agric. Vet. Sci., 5(3): 23-29.
Aguiar, J.L., Bachie, O. and Ploeg, A., 2014. Response of resistant and susceptible bell pepper (Capsicum annuum) to a southern California Meloidogyne incognita population from a commercial bell pepper field. J. Nematol., 46(4): 346-351.
Ahmed, D., Shahab, S. and Safiuddin, 2013. Pathogenic potential of root-knot nematode Meloidogyne incognita and root-rot fungus Fusarium solani on chilli (Capsicum annuum L.). Arch. Phytopathol. Plant Prot., 46(18): 2182-2190. https://doi.org/10.1080/03235408.2013.787750
Blasco, A.G., 2016. Population dynamics of Meloidogyne spp. on tomato and cucumber and biologically-based management strategies. PhD dissertation, Universitat Politècnica de Catalunya Barcelona Tech, Catalonia, Spain.
Bommalinga, S., Narasimhamurthy, T.N., Prahalada, G.D. and Reddy, B.M.R., 2013. Screening of bell pepper cultivars against root-knot nematode Meloidogyne incognita [(kofoid and white) Chitwood]. Int. J. Life Sci. Biotechnol. Pharm. Res., 2(1): 225-228.
Bucki, P., Paran, I., Ozalvo, R., Iberkleid, I., Ganot, L. and Braun Miyara, S., 2017. Pathogenic variability of Meloidogyne incognita populations occurring in pepper-production greenhouses in Israel toward Me1, Me3 and N pepper resistance genes. Plant Dis., 101(8): 1391-1401. https://doi.org/10.1094/PDIS-11-16-1667-RE
CSA, 2010-2022. Report on area and production of major crops, agricultural sample survey, the Federal Democratic Republic of Ethiopia, Volume I, Addis Ababa., Ethiopia.
Daramola, F.Y., Malgas, R. and Malan, A.P., 2021. Occurrence and seasonal changes in the population of root-knot nematodes on Honeybush (sp.). Helminthologia, 58(2): 202-212. https://doi.org/10.2478/helm-2021-0018
Demissie, S., Meressa, B.H., Muleta, D. and Assefa, F., 2022. Biodiversity of parasitic nematodes associated with hot pepper (Capsicum spp.) in Jimma area, Ethiopia. Russ. J. NematoI., 30 (2): 161-173.
Di Vito, M., Cianciotta, V. and Zaccheo, G., 1992. Yield of susceptible and resistant pepper in microplots infested with Meloidogyne incognita. Nematropica, 22: 1-6.
Di Vito, M., Greco, N. and Carella, A., 1985. Population densities of Meloidogyne incognita and yield of Capsicum annuum. J. Nematol., 17(1): 45-49.
Di Vito, M., Greco, N. and Carella, A., 1986. Effect of Meloidogyne incognita and importance of the inoculum on the yield of eggplant. J. Nematol., 18(4): 487-490.
Djian-Caporalino, C., Molinari, S., Palloix, A., Ciancio, A., Fazari, A., Marteu, N., Ris, N. and Castagnone-Sereno, P., 2011. The reproductive potential of the root-knot nematode Meloidogyne incognita is affected by selection for virulence against major resistance genes from tomato and pepper. Eur. J. Plant Pathol., 131: 431-440. https://doi.org/10.1007/s10658-011-9820-4
Ehwaeti, M.E., Phillips, M.S. and Trudgill, D.L., 1998. Dynamics of damage to tomato by Meloidogyne incognita. Fundam. Appl. Nematol., 21(5): 627-635.
Ferris, H., Ball, D., Beem, L. and Gudmundson, L., 1986. Using nematode count data in crop management decisions. Calif. Agric., 40(1): 12-14.
Fourie, H., Mc Donald, A.H. and De Waele, D., 2010. Relationships between initial population densities of Meloidogyne incognita race 2 and nematode population development in terms of variable soybean resistance. J. Nematol., 42(1): 55–61.
Greco, N. and Di Vito, M., 2009. Population dynamics and damage levels. In: Perry ,R.N., Moens, M. and Starr, J.L. (eds.). Root-knot nematodes. CABI. UK. pp. 246-274. https://doi.org/10.1079/9781845934927.0246
Hajihassani, A., Rutter, W.B. and Luo, X., 2019. Resistant pepper carrying N, Me1, and Me3 have different effects on penetration and reproduction of four major Meloidogyne species. J. Nematol., 51: e2019-20. https://doi.org/10.21307/jofnem-2019-020
Hooper, D.J., Hallmann, J. and Subbotin, S.A., 2005. Methods of extraction, processing and detection of plant a soil nematode. In: Luc, M., Sikora, R.A. and Bridge, J. (eds). Plant-Parasitic Nematodes in Subtropical and Tropical Agriculture. CABI. UK. pp. 53-86. https://doi.org/10.1079/9780851997278.0053
Hu, W., Kingsbury, K., Mishra, S. and DiGennaro, P., 2020. A comprehensive transcriptional profiling of pepper responses to root-knot nematode. Genes, 11(12): 1507-15021. https://doi.org/10.3390/genes11121507
Hussain, M.A., Mukhtar, T. and Kayani, M.Z., 2011. Assessment of the damage caused by Meloidogyne incognita on Okra (Abelmoschus esculentus). J. Anim. Plant Sci., 21(4): 857-861.
Lindsey, D.L. and Clayshulte, M.S., 1982. Influence of initial inoculum densities of Meloidogyne incognita on three chile cultivars. J. Nematol., 14: 353-358.
Mandefro, W. and Mekete T., 2002. Root-knot nematodes on vegetable crops in central and western Ethiopia. Pest Mgt. J. Eth., 6: 37-44.
Moosavi, M.R., 2015. Damage of the root-knot nematode Meloidogyne javanica to bell pepper, Capsicum annuum. J. Plant Dis. Prot., 122 (5/6): 244-249. https://doi.org/10.1007/BF03356559
Norton, D.C., 1989. Abiotic soil factors and plant-parasitic nematode communities. J. Nematol., 21(3): 299-307.
Putri, B.R., Santoso, I. and Yasman, Y., 2020. Antagonistic potential of Bacillus siamensis LDR against Aspergillus niger ABP and ART. In AIP conference proceedings, AIP Publishing LLC. 2242(1): 050017. https://doi.org/10.1063/5.0012314
Ravichandra, N.G., 2014. Nematode population threshold levels. In: Ravichandra, N.G. (ed.). Horticultural nematology. Springer, Dordrecht. Netherlands. pp. 101. https://doi.org/10.1007/978-81-322-1841-8_6
Requena, M.E. and Egea-Gilabert, C., 2011. Host-pathogen interaction of root-knot nematode Meloidogyne incognita on pepper in the southeast of Spain. Eur. J. Plant Pathol., 131: 511-518. https://doi.org/10.1007/s10658-011-9826-y
Seinhorst, J.W., 1968. The relationships between population increase and population density in plant parasitic nematodes V. Influence of damage to the host on multiplication. Nematologica, 13: 481-492. https://doi.org/10.1163/187529267X00265
Seinhorst, J.W., 1998. The common relation between population density and plant weight in pot and microplot experiments with various nematode plant combinations. Fundam. Appl. Nematol., 21(5): 459-468.
Teklu, M.G., Meressa, B.H., Radtke, E., Been, T.H. and Hallmann, J., 2016. Damage thresholds and population dynamics of Pratylenchus penetrans on carrot (Daucus carota L. cv. Nerac) at three different seed densities. Eur. J. Plant Pathol., 146: 117-127. https://doi.org/10.1007/s10658-016-0898-6
Teklu, M.G., Schomaker, C.H. and Been, T.H., 2014. Relative susceptibilities of five fodder radish varieties (Raphanus sativus var. oleiformis) to Meloidogyne chitwoodi. Nematol, 16(5): 577-590. https://doi.org/10.1163/15685411-00002789
Thies, J.A. and Fery, R.L., 2000. Characterization of resistance conferred by the N gene to Meloidogyne arenaria races 1 and 2, M. hapla, and M. javanica in two sets of isogenic lines of Capsicum annuum L. J. Am. Soc. Hortic. Sci., 125(1): 71-75. https://doi.org/10.21273/JASHS.125.1.71
Thies, J.A., 2011. Virulence of Meloidogyne incognita to expression of N gene in pepper. J. Nematol., 43(2): 90-94.
To share on other social networks, click on any share button. What are these?





