Improving the Phytosanitary of Fig Plants Infected with Fig Latent Virus by Nanochitosan and Biomagic
Research Article
Improving the Phytosanitary of Fig Plants Infected with Fig Latent Virus by Nanochitosan and Biomagic
Hanaa H.A. Gomaa1*, Dalia Y.A. Amin1, Mona A. Ismail1, Basma Hamdy2, Khaled A. El-Dougdoug3
1Department of Botany and Microbiology, Faculty of Science, Suez Canal University, Ismailia, Egypt; 2Regional Center for Mycology, Al-Azhar University, Cairo, Egypt; 3Department of Microbiology, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
Abstract | Fig viruses have been found as naturally mixing infection in many countries in the world and Egypt. The present study aimed to evaluate the potential effect of nanochitosan and biomagic for treatment of the pathological effects related to virus infection in fig plants. The fig (Ficus carica L.) cv. sultani) plants were grafted by infectious blind eye from Fig latent virus (FLV) infected plants. Infected and healthy plants were foliar sprayed with nanochitosan (ChNPs), biomagic (BM) or combination (ChNPs and BM). Microtome and ultrathin sections were carried out on healthy and infected treated fig leaves. The shoot length, leaf area, fresh and dry weight were determined. Biochemical markers as indicators for systemic acquired resistance; total proteins, salicylic acid, phenol, proline, oxidative enzyme activities (polyphenol oxidase and superoxide dismutase) and virological assessments were assayed. Reduction in the disease severity of treated foliar ChNPs and BM fig plants was recorded. Systemic acquired resistance was detected related to Chlorophyll a and b and carotenoid, Phenol, Proline, salicylic acid and oxidative enzymes contents. The microtome and ultrathin section analysis revealed that improved phytosanitary of cell and tissues of FLV infected fig plants treated with ChNPs and BM, as well as improved shoot length, leaf area and dry weight. Biochemical markers as indicators for systemic acquired resistance were significantly increased including total proteins, salicylic acid, phenol, proline, Peroxidase, polyphenol oxidase and superoxide dismutase activities Nanochitosan and biomagic were treated and improved tissues, cell membranes, chloroplasts, mitochondria, and nucleus damage caused by FLV infection in fig plants. Treatment of fig plants with nanochitosan and biomagic led to a significant increase in plant health, growth characteristics, immunity against virus infection, as well as repair of the effects caused by the FLV infection. It has been suggested that, the induction of systemic acquired resistance was successfully achieved and protected fig plants against FLV infection.
Received | November 26, 2022; Accepted | February 28, 2023; Published | July 12, 2023
*Correspondence | Hanaa H.A. Gomaa, Department of Botany and Microbiology, Faculty of Science, Suez Canal University, Ismailia, Egypt; Email: [email protected]
Citation | Gomaa, H.H.A., D.Y.Z. Amin, M.A. Ismail, B. Hamdy, K.A. El-Dougdoug. 2023. Improving the phytosanitary of fig plants infected with fig latent virus by nanochitosan and biomagic. Journal of Virological Sciences, 11(2): 9-24.
DOI | https://dx.doi.org/10.17582/journal.jvs/2023/11.2.9.24
Keywords | Biomagic, Fig plants, FLV, Nanochitosan, Peroxidase (PO), Polyphenol oxidase (PPO), Superoxide dismutase (SOD). Systemic acquired resistance (SAR)
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Fig plants (Ficus carica L.) belong to family Moraceae, is one of common crops in the Mediterranean Basin, Africa and Southwest Asia (Zohary et al., 2012). A number of viruses have been identified in fig trees and classified as members of the family Flexiviridae, genus Trichovirus (Fig latent virus, FLV-1), genus Emaravirus (Fig mosaic virus, FMV), Closteroviridae (Fig leaf mottle-associated virus 1 and 2, FLMaV-1 and 2, and Fig mild mottle-associated virus, FMMaV), Caulimoviridae (Fig badnavirus 1, FBV-1), Betaflexiviridae (Fig latent virus 1, FLV-1), Partitiviridae (Fig cryptic virus, FCV) and Tymoviridae (Fig fleck-associated virus, FFkaV). FLV is now found in almost all fig orchards worldwide (Martelli, 2011). Therefore, it can result in significant economic losses (Shahmirzaie et al., 2010). Biomagic, as defined, is a ready to use solution that may be sprayed on plants to aid in their development. It’s possible that this will improve the vegetative development of peanut plants. Biomagic’s ability to escape the plant canopy and reach the soil solution and become accessible to microorganisms and plant roots might be a factor in this. Chitosan nanoparticles (CNP) may be able to initiate and enhance immune responses in plants, according to the findings of the current research. The plant’s innate immune response was significantly improved by CNP-treatment of leaves, which stimulated the activity of defence enzymes, upregulated defense related genes, including those of numerous antioxidant enzymes, and raised total phenolic levels. The current study was carried out to evaluate the potential effect of nanochitosan and biomagic for treatment of the pathological effects related to FLV infection in fig plants.
Materials and Methods
Virus source
Fig latent virus (FLV) strain, GenBank Accession number, (MZ076515) was obtained from Department of Botany and Microbiology, Faculty of science, Suez Canal University, Ismailia, Egypt maintained as a latent symptom on fig plants which gave positive RT-PCR results.
Plant sources
Healthy fig plants (Ficus carica, cv. Sultani, family, Moraceae) were obtained from Department of Horticulture, Faculty of Agriculture, Ain Shams University, Egypt.
Virus inhibitors
Chitosan nanoparticles (ChNPs) with size of 15 to 25 nm and spherical shape were purchased from Virology Labs, Department of Microbiology, Faculty of Agriculture, Ain Shams University, in a liquid form. It was uniformly foliar-applied at concentration of 7.5 mL/L. recommended by Nesma et al. (2018).
Biomagic is a combination of essential amino acids, enzymes and plant growth hormones (Registered in 2008, UniChem Seeds Pvt Ltd in India). The supplier company is located in New Delhi, and is one of the leading sellers of listed products. It was uniformly foliar-applied at concentration of 10 g/L recommended by Hafez (2013) and El-Massriy (2009).
Grafting inoculation
Eighty healthy fig plants planted in plastic pot (diameter 30 cm2, one plant per pot) containing sterilized peat moss:sand: vermiculite with ratio (W:W:W) under practical agriculture recommendations. About 20 fresh, semi-hard wood cuttings with 4-6 nodes from FLV infected fig plants were maintained in water. Forty fig plants were inoculated by grafting with chip budding of FLV infected fig plants and ten grafting inoculated with chip budding tissue from healthy fig plants and sealed with parafilm according to Roistacher (1991) and ten healthy fig seedlings were grafting inoculated with bark tissue from healthy fig plants and sealed with parafilm (as a negative control). The inoculated fig seedlings were kept under insect-proof cages in a greenhouse condition at temperatures 24ºC to 27ºC maximum during the daytime and 18ºC to 21ºC minimum at night). Most of the symptoms developed after 25-30 days. The results were confirmed by RT-PCR.
Experimental design was carried out in 80 pots at autumum growing season 2021. The experimental plastic pots (30 cm2 diameter) were arranged in a randomized complete design and ten pot replicates for each treatment (a plant per pot). The layout treatments used in the experiment were divided into two groups as follows:
Group 1 (as a control) included forty fig plants divided into:
- Ten healthy fig plants without treatment.
- Ten healthy fig plants were sprayed with 7.5 mL/ L ChNPs,
- Ten healthy fig plants were sprayed with 10 mL/ L Biomagic.
- Ten healthy fig plants were sprayed with 7.5 mL/ L ChNPs, and 10 mL/ L Biomagic
Group 2 included forty fig plants virus inoculated and divided as follows:
- Ten fig plants virus inoculated without treatment.
- Ten Fig plants were sprayed with 7.5 mL/ L ChNPs.
- Ten Fig plants were sprayed with 10 ml/L Biomagic
- Ten Fig plants were sprayed with 7.5 mL / L ChNPs and 10 mL / L Biomagic.
Assessments of plant immunity
Disease severity of infected fig plants was calculated using the scale rating by formula according to (Yang et al., 1996).
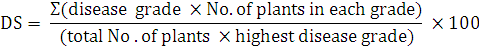
Detection of virus
Total RNA was extracted from 0.5 g treated fig leaves as described by the instruction manual of High Pure RNA tissue kit (Version 1, 2000) from Roche diagnostics GmbH, Germany.
Reverse transcription-polymerase chain reaction (RT-PCR)
Two oligonucleotide primers (CPtr15ʹ, CCATCTTCACCACACAAATGTC3ʹ and CPtr2, 5ʹ CAATCTTCTTGGCCTCCATAAG3ʹ) were synthesized for FLV coat protein gene according to Gattoni et al. (2009). Coat protein gene was amplification by one step RT-PCR according to Latinović et al. (2019). The RT-PCR product was stained and separated on ethidium bromide-agarose gel (1.5 µg/mL). The PCR fragment was visualized on a UV transilluminator and photographed using Gel Documentation System (GELDOC 2000, BioRad, USA). PGEM DNA marker (Promega) was used to determine the size of amplified PCR products.
Determination of transpiration rate
The detached parts of fig leaves were smeared with petroleum jelly and rapidly weighted. They were then exposed in the open air under natural conditions for 2 min and weighted again. The decrease in weight corresponds to the water lost by transpiration during the exposure period was calculated on the basis of fresh weight according to Abd El-Rahman and Batanouny (1965).
Electrolyte leakage
Twenty fig leaves discs were put in a boiling tube containing 10 mL of deionized water and heated at 45°C (ECₐ) and 55°C (ECb) for 30 min and 100°C for 10 min (ECc) in waterbath. The tubes were measured by conductivity meter according to Sullivan and Ross (1979). The electrolyte leakage was calculated by using the formula:
Electrolyte leakage (%) = (ECb-ECa/ECc) × 100
Membrane Stability Index (MSI) was estimated by mixed 200 mg of fig leaves in 10 mL of double distilled water in two sets. One set heated at 40°C for 30 min in water bath and the electrical conductivity (C1) was measured. The second set was boiled at 100°C in water bath for 10 min was also measured (C2) by conductivity meter. MSI calculated using the formula described by Sairam (1994).
MSI= [1-(C1/C2) ×100]
Determination of lipid peroxidation was measured in terms of malondialdehyde (MDA) as described by Heath and Packer (1968). Two mL of 0.1% (w/v) trichloroacetic acid (TCA), 0.5 g of the leaves were homogenized, followed by 20 min of centrifugation at 12,000 rpm. One mL supernatant was mixed with 0.5% equivalent TCA (10%) (w/v) TBARS, heated at 95oC for 30 min, cooled in ice. The absorption of supernatant was measured at 532 nm. After subtraction of the non-specific absorbance at 600 nm, the concentration of MDA was calculated by molar extinction coincident of 155mM/1 cm1.
Photosynthetic pigments
One gram of fresh leaf was extracted by grinding with 10 ml of 80% acetone and centrifuged for 5 min, at 3000 rpm. The supernatant was used for spectrophotometric determination of chlorophyll a, b and carotenoids according to method of Lichtenlhaler (1987).
Phenol compounds: One g of fresh leaves was macerated in 50 mL of 80% ethanol for at least 24 h at 0°C, extraction and determination of phenolic compounds were done according to Daniel and George (1972) using UV-spectrophotometer (UNICO, 2000) at wave length 725 nm.
Proline content: Leaf samples (0.2 g) were homogenized in 5 mL of 3% (w/v) sulfosalicylic acid and centrifuged at 3000 rpm at 4°C for 10 min. The supernatants were used for proline estimation according to Bates et al. (1973) at 520 nm using UV-spectrophotometer (UNICO, 2000).
Enzymes activities: Two g of the terminal buds were homogenized with 10 mL of phosphate buffer pH 6.8 (0.1 M), and then centrifuged at 2oC for 20 min at 20000 rpm in a refrigerated centrifuge. The clear supernatant was taken as the enzymes source (Mukherjee and Choudhuri, 1983).
Peroxidase (POX) activity was assayed by measuring the inhibition of the auto-oxidation of pyrogallol using a method described by Bergmeyer and Bernt (1974) at 470 nm wave length using UV-spectrophotometer (Labomed, inc., 23).
Polyphenol oxidase (PPO) activity was assayed by measuring the inhibition of the auto-oxidation catechol using a method described by Matta and Diamond (1963). The absorbance was measured at 495 nm wave length using UV-spectrophotometer (Labomed, inc.23).
Superoxide dismutase (SOD) activity was determined by measuring the inhibition of auto-oxidation of pyrogallol using a method described by Marklund and Marklund (1974) at 325 nm wave length using UV-spectrophotometer (Labomed, inc. 23).
Light and electron microscopic examination
The lamina, blade and petioles of healthy and infected leaves at the same position and age on fig plants were sectioned into anatomical cross sections to investigate the changes in inoculated leaves with the virus compared with healthy ones.
Light microscopic
The anatomical structure of infected and healthy fig leaves (one cm of blade including the midrib) was taken from the infected leaf and healthy ones. The selected tissue samples were processed according to standard procedures for light microscopic according to Johansen (1940), Corgan and Widmoyer (1971) and Ruzin (1999). Sections were microscopically inspected to detect histological manifestations of noticeable responses resulted from the infection.
Transmission electron microscopy (TEM)
Five slices of one mm2 were excised from symptomatic fresh leave from infected fig plant and healthy ones. The selected tissue samples were processed according to standard procedures for electron microscopy according to Luft (1961) and Martelli et al. (1993). The ultrathin sections were viewed with a JOEL JM 100-C electron microscope (Electron Microscope Unit, Al-Azhar University, Cairo) a Zeiss-910 TEM at an accelerating voltage of 75 kV.
Statistical analysis
Data were statistically analyzed using Two-way ANOVA and Holm-Sidak test (Sigma Plot 12.0) at 0.05 level of probability. The values recorded in the values of the biochemical analysis are means of three replicates.
Results and Discussion
Treatment of fig plants with nanochitosan and biomagic led to reduction of virus infectivity, a significant increase in plant health, growth characteristics, immunity and repair of anatomical and ultrastructural cytopathological effects induced by FLV infection.
Reduction of virus infectivity
Sprayed infected fig plants with ChNPs, biomagic and combination of them led to reduction of symptoms and FLV infectivity. The ChNPs showed high reduction of FLV infectivity compared with biomagic. The percentage of reduction of infection, disease severity 45 days compared to FLV infected virus plants (Table 1 and Figure 1), as well as reduction of FLV replication. PCR produced bands at expected size of 520 bp for CP gene of FLV detected infected fig plant cv. sultani. which showed low fragment density, by infected fig plant treated with ChNPs (+), biomagic (++) and combination with ChNPs (+) biomagic (+) while high fragment density (++++) by untreated infected fig plant (Table 1 and Figure 2). The study further details their mechanism of action. According to virus infectivity and replication, biomagic induced systemic resistance (virucidal) while ChNPs may inhibit virus replication (antivirus) as well as reduction of virus symptoms (Figure 2).
Table 1: Disease severity and virus concentration of treated infected fig plants sprayed with ChNPs and Biomagic under greenhouse condition.
|
Parameters |
Symptoms index |
Virus infectivity |
RT-PCR |
||||||
|
No of infected plants |
mM *(2) |
sM *(4) |
Def. *(6) |
% Inf. |
% Red. |
% Disease severity |
|||
|
Infected plant (Control) |
9 |
2 |
4 |
3 |
90 |
- |
63.00 |
++++ |
|
|
Treated infected plants |
ChNPs |
4 |
3 |
1 |
0 |
40 |
60 |
16.66 |
+ |
|
Biomagic |
5 |
3 |
2 |
0 |
50 |
50 |
23.33 |
++ |
|
|
ChNPs and biomagic |
3 |
2 |
1 |
0 |
30 |
70 |
13.33 |
+ |
|
Total inoculated plants = 40 plant, %Inf=% Infection, % Red = %Reduction. * Degree of Symptoms index (2) mM=mild mosaic, (4) sM = sever mosaic), (6) Def. = deformation). ** Virus concentration was determined at the means of three replicates by DAS-ELISA. (Optical density at 405 nm) Negative control= 0.114; Positive control = 0.475.
Table 2: Effect of virus infection on leaf area, dry weight and shoot length of treated infected fig plants compared with healthy ones (in vivo).
|
Parameters/ Treatments |
Leaf area (Cm) |
Shoot length (Cm) |
Dry weight (g/gfw) |
|
|
Healthy plant (Control) |
Control |
34.5 b |
52.0 b |
0.43 b |
|
ChNPs |
37.4 b |
66.5 d |
0.52 c |
|
|
Biomagic |
42.4 c |
72.4 e |
0.56 d |
|
|
ChNPs + biomagic |
46;4 c |
84.8 f |
0.54 d |
|
|
Infected plant |
Control |
15.3 a |
31.3 a |
0.33 a |
|
Treated infected plants |
ChNPs |
32.5 b |
59 .6 c |
0.51 c |
|
Biomagic |
33.2 b |
67.3 d |
0.65 e |
|
|
ChNPs + biomagic |
35.5 b |
75.3 e |
0.58 d |
|
|
L.S.D 1% |
5.2 |
4.5 |
0.18 |
|
Each value is a mean of 3 replicates. g/gfw = gram per gram fresh weight.
Enhancement of growth parameters
Impact of foliar by antiviral (ChNPs), and virucidal (biomagic) and combination of ChNPs and Biomagic on healthy and infected plants were significantly increased leaf area, shoot length and dry weight compared with non-foliar healthy and infected fig plants (Table 2 and Figure 3).
Plant immunity
Chlorophyll pigments: The chlorophyll a, b, (a+b) and carotenoid contents were significantly decreased in FLV inoculated fig plants. On the other hand, the foliar healthy with ChNPs, biomagic and combination of ChNPs and biomagic showed significant increase in chlorophyll a, b, (a+b) and carotenoid (Table 3).
Total soluble protein contents were significantly increased in sprayed healthy and virus infected fig plants with ChNPs, biomagic and combination of ChNPs and biomagic (Table 4).
Scavenging enzyme activities (SODs, PPOs, POXs and CATs) were increased in healthy and virus infected fig plants sprayed with ChNPs, biomagic and combination of ChNPs and biomagic (Table 4). ChNPs, biomagic and combination of ChNPs and
Table 3: Chlorophyll a and b, total a+b, and carotenoids, contents (mg g-1 FW) of inoculated infected fig plants sprayed with ChNPs, biomagic and combination compared with healthy ones.
|
Parametersl/ Treatment |
Photosynthesis pigments |
|||||||
|
Ch-a mg g-1 FW |
Ch-b mg g-1 FW |
Ch-(a+b) mg g-1 FW |
Carotenoids mg g-1 FW |
|||||
|
Healthy plant |
Control |
13.7 |
6.8 |
20.5 |
4.5 |
|||
|
ChNPs |
15.2 |
8.6 |
22.4 |
5.7 |
||||
|
biomagic |
16.9 |
9.5 |
28.5 |
7.4 |
||||
|
ChNPs+biomagic |
17.2 |
9.3 |
29.4 |
7.9 |
||||
|
Infected plant |
(Control) |
9.4 |
4.7 |
13.6 |
3.6 |
|||
|
Treated infected plants |
ChNPs |
15.6 |
7.8 |
21.6 |
4.9 |
|||
|
biomagic |
17.4 |
8.5 |
29.2 |
6.9 |
||||
|
ChNPs+biomagic |
17.8 |
8.2 |
29.5 |
8.2 |
||||
|
L.S.D. 1% |
1.8 |
1.2 |
2.6 |
1.2 |
||||
Each value means of 3 replicates.
Table 4: Effect of virus infection on POX, CAT, SOD and APX activities (unit g-1 FW) of fig plants sprayed with ChNPs and biomagic compared with non-sprayed plants ones.
|
Parameters/ Treatment |
Protein* |
CAT |
POX |
PPOs |
SOD |
|
|
Healthy plant |
Control |
34.45 |
48 |
42 |
40.5 |
42.2 |
|
ChNPs |
38.24 |
52 |
45 |
46.2 |
45.2 |
|
|
Biomagic |
42.21 |
57 |
52 |
47.8 |
52.3 |
|
|
ChNPs + biomagic |
44.12 |
59 |
57 |
52.3 |
54.2 |
|
|
Infected plant |
Control |
35.42 |
58 |
48 |
56.4 |
48.2 |
|
Treated infected plants |
ChNPs |
37.25 |
54 |
55 |
53.3 |
49.5 |
|
Biomagic |
41.14 |
61 |
58 |
62.5 |
53.7 |
|
|
ChNPs + biomagic |
42.15 |
65 |
62 |
65.3 |
56.5 |
|
|
L.S.D 1% |
2.0 |
2 |
1.2 |
1.8 |
1.5 |
|
Each value means of 3 replicates.
biomagic improved the decrease of scavenging enzyme activities induced by viral infection. The plant resistance related to scavenging enzyme activities against plant viruses which catalyzes the formation of lignin and other oxidative phenols. The ROS (reactive oxygen species) mechanism synthesized for detoxifying under ChNPs and biomagic as well as virus infection response in order to protect cellular membranes and organelles from the damaging effects of ROS.
Physiological processes
Treatment of fig plants with nanochitosan, biomagic and combination ChNPs and biomagic increased membrane features, Electrolyte leakage (EL, Membrane stability index MSI and Relative water content RWC and Malondialdehyde MDA (Table 5). On the other hand, ChNPs, biomagic and combination of ChNPs and biomagic repaired the significant decrease in EL with 1.93,7.88 and 6.39%, MSI with 11.71, 16.74 and 19.459%, RWC with 43.21, 48.18 and 36.989% and MDA with 78.40, 77.70 and 89.109%, respectively (Table 5) due to FLV infection.
Phytochemical contents
Phenol, Free proline and Lipid peroxidation (malondialdehyde, (MDA) were significantly increased in FLV infected compared to healthy fig plants. On the other hand, phenols were significantly increased in healthy (0.122, 0.149, 0.164) and in FLV infected fig plants (0.131, 0.176, 0.182) O.D. Free proline was significantly increased in healthy (0.87, 0.96, 1.22) and in FLV infected fig plants (1.35, 1.64, 1.82) O.D. MDA was significantly increased in healthy (18.5, 20.3, 21.4) and 24.6, 28.2, 30.2 mM/cm1 in FLV infected fig plants sprayed with ChNPs, biomagic and combination of ChNPs and biomagic, respectively (Table 6).
Table 5: Effect of FLV infection on EL, MSI and RWC of fig plants sprayed ChNPs and biomagic compared with non-sprayed plants ones (in vivo) and healthy plants.
|
Parameters/ Treatment |
EL |
MSI |
RWC |
MDA |
|||||
|
% |
% Repair |
% |
% Repair |
% |
% Repair |
% |
% Repair |
||
|
Healthy plant |
Control |
6.72 a |
- |
62.15b |
47.13 |
32.42 b |
28.90 |
42.62 b |
11.13 |
|
ChNPs |
7.73b |
15.02 |
72.16c |
+16.10 |
42.52 c |
31.15 |
59.22 c |
54.41 |
|
|
BM |
7.43 a |
10.56 |
75.52e |
+21.51 |
46.42 c |
43.18 |
62.52 c |
62.24 |
|
|
ChNPs + BM |
7.52b |
11.90 |
75.18e |
+20.96 |
48.32 dc |
49.04 |
68.42 dc |
78.40 |
|
|
Infected plant (Control) |
9.75 c |
31.07 |
42.24a |
- |
25.15 a |
- |
38.35 a |
- |
|
|
Treated infected plants |
ChNPs |
6.85 a |
1.93 |
69.43c |
+11.71 |
46.43 c |
43.21 |
65.55 c |
78.40 |
|
BM |
7.25 a |
7.88 |
72.56d |
+16.74 |
48.04 dc |
48.18 |
68.15 dc |
77.70 |
|
|
ChNPs + BM |
7.15 a |
6.39 |
74.24d |
+19.45 |
44.41 c |
36.98 |
72.52 c |
89.10 |
|
|
L.S.D 1% |
0.8 |
2.2 |
2.5 |
3.2 |
|||||
Each value means of 3 replicates. BM: Biomagic; ChNPs: Nano chitosan.
Table 6: Effect of ChNPs and biomagic on some phytochemical contents in FLV-1infected fig plants compared with healthy ones (in vivo).
|
Parameters/ Treatment |
Phenol (O.D.725 nm) |
Proline (O.D.520 nm) |
MDA (mM/cm1) |
|
|
Healthy plant |
Control |
0.046 |
0.62 |
16.2 |
|
ChNPs |
0.122 |
0.87 |
18.5 |
|
|
Biomagic |
0.149 |
0.96 |
20.3 |
|
|
ChNPs +biomagic |
0.164 |
1.22 |
21.4 |
|
|
Infected plant (Control) |
0.096 |
0.98 |
17.5 |
|
|
Treated infected plants |
ChNPs |
0.131 |
1.35 |
24.6 |
|
Biomagic |
0.176 |
1.64 |
28.2 |
|
|
ChNPs+biomagic |
0.182 |
1.82 |
30.2 |
|
|
L.S.D 1% |
0.24 |
0.13 |
1.2 |
|
Each value means of 3 replicates. Phenol (mg g-1DW), Proline (µg g-1DW), MDA (mmol g-1DW), Salicylic acid (mg g-1DW).
Table 7: Anatomical parameters and counts of blade and petiole of healthy and infected fig leaves.
|
Anatomical parameters |
Fig leaves |
||||||
|
Healthy |
Infected |
% Relative FLV/ infection |
Infected treated |
% Relative treatment |
|||
|
Blade |
Upper epidermis (u) |
52 |
36 |
30.76 |
47 |
30.55 |
|
|
Palisade tissue thickness (u) |
245 |
210 |
14.28 |
236 |
12.38 |
||
|
Spongy tissue thickness (u) |
312 |
195 |
37.5 |
262 |
34.35 |
||
|
Vascular tissue thickness (u) |
375 |
264 |
29.6 |
325 |
23.10 |
||
|
Vascular bundle diameter |
Length (u) |
550 |
350 |
54.54 |
510 |
45.70 |
|
|
Width (u) |
570 |
٣٦٠ |
54.38 |
512 |
42.22 |
||
|
Xylem |
Thickness |
185 |
105 |
34.24 |
175 |
66.66 |
|
|
Row No |
35 |
19 |
65.71 |
30 |
57.71 |
||
|
Phloem |
Thickness (u) |
125 |
95 |
24 |
115 |
24.00 |
|
|
Row No |
5 |
4 |
20 |
5 |
25.00 |
||
|
Fiber thickness (u) |
145 |
75 |
55.17 |
131 |
74.66 |
||
|
Main vein thickness (u) |
1285 |
745 |
42.02 |
1225 |
64.2.9 |
||
|
Lower epidermis (u) |
35 |
24 |
31.42 |
30 |
25.00 |
||
|
Blade thickness (u) |
625 |
412 |
34.08 |
505 |
22.57 |
||
|
Petiole |
Epidermis thickness (u) |
39 |
20 |
58.97 |
35 |
75 .00 |
|
|
Collenchyma thickness (u) |
75 |
39 |
52 |
65 |
66.66 |
||
|
Parenchyma thickness (u) |
320 |
193 |
55.31 |
290 |
84.71 |
||
|
Fiber thickness (u) |
95 |
52 |
52.63 |
91 |
75 .00 |
||
|
Vascular tissue |
Thickness (u) |
675 |
٥٤٥ |
20.15 |
612 |
12.29 |
|
|
Number |
15 |
10 |
33.33 |
13 |
30.00 |
||
|
Xylem |
Thickness (u) |
312 |
245 |
27.34 |
282 |
15.10 |
|
|
Row No |
12 |
9 |
33.33 |
11 |
22.22 |
||
|
Phloem |
Thickness (u) |
72 |
54 |
33.33 |
68 |
25.92 |
|
|
Row No |
4 |
2 |
50.00 |
4 |
50.00 |
||
|
Pith diameter (u) |
1142 |
715 |
59.72 |
1121 |
56.78 |
||
|
Cross section diameter (u) |
5124 |
4325 |
18.47 |
4822 |
11.49 |
||
Correction of the changes in anatomical structure of virus infected fig leaves
Anatomical characters: FLV infection reduced the anatomical structure of fig leaf lamina upper and lower epidermis, palisade, spongy, vascular tissue thickness, vascular bundle diameter, number and thickness of xylem and phloem, fiber, main vein and blade thickness as well as petiole fig leaf (epidermis, collenchyma, parenchyma thickness, vascular bundle diameter, number and thickness of xylem and phloem, pith and cross section diameter). However, the treatment of fig plants with ChNPs and biomagic led to repair of the anatomical structure of lamina and petiole of FLV-infected fig plants (Table 7 and Figure 4).
Repair of ultrastructural cell changes
FLV infection reduced and deformed the organelles of fig leaf cells (Figure 5B, E, H, K) compared with healthy ones (Figure 5A, D, F, J). Treatment of fig plants with ChNPs and biomagic led to repair of deformations and ultrastructural changes of fig leaf cells of FLV-infected fig plants (Figure 5C, F, I, L).
Healthy leaf cells (Figure 5A, D, F, J): The examination of healthy ultrathin sections of mature leaves, showed mesophyll cells with relatively large intercellular spaces. The mesophyll cells are nearly rounded chlorenchyma with uniformly thin cell wall and contained normal nucleus, chloroplasts, and mitochondria (Figure 3A). The chloroplast in healthy cells was typical lens-shaped and bounded by an envelope, enclosing the stromal, grana discs arranged in regular rows and connected to thylakoids membrane. The mitochondria are slightly ovoid and bounded by a smooth envelope enclosing matrix. Some mitochondrial matrix contains electron dense regions and the nucleoids (Figure 5A, D, F, J).
Virus infected leaf cells (Figure 5B, E, H, K): FLV virus induced formation of numerous vesicles in the cytoplasm from degraded cell membranes, chloroplasts, mitochondria, and nucleus that were condensed and located along the cell membrane. Normal nuclei and ruptured endoplasmic reticulum were detected in the infected cells (Figure 5). Treatment of fig plants with nanochitosan and biomagic led to a significant increase in plant health, repair and improvement of the effects caused by Fig latent virus infection. FLV-1 infected leaves showed different cytopathic effects (Figure 5B, E, H, K). The mesophyll tissues have relative small intercellular spaces (Figure 5B, E, H, K). The infected fig leaves revealed the presence of completely destroyed cells (Figure 5). The mesophyll tissue is large and some cells are lacking chlorenchyma with thin cell wall and contained deformed membranes of nucleus, chloroplasts and mitochondria compared with healthy mesophyll tissue (Figure 5B, E, H, K).
Cell wall in healthy cell with uniformly thin cell wall was complete and composed of three layers. While in infected cell it was destroyed, incomplete and layers thickness (Figure 5B, E, H, K).
The chloroplasts alterations
Infected fig leaf cells were showed alterations in chloroplasts compared with healthy ones. Such as slightly elongated chloroplasts with irregular rows of grana and decreased in number (Figure 5B, E, H, K) unusual numbers of plastoglobules in grana and stroma and destructed regions in chloroplastids which do not organized into grana and thylakoid system (Figure 5B, E, H, K).
Nucleus
It was showed with several dark stained botches or without chromatin (Figure 5) and sometimes nucleolus appeared deformed shape (Figure 5B, E, H, K). The cell wall lignification due to small size of intercellular spaces (IS) between mesophyll cells (Figure 5) compared with healthy ones (Figure 5B, E, H, K).
Cytoplasm
Many cytoplasmic vesicles formed by imagination of the plasmalemma or tonoplast were presence in leaf infected with FLV only (Figure 5B, E, H). Virus particles were found in infected cells surrounded with plasma membrane (Figure 5B, E, H). As well as inclusion bodies intracellular structures with different types related to viral infection (Figure 5B). The amorphous inclusions which aggregate of single small rounded particles (Figure 5B, E, H) in cytoplasm and vessels cylindrical inclusion bodies in mitochondria, multiple membranes bodies appeared as tubules in their longitudinal section (Figure 5B, E, K) and frequently associated with an internal core either simple or compound and usually appeared along plasma membrane into vacuoles.
Treatment of fig plants with nanochitosan and biomagic led to a significant increase in plant health, growth characteristics, immunity against virus infection as well as repair of the effects caused by the FLV infection. It has been suggested that, the induction of systemic acquired resistance was successfully achieved; it also protected fig plants against FLV infection.
Relative water content (RWC) was significantly increased in infected fig plants sprayed with ChNPs, biomagic and their combination of ChNPs and biomagic compared to healthy and infected plants. Fig plants under the influence of FLV with temperature, and relative humidity at winter season (Abd El-Rahman and Batanouny, 1965) and (Moursy, 2013) showed different responses in the transpiration rate comparing with healthy plants. These results are in agreement with Radwan et al. (2007) who demonstrated that the effect of diseases on plants may increase or decrease transpiration rate of infected plants, depending on the mode of infection. Also, it showed different responses in the rate of electrical leakage and the cell membrane stability compared with healthy plants. Evidence suggests that membranes are the primary sites of salinity injury to cells and organelles (Candan and Tarhan, 2003) because ROS can react with unsaturated fatty acids to cause peroxidation of essential membrane lipids in plasmalemma or intracellular organelles (Karabal et al., 2003). Peroxidation of plasmalemma leads to the leakage of cellular contents, rapid desiccation and cell death. Stability of biological membranes has been taken as an effective screening tool to assess the salinity stress effects (Kukreja et al., 2005).
The chlorophylls a, b and carotenoid contents of infected fig leaves were decreased compared to virus infected fig plants treated with ChNPs and biomagic compared with healthy ones. These results were in agreement with Badarau et al. (2012), who demonstrated that plants mechanically infected with FLV-1 suffered significantly harmful effects on pigment contents. Concerning the changes in the leaves pigment contents, foliar mosaic represents a common symptom of primary infection with FLV-1. Also, Raithak and Gachande, (2012) reported that the level of depletion of chlorophyll, ß-carotene and lycopene was found to be varied depending upon virus infection of fig plants. This result in agreement with (Shalitin and Wolf, 2000) and (Tajul et al., 2011), they reported that virus infections is known to affect plant physiology dramatically, including decreased photosynthesis, increased respiration and altered carbohydrate levels.
Total soluble protein content levels differed in virus infected, virus infected and treated with ChNPs and biomagic compared with healthy leaves of fig plants. Since virus infection unexpectedly increased the incorporation of proteins. It is probable that virus infection increased protein accumulation via the incorporation. This cannot confirm the explanation of Prasad et al. (1985) in which they attributed increased protein content partly due to proteinous nature of virus itself. A decrease of total soluble proteins due to viral infection has been reported by (Taiwo and Akinjogunla, 2006). Plant acclimation to stress is associated with profound changes in their proteome and metabolome. Since proteins are directly involved in plant response to biotic stresses, proteomics studies can significantly contribute to unravel the possible relationships between protein abundance and plant pathogen interactions (Moshe et al., 2012).
Phytochemical contents, phenol, proline and MDA were significantly increased in FLV infected and treated with ChNPs, biomagic and combination, compared with non-treatment and healthy fig plants ones. The current study suggested that treated FLV infected fig plants with ChNPs and biomagic compared with healthy ones leave of fig plants induced oxidative stress which enforces the host to produce high level of reactive oxygen species (ROS) where ROS play a central role in plant pathogen defense. These results in agreement with (Treutter, 2006; Kumar et al., 2010; Rai et al., 2011), they reported that phenols play an important role in host pathogen interaction, disease development and defense reaction of infected plants. Hence, the increased quantity of phenolics in the infected parts of pumpkins presumably appears to contribute towards the resistance against viral infection. Phenolic compounds including flavonoids are structurally diverse group of plant secondary products that can play a variety of roles in plant defense against pathogens, such as phytoanticipins, phytoalexins, structural barriers and activators for plant defense genes (Junqueria et al., 2004; Treutter, 2006). The antioxidant activity of phenolic compounds can play an important role in neutralizing ROS (Zheng and Wang, 2001). Also, proline was increased in virus infected, virus infected and treated with ChNPs and biomagic compared with healthy ones leaves of fig plants compared to healthy ones. Increased levels of proline in plants correlate with enhanced stress tolerance (Ashraf and Foolad, 2007; Mazid et al., 2011). Proline and other amino acids are the most common compatible solutes that occur in a wide variety of plants. They provide protections against stress by maintaining redox homeostasis, scavenging free radicals (Haque et al., 2008).
Whereas, (Hammond-Kosack and Jones, 2000; Ali et al., 2006) reported that during this response, ROS are produced by plant cells via the enhanced enzymatic activity of plasma-membrane. Overall, ROS amounts increase to critical levels and induce programmed cell death (PCD)
In addition, phenol oxidizing enzymes such as POX and PPO are associated with many diseases Ali et al. (2006) suggested that the increased POX activity following virus infection was a reflection of physiological changes associated with, but not responsible for, induced POX participates in a variety of plant defense mechanisms in which H2O2 is often supplied by an oxidative burst, a common event in defense responses (Dixon and Lamb, 1990). The cell wall of plants appears to be a major site for defense related peroxidase polymerization reactions such as lignification (Hammerschmidt and Kuc, 1982) and cross-linking of structural cell wall protein (Fry, 1986). POXs comprise one important class of PR proteins implicated in these (defense responses) in which an important role is to catalyze the formation of phenolic radicals at the expense of H2O2 and oxidize phenolic monomers to form lignin (Gaspar et al., 1985; Goldbach et al., 2003). Scavenging enzymes were increased mostly in FLV infected fig plants compared to healthy ones. Since many defense enzymes are involved in defense reaction against plant pathogens. These include oxidative peroxidase (PO) and polyphenol oxidase (PPO), which catalyzes the formation of lignin and other oxidative phenols that contribute to the formation of (defense barriers for reinforcing the cell structure (Hernández et al., 2001; Sairam et al., 2005). The induction of antioxidant enzymes, such as SODs, POXs and CATs is the most common mechanism for detoxifying ROS synthesized during stress response in order to protect cellular membranes and organelles from the damaging effects of ROS (reactive oxygen species) (Khalil, 2011). Lastochkina (2019) reported that PGPB was proposed to reduce MDA to a minimum level to counteract salinity conditions. Proline reduces the detrimental effects of salinity by mediating ROS scavenging to maintain proteins and other essential biomolecular structures, in addition to the crucial function of proline, citric acid, and total soluble sugar levels in the maintenance of cell water balance (Abu-Shahba et al., 2021; Mowafy et al., 2021).
ROS molecules must be detoxified to reduce injury under stressful conditions. In addition, the efficient destruction of ROS requires the coordination of many antioxidant enzymes (Sofy et al., 2021). Multiple scavenging enzymes, like CAT, POX, and PPO, reduce the impact of ROS. On the other hand, many antioxidant enzymes are fully dedicated to maintaining ROS homeostasis, while others are involved in growth, redox control of target proteins, and detoxification processes (El-Beltagi et al., 2018; El-Beltagi et al., 2020). Antioxidant enzymes increase, implying that more and more ROS is needed, and such ROS effects are decreased. CAT plays an essential role in the elimination of H2O2 from the cell’s various organelles. POX is also engaged in H2O2 scavenging and plays an essential function in stress tolerance (Mohamed et al., 2018). ROS-scavenging enzymes like CAT and PPO were significantly increased in different crops inoculated by PGP microbial isolates in saline conditions (Hmaeid et al., 2019).
The architectural organization of mesophyll cells was apparently well preserved and organelles, like nuclei and mitochondria, had a normal aspect. Chloroplasts, however, were misshapen, swollen and exhibited a disarranged membrane system. The ground cytoplasm of cells of all samples examined contained a great number of flexible virus-like particles scattered in the cytosol or arranged in bundles. By (Appiano, 1982; Martelli et al., 1993), which are thought to be particles of fig mosaic virus (FMV) (Elbeaino et al., 2009). Treatment of fig plants with nanochitosan and biomagic led to a significant increase in plant growth, the changes in anatomical tissue and ultrathin cell structural, immunity against virus infection as well as, repair of the effects caused by the FLV infection.
Conclusions and Recommendations
We recommend the use of Nanochytosan and Biomagic against plant virus infection.
- Biomagic strengthens and supplies plant growth, both in anatomical structure and appearance, and increases plant resistance to viral infection.
- Nanocytosan has an effective role as antiviral, as there was an increase in leaf area, plant length and dry weight compared to non-sprayed plants.
- Periodic examination of fig trees (and any inffected tree labeled) as for the apparent symptoms, (and that is daily) and serology examination
- The examination begins with healthy and then infected trees to prevent the spread of infection.
Acknowledgement
Not applicable
Novelty Statement
Our Results showFig plants sprayed with ChNPs, biomagic, and the combination of the two showed considerably elevated activity in the antioxidant enzymes catalase, peroxidase, polyphenoloxidase, and superoxide dismutase. They found that in Fig plants sprayed with ChNPs, bio magic as well as the combination before and post of viral inoculation challenge with FLV, Physiological processes were altered.
Compared to healthy and infected plants, the electrolyte leakage in infected fig plants was much higher when sprayed with ChNPs, biomagic, or a combination of ChNPs + biomagic. When compared to healthy individuals, the FLV-infected individuals had a significantly higher membrane stability index (MSI) than those who were not.
Author’s Contribution
Prof. Khaled El-dougdoug devised the project, the main conceptual ideas and proof outline, conceived, and planned the experiment. Prof. Hanaa Hussien discussed the results and contributed to the interpretation of the results. Prof. Mona Ismail contributed to the final manuscript. Basma Hamdy contributed to sample preparation. Dalia Yousri carried out the experiments, wrote the manuscript with input from all authors. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abd El-Rahman, A.A. and Batanouny, K.H., 1965. Transpiration of desert plants under different environmental conditions. J. Ecol., 53(2): 267-272. https://doi.org/10.2307/2257974
Abu-Shahba, M.S., Mansour, M.M., Mohamed, H.I., and Sofy, M.R., 2021. Comparative cultivation and biochemical analysis of iceberg lettuce grown in sand soil and hydroponics with or without microbubbles and macrobubbles. J. Soil Sci. Plant Nutr., 21(1): 389-403. https://doi.org/10.1007/s42729-020-00368-x
Ali, S.H., Eisa, S.S. and El-Dougdoug, K.A., 2006. Role of reactive oxygen species and anti-oxidants in hypersensitive local virus-infected plants. J. Agric. Sci. Mansoura Univ., 31: 6465-6480. https://doi.org/10.21608/jssae.2006.225031
Appiano, A., 1982. Cytological observations on leaves of fig infected with fig mosaic. Caryologia, 35: 152.
Ashraf, M. and Foolad, M.R., 2007. Roles of glycine betaine and proline in improving plant abiotic stress tolerance. Environ. Exp. Bot., 59: 206-216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Assaha, D.V., Ueda, A., Saneoka, H., Al-Yahyai, R., and Yaish, M.W., 2017. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol., 8: 509. https://doi.org/10.3389/fphys.2017.00509
Badarau, C.L., Chiru, N., Dams, A.F. and Nistor, A., 2012. Effects of Saturejahortensis oil treatments and exogenous H2O2 on potato virus Y (PVY) Infected Solanumtuberosum L. plants under drought conditions.
Bates, L.S., Waldren, R.P., and Teare, I.D., 1973. Rapid determination of free proline for water-stress studies. Plant Soil, 39(1): 205-207. https://doi.org/10.1007/BF00018060
Bergmeyer, H.U. and Bernt, E., 1974. Lactate dehydrogenase. UV-assay with pyruvate and NADH. In: Bergmeyer, H.U., Ed., Methods of Enzymatic Analysis, Academic Press, New York, pp. 574-578. https://doi.org/10.1016/B978-0-12-091302-2.50010-4
Brilli, F., Pollastri, S., Raio, A., Baraldi, R., Neri, L., Bartolini, P., Podda, A., Loreto-Maserti, B.E., Balestrini, R., 2019. Root colonization by Pseudomonas chlororaphis primes tomato (Lycopersicumesculentum) plants for enhanced tolerance to water stress. J. Plant Physiol., 232: 82-93. https://doi.org/10.1016/j.jplph.2018.10.029
Candan, N., and Tarhan, L., 2003. The correlation between antioxidant enzyme activities and lipid peroxidation levels in Menthapulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant Sci., 163: 769-779. https://doi.org/10.1016/S0168-9452(03)00269-3
Corgan, J.N., and Widmoyer, F.B., 1971. The effect of gibberellic acid on flower differentiation, data of bloom, and flower hardiness of peach J. Am. Sco., 96: 54-57. https://doi.org/10.21273/JASHS.96.1.54
Dalia, Y.Z.A., 2021. Application of Defensive strategies on virus infected fig plants, MSc, Dept. of Microbiology, Fac. Sci. Suez Canal Univ. pp. 120.
Daniel, H.D. and George, C.M., 1972. Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. J. Am. Soc. Hortic. Sci., 97: 651-654. https://doi.org/10.21273/JASHS.97.5.651
Dixon, R.A. and Lamb, C.J., 1990. Molecular communication in interactions between plants and microbial pathogens. Ann. Rev. Plant Mol. Biol., 41: 339-367. https://doi.org/10.1146/annurev.pp.41.060190.002011
Elbeaino, T., Digiaro, M., Alabdullah, A., De Stradis, A., Minafra, A., Mielke, N., Castellano, M.A., and Martelli, G.P., 2009. A multipartite single-stranded negative-sense RNA virus is the putative agent of fig mosaic disease. J. Gen. Virol., 90: 1281-1288. https://doi.org/10.1099/vir.0.008649-0
El-Beltagi, H.S., Mohamed, H.I., Megahed, B.M., Gamal, M., and Safwat, G., 2018. Evaluation of some chemical constituents, antioxidant, antibacterial and anticancer activities of Beta vulgaris L. root. Fresenius Environ. Bull., 27(9): 6369-6378.
El-Beltagi, H.S., Sofy, M.R., Aldaej, M.I., and Mohamed, H.I., 2020. Silicon alleviates copper toxicity in flax plants by up-regulating antioxidant defense and secondary metabolites and decreasing oxidative damage. Sustainability 12(11): 4732. https://doi.org/10.3390/su12114732
El-Massriy, M.M.A., 2009. Production of lettuce using organic, bio- and mineral fertilization under salineconditions. Ph. D. thesis. Dept. Hortic. Fac. Agric. Ainm. Shams University, Cairo, Egypt.
Flock, R., and Wallace, J., 1955. Transmission of fig mosaic by the eriophyid mite Aceriaficus. Phytopathology, 45: 52–54.
Fry, S.C., 1986. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Ann. Rev. Plant Physiol., 37: 165-183. https://doi.org/10.1146/annurev.pp.37.060186.001121
Gaspar, T., Penel, C., Castillo, F.J., and Greppin, O., 1985. A two-step control of basic and acidic peroxidases and its significance for growth and development. Physiol. Plant, 64: 418-423. https://doi.org/10.1111/j.1399-3054.1985.tb03362.x
Gattoni, G., Minafra, A., Castellano, M.A., De Stradis, A., Boscia, D., Elbeaino, T., Digiaro, M. and Martelli, G.P., 2009. Some properties of fig latent virus 1, a new member of the family Flexiviridae. J. Plant Pathol., 91: 555-564.
Goldbach, R., Bucher, E., and Prins, M., 2003. Resistance mechanisms to plant viruses: An overview. Virus Res., 92: 207-212. https://doi.org/10.1016/S0168-1702(02)00353-2
Hafez, M.R., 2013. Effect of some biological components on Jerusalem Artichoke (Helianthus tuberosus L.) productivity under north Sinai conditions. J. Appl. Sci. Res., 9(1): 804-810.
Hammerschmidt, R. and Kuc, J., 1982. Lignification as a mechanism for induced systemic resistance in cucumber. Physiol. Plant Pathol., 20: 61-71. https://doi.org/10.1016/0048-4059(82)90024-8
Hammond-Kosack, K.H., and Jones, J.D.G., 2000. Responses to plant pathogens. In: Biochemistry and molecular biology of plants. Am. Soc. Plant Physiol., Rockville-Maryland.
Haque, A.M.M., Christopher, O. and Akinbile, 2008. Arsenic contamination in irrigation water for rice production in Bangladesh: A review.
Heath, R.L., and Packer, L., 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125: 189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hernández, J.A., Talavera, J.M., Martínez-Gómez, P., Dicenta, F., and Sevilla, F., 2001. Response of antioxidative enzymes to plum pox virus in two apricot cultivars. Physiol. Plant., 111: 313-321. https://doi.org/10.1034/j.1399-3054.2001.1110308.x
Hmaeid, N., Wali, M., Metoui-Ben, M.O., Pueyo, J.J., Ghnaya, T., and Abdelly. 2019. Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl. Soil Ecol., 133: 104-113. https://doi.org/10.1016/j.apsoil.2018.09.011
Johansen, D.A., 1940. Plant microtechnique, MC. Grow Hill Book CO., New York.
Jones, R.A.C., and Smith, L.J., 2000. Inheritance of hypersensitive resistance to Bean yellow mosaic virus in narrow-leafed lupin (Lupinusangustifolius). Ann. Appl. Biol., 146: 539-543. https://doi.org/10.1111/j.1744-7348.2005.040148.x
Junqueria, A., Bedendo, I. and Pascholati, S., 2004. Biochemical changes in corn plants infected by the maize bushy stunt phytoplasma. Physiol. Mol. Plant Pathol., 65: 181-185. https://doi.org/10.1016/j.pmpp.2005.01.005
Karabal, E., Yucel, M. and Oktem, H.A., 2003. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci., 164: 925-933. https://doi.org/10.1016/S0168-9452(03)00067-0
Khalil, R.R., 2011. Physiological effects of stigmastereol on growth, productivity and certain metabolic activities of two economic crop plants grown under salinity stress. Ph.D. thesis. Plant Pathol. Dept. Fac. Sci. Banha Univ. Egypt.
Kukreja, S., Nandwal, A.S., Kumar, N. and Sharma, S.K., 2005. Plant water status, H2O2 scavenging enzymes, and ethylene evolution and membrane integrity of Cicerarietinum roots as affected by salinity. Plant Biol., 49: 305. https://doi.org/10.1007/s10535-005-5308-4
Kumar, A., Singh, S., Gaurav, A.K., Srivastava, S., and Verma, J.P., 2020. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol., 11: 1216. https://doi.org/10.3389/fmicb.2020.01216
Kumar, A., Mali, P.C. and Manga, V.K., 2010. Changes of some phenolic compounds and enzyme activities on infected pearl millet caused by Sclerosporagraminicola. Int. J. Plant Physiol. Biochem., 2(1): 6-10.
Lastochkina, 2019. Bacillus subtilis-mediated abiotic stress tolerance in plants. In: (eds. Islam, M.T., Rahman, M.M., Pandey, P., Boehme, M.H., Haesaert, G.,) Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol: Volume 2. Springer International Publishing, Cham, pp. 97-133. https://doi.org/10.1007/978-3-030-15175-1_6
Latinović, J., Radišek, S., Bajčeta, M., Jakše, J. and Latinović, N., 2019. Viruses associated with fig mosaic disease in different fig varieties in montenegro. Plant Pathol. J., 35(1): 32-40. https://doi.org/10.5423/PPJ.OA.04.2018.0058
Lichtenthaler, H.K., 1987. Chlorophyll and cartenoidspigments of photosynthetic biomembranes. Methods Enzymols, 148: 350-282. https://doi.org/10.1016/0076-6879(87)48036-1
Luft, J.H., 1961. Improvement in epoxy resin embedding method. J. Biophys. Biochem. Cytol., 9: 409-414. https://doi.org/10.1083/jcb.9.2.409
Marklund, S. and Marklund, G., 1974. Involvement of superoxide anion radical in autoxidation of pyrolgallol and convenient assay for superoxide dismatase. Eur. J. Biochem., 47: 469-474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Martelli, G.P., Castellano, M.A., and Lafortezza, R., 1993. An ultrastructural study of fig mosaic. Phytopathol. Mediterr., 32: 33-43.
Martelli, G.P., 2011. Fig mosaic disease and associated pathogens. In: (eds. Hadidi, A., Barba, M., Candresse, T., Jelkmann, W.,). Virus and virus-like diseases of pome and stone. J. Plant Pathol., 91(3): 555-564.
Matta, A. and Dimond, A.E., 1963. Symptoms of Fusarium wilt in relation to quality of fungus and enzyme activity in tomato stems. Phytopathology, 53: 574-575.
Mazid, M., Khan, T.A., and Mohammad, F., 2011. Role of Nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: A synergistic signaling approach. J. Stress Physiol. Biochem., 7(2): 2011.
Megahed, A., El-Dougdoug, K., Othman, B., Lashin, S., Ibrahim, M., and Sofy, A., 2012. A new Egyptian satellite strain of cucumber mosaic cucumovirus. Int. J. Virol., 8(3): 240-257. https://doi.org/10.3923/ijv.2012.240.257
Megahed, A., KhA, E-D., Othman, B., Lashin, S., Ibrahim, M., Sofy, A., 2013. Induction of resistance in tomato plants against tomato mosaic to bamovirus using beneficial microbial isolates. Pak. J. Biol. Sci., 16(8): 385-390. https://doi.org/10.3923/pjbs.2013.385.390
Mohamed, H.I., El-Beltagi, H.S., Aly, A.A., and Latif, H.H., 2018. The role of systemic and non systemic fungicides on the physiological and biochemical parameters in Gossypiumhirsutum plant, implications for defense responses. Fresenius Environ. Bull., 7(12): 8585-8593.
Moshe, A., Jens, P., Brotman, Y., Mikhail, K., Iris, S., Henrykl, C. and Renal, G., 2012. Stress responses to tomato yellow leaf curl virus (TYLCV) infection of resistant and susceptible tomato plants are different. Metabolomics Sl: 006.
Moursy, M., 2013. A study of floristic composition and ecological gradients in Saint Katherine Mountain, Saint Katherine protectorate, South Sinai, Egypt. Ph.D. Thesis. Botany and Micro. Dept. Faculty of Sci. Al-Azhar University. Egypt.
Mowafy, A.M., Fawzy, M.M., Gebreil, A., and Elsayed, A., 2021. Endophytic Bacillus, Enterobacter, and Klebsiella enhance the growth and yield of maize. Acta Agric. Scand. B Soil Plant Sci., 71(4): 237-246. https://doi.org/10.1080/09064710.2021.1880621
Mukherjee, S.P., and Choudhuri, M.A., 1983. Implication of water stress-induced changes in level of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant, 58: 166-170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x
Nesma, I.H., Sahar, A.Y., Othman, B.A., and El-Dougdoug, K.A., 2018. Preparation, characterization of chitosan nanoparticles and it effect on Tobacco mosaic virus infectivity in vitro. Int. Conf. Adv. Technol. Appl. Agric. Int. Res. Center, pp. 27-29.
Ngalimat, M.S., Mohd, H.E., Zulperi, D., Ismail, S.I., Ismail, M.R., Mohd, Z.N.A.I., Saidi, N.B., Yusof, M.T., 2021. Plant growth-promoting bacteria as an emerging tool to manage bacterial rice pathogens. Microorganisms, 9(4): 682. https://doi.org/10.3390/microorganisms9040682
Prasad, B., Verma, O.P. and Daftari, L.N., 1985. Effect of leaf curl virus infection on metabolism of sesame leaves. Ind. Phytopathol., 38: 343-345.
Radwan, D.E.M., Fayez, K.A., Mahmoud, S.Y., Hamad, A. and Lu, G., 2007. Physiological and metabolic changes of Cucurbitapepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiol. Biochem., 45: 480–489. https://doi.org/10.1016/j.plaphy.2007.03.002
Rai, G.K., Singh, K.R., Rai, P.K. and RAI, S.K., 2011. Peroxidase, polyphenol oxidase activity, protein profile and phenolic content in tomato cultivars tolerant and susceptible to Fusarium F. sp. Lycoper Sci. Pak. J. Bot., 43(6): 2987-2990.
Raithak, P.V. and Gachande, B.D., 2012. Changes in pigment contents of virus infected tomato plant. Botany Research Laboratory and Plant Disease Clinic, N.E.S. Science College, Nanded-431605-India. Asian J. Biol. Biotechnol.,
Roistacher, C., 1991. Graft-transmissible diseases of citrus. Hand Book for Detection and Diagnosis, FAO. Roma, 286.
Ruzin, S.E., 1999. Staining techniques. In: (eds. Ruzin, S.E.,). Plant Microtechnique and microscopy. New York; Oxford University Press, pp. 87-116.
Sairam, R.K., 1994. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol., 3: 584–593.
Sairam, R.K., Srivastava, G.C., Agarwal, S. and Meena, R.C., 2005. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant., 49: 85-91. https://doi.org/10.1007/s10535-005-5091-2
Shahmirzaie, M., Rakhshandehroo, F., Zamanizadeh, H.R., Minafra, A., and Martelli, G.P., 2010. First report of Fig latent virus 1 (FLV-1) from Fig trees in some provinces of Iran. 19th Iran. Plant Prot. Cong., 31 July- 3 August 2010.
Shalitin, D., and Wolf, S., 2000. Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol., 123: 597-604. https://doi.org/10.1104/pp.123.2.597
Sofy, M.R., Aboseidah, A.A., Heneidak, S.A., and Ahmed, H.R., 2021. ACC deaminase containing endophytic bacteria ameliorate salt stress in Pisumsativum through reduced oxidative damage and induction of antioxidative defense systems. Environ. Sci. Poll. Res., pp. 1-21. https://doi.org/10.1007/s11356-021-13585-3
Sullivan, C.Y., and Ross, W.M., 1979. Selecting for drought and heat resistance in grain sorghum. In: Mussell, H. and Staples, R.,). Stress Physiology in Crop Plants. New York, USA: John Wiley.
Taiwo, M.A., and Akinjogunla, O.J., 2006. Cowpea viruses: Quantitative and Qualitative effects of single and mixed viral infections. Afr. J. Biotech., 5: 1749–1756.
Tajul, M.I., Naher, K., Hossain, T., Siddiqui, Y. and Sariah, M., 2011. Tomato yellow leaf curl virus (TYLCV) alters the phytochemical constituents in tomato fruits. AJCS, (5): 575-581.
Treutter, D., 2006. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett., 4: 147–157. https://doi.org/10.1007/s10311-006-0068-8
Walia, J.J., Salem, N.M., and Falk, B.W., 2009. Partial sequence and survey analysis identify a multipartite, negative-sense RNA virus associated with fig mosaic. Plant Dis., 93: 4–10. https://doi.org/10.1094/PDIS-93-1-0004
Wang, M., Ding, F., Zhang, S., 2020. Mutation of SlSBPASE aggravates chilling induced oxidative stress by impairing glutathione biosynthesis and suppressing ascorbate-glutathione recycling in tomato plants. Front. Plant Sci., 11: 2135. https://doi.org/10.3389/fpls.2020.565701
Yang, X., Liangyi, K., and Tien, P., 1996. Resistance of tomato infected with cucumber mosaic virus satellite RNA to potato spindle tuber viroid. Ann. Appl. Biol., 129: 543-551. https://doi.org/10.1111/j.1744-7348.1996.tb05775.x
Zhang, Y., Li, Y., Hassan, M.J., Li, Z., and Peng, Y., 2020. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol., 20(1): 1-12. https://doi.org/10.1186/s12870-020-02354-y
Zheng, W., and Wang, S.Y., 2001. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem., 49: 5165-517. https://doi.org/10.1021/jf010697n
Zohary, D., Hopf, M., and Weiss, E., 2012. Domestication of plants in the old world: The origin and spread of domesticated plants in southwest Asia, Europe, and the Mediterranean Basin. Oxford University Press, Demand. (Managing editor Zhang Juan). https://doi.org/10.1093/acprof:osobl/9780199549061.001.0001
To share on other social networks, click on any share button. What are these?




