Occurrence and Histopathology of Henneguya sp. (Cnidaria: Myxozoa) in the Air Breathing Organ of the Sharptooth Fish Clarias gariepinus (Clariidae)
Research Article
Occurrence and Histopathology of Henneguya sp. (Cnidaria: Myxozoa) in the Air Breathing Organ of the Sharptooth Fish Clarias gariepinus (Clariidae)
Nounagnon Darius Tossavi1,2*, Mariette Sindété3, Nike Fumilayo Aladetohun4, Marie-Line Escande5, Adam Gbankoto3
1Unité de Recherche en Aquaculture et de Gestion des Pêcheries, Ecole d’Aquaculture, Université Nationale d’Agriculture, BP: 55 Porto-Novo, Bénin; 2Laboratoire de Parasitologie et Ecologie Parasitaire, Département de Zoologie, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, 01 BP 526 Cotonou, Bénin; 3Laboratoire de Physiologie et Pharmacologie Expérimentales, Département de Physiologie Animale, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, 01 BP 526 Cotonou, Bénin; 4Department of Fisheries Technology, Federal College of Fisheries and Marine Technology, Ahmadu Bello way, Lagos, Nigeria; 5Observatoire Océanologique de Banyuls, UPMC, Université Paris 06, UMR 7232, Avenue Fontaulé, F-66650 Banyuls-sur-Mer, France.
Abstract | For around 49 fish species, respiration is partly ensured by a specific organ called Air Breathing Organ (ABO). ABO replaces fish gills in hypoxic conditions. The present study is dedicated to investigate the occurrence and potential physiological effects due to a myxosporean parasite infecting ABO in Clarias gariepinus. To achieve this goal, a total of 339 C. gariepinus was investigated for myxosporean infection. Fish sex, size and weight were recorded and light and electron microscopes were used to identify the parasite and to enlighten the physiological impacts. The prevalence was accessed as a function of fish size and climatic seasons. Water physicochemical parameters were collected and its correlation with the prevalence of infection was assessed. The mean prevalence was 10.91% (37/339). No significant difference was observed either between males and females (χ2=0.136; df=3; p=0.987) or between seasons (χ2=1.772; df = 3; p=0.621). However, significant difference was recorded between fish class weight (χ2=11.781; df = 5; p=0.038). Longitudinal and transverse sections in the ABO revealed cysts establishment in the elastic cartilage of ABO and its cartilage erosion. This leads to limitation of fish’s ability to exchange air gases by reduction the folds. Therefore, a crucial attention could be paid to this parasite and develop effective control measures to mitigate its impact on fish health and production.
Keywords | Clarias gariepinus, Suprabranchial organ, Prevalence, Electron microscope, Pathology, Physicochemical parameters
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | November 07, 2024; Accepted | December 24, 2025; Published | December 27, 2024
*Correspondence | Nounagnon Darius Tossavi, Unité de Recherche en Aquaculture et de Gestion des Pêcheries, Ecole d’Aquaculture, Université Nationale d’Agriculture, BP: 55 Porto-Novo, Bénin; Email: tndarius@yahoo.com
Citation | Tossavi ND, Sindété M, Aladetohun NF, Escande M-L, Gbankoto A (2024). Occurrence and histopathology of Henneguya sp. (Cnidaria: Myxozoa) in the air breathing organ of the Sharptooth fish Clarias gariepinus (Clariidae). J. Adv. Parasitol. 11: 31-39.
DOI | https://dx.doi.org/10.17582/journal.jap/2024/11.31.39
ISSN | 2311-4096
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Animal protein contains essential amino acids that are important for a healthy and well-balanced human diet (Elmadfa and Meyer, 2017). Free-ranging land animals are no longer a significant source of protein, and the production costs of farm-raised animals continue to increase as land becomes more expensive (Guo and Woo, 2009). Fish is a more or less complete food because it contains fat, minerals, oils, and vitamins (Ayanda, 2009), as well as easily digestible proteins (Guo and Woo, 2009). Fish makes up almost 20% of animal proteins worldwide for about 3 billion people and approximately 15% of these same proteins for 4.3 billion people (FAO, 2012). Fish culture is consequently a good option, as production costs are lower over time, representing the best alternative to fill the gap between natural fish catch and the estimated needs of consumers in animal protein consumption. Aquaculture is an important socio-economic sector, especially for rural communities, contributing to livelihoods, food security, and poverty alleviation (Edwards et al., 2002).
Clarias gariepinus Burchell, 1822 (Clariidae) is one of the species possessing a pair of suprabranchial chambers located in the dorsal-posterior part of the branchial cavity, having extensions from the upper parts of the second and fourth gill arches, forming the arborescent organs. This structure is an air-breathing organ (ABO) that could allow this fish species for atmospheric breathing (Teugels, 2011). Beyond the abundant mucus production as a living adaptation in adverse environments (Donnelly, 1973), C. gariepinus developed an ability to move between environments and bury itself during drought (Cambray, 2003). Considering their capacity to access abundant oxygen in the air, it appears that fish that breathe atmospheric air grow faster than non-air-breathing species (Lefèvre et al., 2014). Besides, for more decades, Lazard and Legendre (1994) claimed its economic importance. For those reasons, C. gariepinus can be cultivated in regular farming conditions or at slightly higher densities (Awe, 2017). Nkamigbo et al. (2014) have therefore estimated for two or three times the value of Clarids more than Tilapia’s. Therefore, Pasch and Palm (2021) suggested that C. gariepinus farming is sustainable and economically profitable aquaculture.
However, C. gariepinus could be heavily infected by myxosporean parasites, especially by Henneguya species, like any other fish. This requires great care to prevent parasites that can induce diseases and affect its production, particularly in the cases of increased fish farm activities (Michel, 1989; Meyer, 1991; Bondad-Reantaso et al., 2005). The Myxozoan phylum brings together the most common parasites of fish (Schmahl et al., 1989; Feist and Longshaw, 2006). The genus Henneguya is the third most diverse in myxozoan species (Lom and Dyková, 2006), and numerous species have been reported infecting wild and farmed fish in many regions around the world (Azevedo and Matos, 2003; Martins and Onaka, 2006; Eiras et al., 2008; Tossavi et al., 2015) with considerable economic impact (McCraren et al., 1975; Yokoyama et al., 2003). Recently, there are reports on myxozaon infection from a diversity of fish species. Gbankoto et al. (2015) and Sokolov et al. (2019) recorded gonad infection by Myxobolus sp. and Hennegya testicularis, respectively. A high mortality rate was shown in hybrid tilapia infected by Myxobolus bejeranoi n. sp. (Lovy et al. 2018). In the cultured Lates calcarifer, Brkhanuddin et al. (2020) revealed a single henneguyosis infection and attested Henneguya is currently a problem for the fish. Moreover, in infected gill filaments, the plasmodia caused swelling or deformation and reduction in respiratory surface area by compression of lamellae (Correya et al., 2021).
In fish, a best localization of Henneguya species is gills as the main site of gas exchange in almost all fishes (Dinh-Hung, 2022). Zayed and Mohamed (2004) assured that the gill surface area is greater in the Nile tilapia than in C. gariepinus, whereas it can survive for a long period either in the hypoxic water or outside the aquatic environment (Chandra and Banerjee, 2004) due to ABO. As gills, ABO is frequently known to be infested by pathological species of Henneguya. Although the process of accurately identifying a Henneguya species recorded in ABO is still ongoing, some interesting information has already been gathered using light and electron microscopy to investigate its morphology and the pathology that it produces. The aim of this study was to assess the prevalence of the parasite and its potential damages on the ABO. The correlation of the infection rate of this parasite with the sex and weight of C. gariepinus was also investigated.
MATERIALS AND METHODS
Study area, fish collection and water quality
From November 2011 to December 2012, specimens of C. gariepinus were bimonthly sampled from Agonlin-Lowé (Adjohoun) located in latitude 06° 39′ N and longitude 02° 28′ E (Figure 1). It represents the middle sector of the Ouémé valley where the climate is subequatorial with a long wet season (LWS) from April to July followed with a short dry season (SDS) from August to September and a long dry season (LDS) for only October and November and finally the short wet season from December to March. The atmospheric temperature is running from 27.8 to 31.4°C. After collection, fish were then carried to the laboratory. Every fish length, weight and sex were recorded. Physicochemical parameters including pH, temperature (T°C), and Dissolved Oxygen (O2), were measured in-situ using a pH-meter multiparameter pH/Oxi340i/SET. Then, water samples were collected in bottles covered with aluminium foil in order to assess the concentrations for Nitrites (NO2), Nitrates (NO3), Ammonium (NH4), orthophosphate (PO4), and suspend solids (SS).
Parasite observation, histology and electron microscopy analysis
Fish were euthanized using Tricaine methanesulfonate (MS-222) for dissection (Anon, 2020). The ABO was delicately removed from the dorsal-posterior chamber of the branchial system. The cysts were carefully cut from the organ and placed in a saline solution. When a cyst burst, a drop of fresh myxospores was observed using the light microscope (Optim Microscope Jeulin, model n° 571 006) and photographed. Based on the spore morphology and morphometric data including polar capsule length and spore total length and spore body length recorded following Lom and Arthur (1989), tissue tropism other available species, we concluded parasite has not been appreciated as a novel species. It should be recognized as Henneguya sp. as the molecular techniques will be employed.
Any smears containing spores were stained with May-Grünwald Giemsa solution. Then, the smears were secured using Canada Balsam medium. The process for histological analysis and transmission microscopy are as described by Tossavi et al. (2015).
Data analyses
The prevalence of infection was determined according to climatic seasons following the formula below. The probable influence of the water quality on the prevalence was assessed using the Pearson correlation coefficient. The chi-square test (χ2) was used to prove the variability between male and female infections on the one hand and between host sex and climate seasons on the other hand. The statistical analyses were carried out using the Minitab 18 Demo software; difference were considered significant with a CI equal 95%.
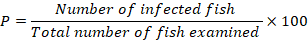
RESULTS and Discussion
Infection rate and water quality
This research on Henneguya sp. infection reported a prevalence equivalent 10.91%. The maximum seasonal prevalence was recorded in LDS while the minimum was obtained in LWS. Females specimens seemed to be more infected than males in SDS and SWS. There was no prevalence according to sex upper to 14% while the it was the lowest in LWS (Figure 2). Significant difference (χ2 = 0.136; df = 3; p = 0.987) was observed neither in the prevalence between males and females (Figure 2), nor between seasons (χ2 = 1.772; df = 3; p = 0.621). Whereas, the occurrence of Henneguya sp. has decreased from fish in the largest weight class to fish in the smallest weight class, from around 20% to 5% (Figure 3). A significant difference was recorded (χ2 = 11.781; df = 5; p = 0.038).
The data shown in Table 1 refer to the physicochemical parameters measured during this study. No significant variations were detected. Furthermore, no significant difference was reported between the total prevalence of Henneguya sp. and the mean value of the physicochemical parameters, except that a negative influence was perceived because of NH4 (Table 2).
Table 1: Physicochemical parameters of Adjohoun (Agonlin-Lowé) in the ouémé river and seasonal prevalence of Henneguya sp. Tossavi et al. (2015), Amended.
|
Water parameters |
LDS |
LWS |
SDS |
SWS |
|
T°C |
30.55 |
28.85 |
26.5 |
27.7 |
|
pH |
7.26 |
7.05 |
7.2 |
7.56 |
|
O2 |
3.24 |
1.54 |
2.78 |
3.46 |
|
NH4 |
0.29 |
1.03 |
0.98 |
0.44 |
|
NO2 |
0.25 |
0.04 |
0.17 |
0.02 |
|
NO3 |
1.1 |
5.79 |
17 |
1.1 |
|
PO4 |
0.04 |
0.36 |
1.28 |
0.57 |
|
SS |
27 |
51.5 |
59 |
40 |
|
PREV |
13.69 |
6.66 |
12.09 |
10.44 |
LDS: Long Dry Season; LWS: Long Wet Season; SDS: Short Dry Season; SWS: Short Wet Season; PREV: Prevalence expresses as percentage.
Table 2: Pearson correlation between prevalence and water physicochemical parameters in Adjohoun (Agonlin-Lowé).
|
T°C |
pH |
O2 |
NH4 |
NO2 |
NO3 |
PO4 |
SS |
|
|
r |
0.138 |
0.355 |
0.801 |
-0.619 |
0.808 |
0.041 |
0.055 |
-0.469 |
|
p |
0.862 |
0.645 |
0.199 |
0.381 |
0.192 |
0.959 |
0.945 |
0.531 |
r: Coefficient of correlation; p: Probability.
Myxospore description
The study found an average of 9 (3 to 17) cysts per infected fish. The cysts were soft and yellowish or whitish coloured with a round or spherical shape. Cysts diameter was about 2 to 6 mm. The cysts were found attached to the gill arches (a), specifically at the tip of the bulb of the ABO (Figure 4b) and sometimes along the extension of the mean stem (Figure 4c).
Mature spores were viewed elongated (Figure 4d). The total length of the spores was 36.8 (3 2 to 41.7) µm whereas the length of the valvular cell was 13.7 (13.1-16) µm and its width was 4.9 (4-7) µm. The spores have two elongated and equal-sized polar capsules that tapered at the anterior, occupying about half of the spore. The spore body was fusiform in front view and had a thin sutural line joining the two valves (Figure 4e, f). The surface of the valves was smooth and had two thin and equal caudal processes measuring from 16 to 21.5 µm in length. In valvular view, the spores presented a protruding anterior rostrum (Figure 4e, f).
Histopathological studies
The study carried out transmission electron microscopic observations on the ABO regarding to understand enough the host-parasite interaction. The ABO was found to consist of a dense connective tissue surrounding elastic cartilage in which myxospores were established (Figure 5a). For healthy ABO, anatomic structures like folds and intervening spaces were observed in the elastic cartilage (Figure 5b), whereas in infected ABO, these structures were progressively destroyed as the myxospores have grown and multiplied (Figure 5c). This resulted in the complete disappearance of the folds and intervening spaces, leaving a clear field for the myxospores (Figure 5c, d). A section in the infected bulb confirms the damage and progression of destruction of the cartilage until it becomes completely loose (Figure 5d).
Transverse sections of the ABO observed by transmission electron microscopy showed an external thick layer of dense connective tissue covering the loose connective tissue (Figure 6a). The elastic cartilage was separated from the loose connective tissue by a thin layer of fibrils known as the perichondrium (Figure 6b, c). The parasite-host interface showed the presence of a few mature myxospores in the elastic cartilage, with the perichondrium almost eroded (Figure 6c). In fact, it had completely disappeared in some areas, leaving wide empty spaces in the elastic cartilage (Figure 6d).
Parasite identity and infection rate associated to environmental quality
According to Molnár et al. (2002), only zoological methods are not enough to determine the validity of morphologically similar myxospores, which are also likely to have an affinity for the same tissues and taxonomically closely affiliated host species. As suggested by Adriano et al. (2009), this same concept can be applied to morphologically similar myxosporea found infecting different organs in the same host species, since the site of infection cannot be used as a taxonomic criterion. To overcome the limitations of morphological identification of the studied species, it will be considered as Henneguya sp. as a precaution until research its molecular identification will be provided.
According to the literature, a prevalence of 10.91%, provided from the present work, was the lowest recorded from ABO (El-Mansy and Bashtar, 2002; Reed et al., 2003; Abdel-Baki et al., 2011; Morsy et al., 2012). Moreover, Gbankoto et al. (2001), Milanin et al. (2010), and Barassa et al. (2012) pointed out no sex influence on the prevalence. However, Muzzal (1995) and Gbankoto et al. (2003) reported a significant difference in prevalence between host sexes. Regarding insignificant seasonal prevalence similar results were shown with other gill myxosporean parasites in Benin (Gbankoto et al., 2001).
El-Mansy and Bashtar (2002) suggested that the highest infection rate was associated with a decrease in length and weight of the fish. The present results are rather in accordance with Mitchell (1988), who reported that parasite infection was high in adult fish. However, those conclusions should be based on the length characterization of the studied sample. Molnár (1998) also recorded the highest prevalence of Henneguya creplini Gurley, 1894 in pikeperch (Stizostedion lucioperca) larger than 40 cm.
The physicochemical parameters were not correlated with the seasonal prevalence. Also, Tossavi et al. (2015) have made the same conclusion regarding intestine infection in C. gariepinus. It could be suggested that physicochemical parameters minimized the environmental effect on C. gariepinus in the culture system (Lefèvre et al., 2014) opposing Ernst et al. (2005) and Khidr et al. (2012), who attested that seasonal prevalence of fish parasites was supposed to be influenced by environmental factors. Therefore, the influence due to NH4 could be justify with Narr et al. (2019) who demonstrated the effects of parasite load and prevalence based on the presence of particulate nutrients. However, it was important to note that insufficiently aerated water and combined use of low-quality feed and poor water exchange leads to high organic and hence microbial loading (Lefèvre et al., 2011) in rearing conditions. Pollution and poor environmental conditions which often address fish immunity making them more susceptible to parasitic and diseases (Hecht et al., 2007). More specifically, due to the intermediate host, farming in ponds or fissured basins exposes fish to myxosporidial infections.
Histology and potential damage pathology
In contrast to regular cases of myxosporidian infection, the histological analysis revealed that the development of Henneguya sp. did not occur in the wall of the ABO. As the cysts were established in the core of the bulb end, it has demonstrated the affinity of the parasite to the tissue. The cyst was shown to contain immature and sporogenic stages at the periphery, whereas its centre was filled with mature myxospores, as suggested by El-Mansy and Bashtar (2002) and Abdel-Ghaffar et al. (2008). The increase in cyst size and maturation of spores weakned the interspaces, which were subsequently destroyed, along with the folds. As concerned, the parasite has lodged in the tuberculae eating the interspaces and progressed towards the superficial surface. A similar mode of degradation has been observed in the fish with Henneguya developing in the inner wall of the intestine epithelium where destruction started before going to the circular muscle (Tossavi et al., 2015). This led to severe damage and deformations of the endothelial cells lining the elastic cartilage of the ABO cells. In the same way, Adriano et al. (2005), who reported the thickness of the externa tunica and the granulomatous layer around the cyst. Whatever it is, Banerjee (2007) have shown dilatation of blood vessels, which degenerates into haemorrhage and respiratory failure, as in the case of proliferative gill sphaerodiosis.
According to Teugel (2011), C. gariepinus depends more on atmospheric O2 than most facultative fish air-breathers like an obligate air-breather (Das, 1927; Singh and Hughes, 1971) although it was shown that its gills are less efficient in extracting O2 from the ventilator water current (Fernandes et al., 1994). The establishment of the cyst in the cartilaginous tissue of the ABO in catfish is responsible for serious lesions in the elastic cartilage, leading to respiratory functional disorders, loss of appetite, and weight (El-Mansy and Bashtar, 2002; Adamek-Urbanska et al., 2021). For these reasons, any infection occurring in the ABO is a substantial phenomenon of reducing gas exchanger surface. Given that C. gariepinus is an important farmed fish worldwide, it is possible that if the parasite became established, it could have a serious impact on fish health.
It is important to access a large knowledge of the ultrastructure of the cyst wall (Current and Janovy, 1976) in order to understand the stress due to the pathogenicity of the parasite on its host. In this study, the cyst wall border was not observed. This is in disagreement with other myxosporeans described by El-Mansy and Bashtar (2002). This situation has made the destruction of the ABO internal structure evident which has rapidly gone through the perichondrium closed to the loose connective tissue. Furthermore, the entire ABO, including an internal cartilaginous core, implies additional support that prevents the collapse of the respiratory lamellae when the fish faces the loss of water. The respiratory surface is then freely exposed, so that the fish can continue aerial exchange of gases for fairly long periods (Ahmed et al., 2008).
In ponds, myxosporean infections are the most common causes of mortality, thereby affecting fish production drastically. The presence of cysts could never represent a good motivation to enhance gill functions. ABO infection by Henneguya sp. and drastically reduces the respiratory surface. As experimented by Zaccone et al. (2018) with other air-breathing fishes, it may be important to investigate the implication of neuroepithelial cells in this phenomenon.
ACKNOWLEDGMENTS
We express sincere gratitude to Professor Anne Cécile Ribou and François Lantoine for providing technical assistance. We also want to thank Mr. Nestor Sakiti and his boundless passion for Myxosporidia research in Benin, please enjoy a well-deserved retirement. Finally, we greatly appreciate the revision carried by the guest reviewers to improve the quality of this manuscript.
NOVELTY STATEMENT
The research provided details in the possible destruction capability of a Hennegua species on the air breathing organ of C. gariepinus species associated to its prevalence and influence from water physicochemical parameters.
AUTHOR’S CONTRIBUTION
TND: Designed the study, collected samples and data in laboratory, carried out the transmission electron microscope observation, prepared figures and drafted the manuscript.
SM: Assisted in samples collection and parasite fixation and prepared tables.
ANF: Assisted for sample collection, for data curation and revised the manuscript.
EML: Supervised the TEM observations, and critically revised the manuscript.
GA: Co-designed the study and critically revised the manuscript.
All authors contributed to improve the quality of the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdel-Baki AS, Sakran T, Zayed E (2011). Validity, impacts and seasonal prevalence of Henneguya species infecting catfish Clarias gariepinus from River Nile, Egypt. Parasitol. Res., 109: 119-123. https://doi.org/10.1007/s00436-010-2234-y
Abdel-Ghaffar F, Abdel-Baki AS, Bayoum E, Bashtar AR, Al-Quraishy S, Morsy KS, Alghamdy A, Mehlhorn H (2008). Light and electron microscopic study on Henneguya suprabranchiae Landsberg, 1987 (Myxozoa: Myxosporea) infecting Oreochromis niloticus, a new host record. Parasitol. Res., 103: 609-617. https://doi.org/10.1007/s00436-008-1019-z
Adamek-Urbanska D, Blazewicz E, Sobien M, Kasprzak R, Kamaszewski M (2021). Histological study of suprabranchial chamber membranes in Anabantoidei and Clariidae fishes. Animals (Basel), 11(4): 1158. https://doi.org/10.3390/ani11041158
Adriano EA, Arana S, Alves AL, Silva MRM, Ceccarelli PS, Henrique-Silva F, Maia AAM (2009). Myxobolus cordeiroi n. sp., a parasite of Zungaro jahu (Siluriformes: Pimelodiade) from Brazilian Pantanal: Morphology, phylogeny and histopathology. Vet. Parasitol., 162: 221-229. https://doi.org/10.1016/j.vetpar.2009.03.030
Adriano EA, Arana S, Cordeiro NS (2005). Histology, ultrastructure and prevalence of Henneguya piaractus (Myxosporea) infecting the gills of Piaractus mesopotamicus (Characidae) cultivated in Brazil. Dis. Aquat. Organism., 64: 229–235. https://doi.org/10.3354/dao064229
Ahmed AE, Mohamed K, Ahmed SA, Masoud F (2008). Anatomical, light and scanning electron microscopic studies on the air breathing dendritic organ of the sharptooth catfish (Clarias gariepinus). J. Vet. Anat., 1: 29-37. https://doi.org/10.21608/jva.2008.45452
Anonymous (2020). Guideline for the preparation and use of MS222 (TMS, tricaine methanesulfonate) for animal procedures. Reviewed and approved by FAU IACUC on august 28th, 2020. https://www.fau.edu/research-admin/comparative-medicine/files/guidelines-for-the-preparation-and-use-of-ms222-final.pdf, consulted on 12, 20th, 2024
Awe ET (2017). Hybridization of snout mouth deformed and normal mouth african catfish Clarias gariepinus. Anim. Res. Inter., 14(3): 2804-2808.
Azevedo C, Matos E (2003). Fine structure of Henneguya pilosa sp. n. (Myxozoa: Myxosporea), parasite of Serrasalmus altuvei (Characidae), in Brazil. Fol. Parasitol., 50: 37-42. https://doi.org/10.14411/fp.2003.006
Banerjee TK (2007). Histopathology of respiratory organs of certain air-breathing fishes of India. Fish Physiol. Biochem., 33: 441–454. https://doi.org/10.1007/s10695-007-9170-5
Barassa B, Adriano EA, Cordeiro NS, Arana S, Ceccarelli PS (2012). Morphology and host-parasite interaction of Henneguya azevedoi n. sp., parasite of gills of Leporinus obtusidens from Mogi-Guaçu River, Brazil. Parasitolol. Res., 11: 887-894. https://doi.org/10.1007/s00436-011-2571-5
Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M (2005). Disease and health management in Asian aquaculture. Vet. Parasitol., 132: 249-272. https://doi.org/10.1016/j.vetpar.2005.07.005
Borkhanuddin MH, Cech G, Molnár K, Shaharom-Harrison F, Duy Khoa TN, Atkinson SD, Székely C 2020. Henneguya (Cnidaria: Myxosporea: Myxobolidae) infections of cultured barramundi, Lates calcarifer (Perciformes: Latidae) in an estuarine wetlands system of Malaysia: description of Henneguya setiuensis n. sp., Henneguya voronini n. sp. and Henneguya calcarifer n. sp. Parasitology Res 119, 85–96, https://doi.org/10.1007/s00436-019-06541-1
Cambray JA (2003). The need for research and monitoring on the impacts of translocated sharptooth catfish, Clarias gariepinus, in South Africa. Afr. J. Aquat. Sci., 28: 191-195. https://doi.org/10.2989/16085910309503786
Chandra S, Banerjee TK (2004). Histopathological analysis of the respiratory organs of Channa striata subjected to the air exposure. Vet. Arhiv., 74: 37-52.
Correya MS, Vijayagopal P, Sanil NK (2021). Morphological and molecular description of a new species of Myxobolus (Myxosporea: Myxobolidae) infecting Planiliza macrolepis (Smith, 1846) from India. J. Parasit. Dis., 45: 887–896. https://doi.org/10.1007/s12639-021-01376-z
Current WL, Janovy JJ (1976). Ultrastructure of interlamellar Henneguya exilis in the channel catfish. J. Parasitol., 62: 975-998. https://doi.org/10.2307/3279193
Das BK (1927). The bionomics of certain air-breathing fishes of India, together with an account of the development of their air-breathing organs. Philos. Trans. R. Soc. B Biol. Sci., 216: 183-217. https://doi.org/10.1098/rstb.1928.0003
Dinh-Hung N, Dong HT, Soontara C, Rodkhum C, Nimitkul S, Srisapoome P, Kayansamruaj P, Chatchaiphan S (2022). Co-infection of Candidatus Piscichlamydia Trichopodus (Order Chlamydiales) and Henneguya sp. (Myxosporea, Myxobolidae) in Snakeskin Gourami Trichopodus pectoralis (Regan 1910). Front. Vet. Sci., 9: 847977. https://doi.org/10.3389/fvets.2022.847977
Donnelly BG (1973). Aspects of behavior in the catfish, Clarias gariepinus (Pisces: Clariidae), during periods of habitat desiccation. Arnol. Rhodesia, 6: 1-8.
Edwards P, Little DC, Demaine H (2002). Rural aquaculture. CABI Publishing, UK. https://doi.org/10.1079/9780851995656.0000
Eiras JC, Takemoto RM, Pavanelli GC (2008). Henneguya caudicula n. sp. (Myxozoa, Myxobolidae) a parasite of Leporinus lacustris (Osteichthyes, Anostomidae) from the High Parana River, Brazil, with a revision of Henneguya spp. infecting South American fish. Acta Protozool., 47: 149–154. https://doi.org/10.1016/j.vetpar.2008.10.020
Elmadfa I, Meyer AL (2017). Animal proteins as important contributors to a healthy human diet. Ann. Rev. Anim. Biosci., 5(1): 111-131. https://doi.org/10.1146/annurev-animal-022516-022943
El-Mansy A, Bashtar AR (2002). Histopathological and ultrastructural studies of Henneguya suprabranchiae Landsberg, 1987 (Myxosporea: Myxobolidae) parasitizing the suprabranchial organ of the freshwater catfish Clarias gariepinus Burchell, 1822 in Egypt. Parasitol. Res., 88: 617-626. https://doi.org/10.1007/s00436-002-0598-3
Ernst II, Whttingtton D, Corneillte S, Talbot C (2005). Effects of temperature, salinity, desiccation and chemical treatments on egg embryonation and hatching success of Benedenia seriolae (Monogenea: Capsalidae), a parasite of farmed Seriola spp. J. Fish Dis., 28: 157-164. https://doi.org/10.1111/j.1365-2761.2004.00605.x
FAO (2012). La situation mondiale des pêches et de l’aquaculture Rome, pp. 241.
Feist SW, Longshaw M (2006). Phylum Myxozoa. In: Woo, PTK. (Ed.), Fish Diseases and Disorders, 1: Protozoan and Metazoan Infections. Second ed. CAB International, UK; pp. 230-296. https://doi.org/10.1079/9780851990156.0230
Fernandes MN, Rantin FT, Kalinin AL, Moron SE (1994). Comparative study of gill dimensions of three erythrinid species in relation to their respiratory function. Can. J. Zool., 72: 160-165. https://doi.org/10.1139/z94-020
Gbankoto A, Pampoulie C, Marques A, Sakiti GN (2001). Occurrence of myxosporean parasites in the gills of two tilapia species from Lake Nokoué (Benin, West Africa): effect of host size and sex, and seasonal patterns of infection. Dis. Aquat. Org., 44: 217-222. https://doi.org/10.3354/dao044217
Gbankoto A, Pampoulie C, Marques A, Sakiti GN, Dramane KL (2003). Infection patterns of Myxobolus heterospora in two tilapia species (Teleostei: Cichlidae) and its potential effects. Dis Aquatic Org., 55: 125-131. https://doi.org/10.3354/dao055125
Gbankoto A, Tossavi ND, Sindété M, Sakiti GN, Moutaïrou K, Ribou A-C. 2015. Some pathophysiological insights into ovarian infestation by Myxobolus sp. (Myxozoa: Myxosporea) in Clarias gariepinus (Clariids: Silurids) from Bénin (West Africa). Parasitol Res (2015) 114:2941-2949, DOI 10.1007/s00436-015-4496-x
Guo FC, Woo PTK (2009). Selected parasitosis in cultured and wild fish. Vet. Parasitol., 163: 207-216. https://doi.org/10.1016/j.vetpar.2009.06.016
Hecht T, Endermann F (2007). The impact of parasites, infections and diseases on the développement of aquaculture in Sub-Saharan Africa. J. Appl Ichthyol., 14: 3-4. https://doi.org/10.1111/j.1439-0426.1998.tb00644.x
Khidr AA, Said AE, Samak OAA, Sheref SEA (2012). The impacts of ecological factors on prevalence, mean intensity and seasonal changes of the monogenean gill parasite, Microcotyloides sp. infesting the Teraponputa fish inhabiting coastal region of Mediterranean Sea at Damietta region. J. Basic Appl. Zool., 65: 109-115. https://doi.org/10.1016/j.jobaz.2012.06.003
Lazard J, Legendre M (1994). La pisciculture africaine: Enjeux et problèmes de recherche. Cah Agricult., 3: 83-92
Lefèvre S, Huong DTT, Ha NTK, Wang T, Phuong NT, Baylay M (2011). A telemetry study of swimming depth and oxygen level in a Pangasius pond in the Mekong Delta. Aquaculture, 315: 410-413. https://doi.org/10.1016/j.aquaculture.2011.02.030
Lefèvre S, Wang T, Jensen A., Cong NV, Huong DTT, Phuong NT, Baylay M (2014). Air-breathing fishes in aquaculture. What can we learn from physiology? J. Fish Biol., 84: 705-731. https://doi.org/10.1111/jfb.12302
Lom J, Arthur JR (1989). A guideline for the preparation of species descriptions in Myxosporea. J. Fish Dis., 12: 151-156. https://doi.org/10.1111/j.1365-2761.1989.tb00287.x
Lom J, Dyková I (2006). Myxozoan genera: Definition and notes on taxonomy, life-cycle terminology and pathogenic species. Fol. Parasitol., 53: 1-36. https://doi.org/10.14411/fp.2006.001
Lövy A, Smirnov M, Brekhman V. Tamir Ofek, Lotan (2018). Morphological and molecular characterization of a novel myxosporean parasite Myxobolus bejeranoi n. sp. (Cnidaria: Myxosporea) from hybrid tilapia in Israel. Parasitol. Res., 117(2): 491–499. https://doi.org/10.1007/s00436-017-5725-2
Martins ML, Onaka EM (2006). Henneguya garavelli n. sp. and Myxobolus peculiaris n. sp. (Myxozoa: Myxobolidae) in the gills of Cyphocharax nogelli (Osteichthyes: Curimatidae) from Rio do Peixe reservoir, Sao José do Rio Pardo, São Paulo, Brazil. Vet. Parasitol., 137: 253-261. https://doi.org/10.1016/j.vetpar.2005.12.023
McCraren JP, Landolt ML, Hoffman GL, Meyer FP (1975). Variation in response of channel catfish to Henneguya sp. infections (Protozoa: Myxosporidia). J. Wildl. Dis., 11: 2-7. https://doi.org/10.7589/0090-3558-11.1.2
Meyer FP (1991). Aquaculture disease and health management. J. Anim. Sci., 69: 4201-4208. https://doi.org/10.2527/1991.69104201x
Michel C (1989). Pathology of tilapias. Aquat. Liv. Resour., 2: 117-126. https://doi.org/10.1051/alr:1989014
Milanin T, Eiras JC, Arana S (2010). Phylogeny, ultrastructure, histopathology and prevalence of Myxobolus oliveirai sp. nov., a parasite of Bryconhilarii (Characidae) in the Pantanal wetland, Brazil. Memor Instituto Oswaldo Cruz, 105: 762-769. https://doi.org/10.1590/S0074-02762010000600006
Mitchell LG (1988). Myxobolid parasites (Myxozoa: Myxobolodidae) infecting fishes of western Montana, with notes on histopathology, seasonality, and intraspecific variation. Can. J. Zool., 67: 1915-1922. https://doi.org/10.1139/z89-274
Molnár K (1998). Taxonomic problems, seasonality and histopathology of Henneguya creplini (Myxosporea) infection of the pikeperch Stizoztedion lucioperca in Lake Balaton. Fol. Parasitol., l45: 261-269.
Molnár K, Eszterbauer E, Székely C (2002). Morphological and molecular biological studies on intramuscular Myxobolus spp. of cyprinid fish. J. Fish Dis., 25: 643-652. https://doi.org/10.1046/j.1365-2761.2002.00409.x
Morsy K, Abdel-Ghaffar F, Bashtar A-R, Mehlhorn H, Al-Quraishy S, Abdel-Gaber R (2012). Morphology and small subunit ribosomal DNA sequence of Henneguya suprabranchiae (Myxozoa), a parasite of the catfish Clarias gariepinus (Clariidae) from the River Nile, Egypt. Parasitolol. Res., 111: 1423-1435. https://doi.org/10.1007/s00436-012-2976-9
Muzzal PM (1995). Distribution of Myxobolus scleroperca (Myxobolidae: Myxosporea) in yellow perch (Perca flavescens) in the Great Lakes. J. Parasitol., l81: 498-499. https://doi.org/10.2307/3283842
Narr CF, Ebert D, Bastille-Rousseau G, Frost PC (2019). Nutrient availability affects the prevalence of a microsporidian parasite. J. Anim. Ecol., 88: 579-590. https://doi.org/10.1111/1365-2656.12945
Nkamigbo DC, Ovuomarie OS, Maduka JU, Isibor AC (2014). Economic efficiency and profitability of catfish (Clarias gariepinus) production in Isoko area of Delta State, Nigeria. J. Agric. Vet. Sci., 6(2): 32-40.
Pasch J, Palm HW (2021). Economic analysis and improvement opportunities of African Catfish (Clarias gariepinus) aquaculture in Northern Germany. Sustainability, 13: 13569. https://doi.org/10.3390/su132413569
Reed CC, Basson L, Van As LL (2003). Myxozoans infecting the sharptooth catfish, Clarias gariepinus in the Okavango River and Delta, Botswana, including descriptions of two new species, Henneguya samochimensis n. sp. and Myxobolus gariepinus n. sp. Fol. Parasitol., 50: 183-189. https://doi.org/10.14411/fp.2003.033
Schmahl G, Mehlhorn H, Taraschewski H (1989). Treatment of fish-parasites. 7. Effects of sym. triazinone (Toltrazuril) on developmental stages of Myxobolus sp. Bütschli, 1882 (Myxosporea, Myxozoa): A light and electron microscopic study. Eur. J. Protistol., 25: 26-32.
Singh BN, Hughes GM (1971). Respiration of an air-breathing catfish Clarias batrachus (Linn.). J. Exp. Biol., 55: 421-434. https://doi.org/10.1242/jeb.55.2.421
Sitjá-Bobadilla A (2009). Can myxosporean parasites compromise fish and amphibian reproduction? P R. Soc. B-Biol. Sci., 276: 2861–2870. https://doi.org/10.1098/rspb.2009.0368
Sokolov SG, Lebedeva DI, Murzina SA, Parshukov AN, Bystrova KA, Ieshko EP (2019). Morphology and phylogeny of Henneguya oviperda infecting oocytes of Esox lucius, with description of parasite-induced histopathology. Dis. Aquat. Org., 133(2): 91–98. https://doi.org/10.3354/dao03331
Teugels GA (2011). Systematic revision of the African species of the genus Clarias (Pisces: Clariidae). Ann Mus Roy Afr. Centr. 1986; 247:1-199. Belão TC, Leite CAC, Florindo LH, Kalinin AL, Rantin FT. Cardiorespiratory responses to hypoxia in the African catfish, Clarias gariepinus (Burchell 1822), an air-breathing fish. J. Comp. Physiol. B., 181: 905-916. https://doi.org/10.1007/s00360-011-0577-z
Tossavi ND, Gbankoto A, Yessoufou A, Escande M-L, Dimitri G, Ribou AC, Moutaïrou K, Sakiti GN (2015). Histopathological and ultrastructural studies of a Henneguya species (Myxozoa: Myxosporea) infesting the intestine of Clarias gariepinus from Benin (West Africa). Parasitol. Res., 114: 861-872. https://doi.org/10.1007/s00436-014-4249-2
Yokoyama H, Kawakami H, Yasuda H, Tanaka S (2003). Henneguya lateolabracis sp. n. (Myxozoa: Myxosporea) the causative agent of cardiac henneguyosis in Chinese sea bass Lateo labrax sp. Fish Sci., 69: 1116-1120. https://doi.org/10.1111/j.0919-9268.2003.00736.x
Zaccone G, Lauriano ER, Capillo G, Kuciel M (2018). Air-breathing in fish: Air breathing organs and control of respiration: Nerves and neurotransmitters in the air-breathing and the skin. Acta Histochem., 120(7): 630-641. https://doi.org/10.1016/j.acthis.2018.08.009
Zayed AE, Mohamed SA (2004). Morphological study on the gills of two species of freshwater fishes: Oreochromis niloticus and Clarias gariepinus. Ann. Anat., 186: 295-304. https://doi.org/10.1016/S0940-9602(04)80044-X
To share on other social networks, click on any share button. What are these?






