Impact of Fig-Enriched Tris-Albumin Extender on the Quality of Boer Buck Spermatozoa
Research Article
Impact of Fig-Enriched Tris-Albumin Extender on the Quality of Boer Buck Spermatozoa
Lalu Ahmad Zaenuri*, Rodiah Rodiah
Faculty of Animal Science University of Mataram, Majapahit Street, No. 62, Mataram Lombok, 83125, West Nusa Tenggara, Indonesia.
Abstract | This study aims to evaluate the effect of additional fig fruit filtrate in a Tris-albumin-based extender on the motility, viability, and morphology of Boer buck spermatozoa. The semen used in this study was collected from a 3-year-old Boer buck weighing 85 kg, which was well-trained, fertile, and free of both internal and external parasites. Ten semen samples were collected using an artificial vagina every four days over a period of ten consecutive days. The collected semen was divided into four tubes based on to the treatment extenders: FF0 (0 ml), FF1 (4 ml), FF2 (6 ml), and FF3 (8 ml) of fig fruit filtrate, each added to 100 ml of a Tris-albumin-based extender (v/v). Each tube was then divided into six aliquots according to the assessment frequencies. The spermatozoa concentration was 75 × 10^6 per 0.25 mL. Progressive motility, viability, and morphology were assessed at 0 hours and every two hours up to 10 hours after dilution and storage at 27°C. The statistical significance of the results was assessed using a one-way analysis of variance (ANOVA). significantly different results were further tested with Ducan’s test with SPSS version16.0 software. The results indicate that the mean percentages of progressive motility in FF0, FF1, FF2, and FF3, measured 10 hours post-extension and preserved at 27ºC, were 45.04±2.74, 43.04±4.18, 47.05±5.70, and 53.80±1.74, respectively. The mean percentages of sperm viability were 48.09±3.15, 50.52±2.50, 50.12±2.83, and 57.88±3.85, respectively. The mean percentages of abnormal sperm were 15.21±3.26, 14.15±2.16, 13.03±1.11, and 12.12±1.22, respectively. The results of the study suggest that the addition of 8 ml (v/v) of tris-albumin-based extender has a significant preservative effect on all three indicators: progressive motility, viability, and morphology of spermatozoa, making it suitable for artificial insemination (AI) for up to 10 hours after dilution and storage at 27°C. It would be advantageous to conduct more comprehensive research to investigate the effects of varying FF concentrations in different storage conditions, such as room temperature, refrigeration, and freezing, on semen preservation. This would not only be beneficial for AI programs, but also contribute to the existing literature on semen preservation extenders.
Keywords | Tris, Albumin, Fig fruit filtrate, Spermatozoa, Goat
Received | October 11, 2024; Accepted | November 13, 2024; Published | December 03, 2024
*Correspondence | Lalu Ahmad Zaenuri, Faculty of Animal Science University of Mataram, Majapahit Street, No. 62, Mataram Lombok, 83125, West Nusa Tenggara, Indonesia; Email: ahmadzaenuri@unram.ac.id
Citation | Zaenuri LA, Rodiah R (2025). Impact of fig-enriched tris-albumin extender on the quality of boer buck spermatozoa. Adv. Anim. Vet. Sci., 13(1): 26-35.
DOI | https://dx.doi.org/10.17582/journal.aavs/2025/13.1.26.35
ISSN (Online) | 2307-8316
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
One approach to enhancing the genetic quality of native goats in Indonesia is the use of artificial insemination (AI) technology. Breeders who utilize AI can access liquid or frozen semen from males with superior genetic traits, reducing costs associated with maintaining male buck (Sriana, 2018). Additionally, the AI method can help prevent the transmission of diseases, particularly venereal diseases, and facilitate better selection of goats (Zaenuri and Rodiah, 2016). To achieve a high conception rate, it is essential to use high-quality semen. Factors that influence the success of artificial insemination include the collection and processing of semen in liquid or frozen packaging, as well as the appropriate insemination techniques and timing (Zaenuri et al., 2022). Semen preservation has become an inseparable part of the modern livestock industry (Chunrong et al., 2019), and one of the most obvious benefits, as explained by Agarwal et al. (2004), is the distribution of superior breeds for improving the genetic quality and productivity of local livestock, which ultimately, by increasing livestock productivity, will increase the contribution of livestock to meet human needs, which are always increasing from time to time.
An alternative to frozen semen for artificial insemination is liquid semen. The challenge is that if the extender is not suitable, the quality of liquid semen can deteriorate rapidly. Tris-egg yolk is the most commonly used type of semen extender (Evans and Maxell, 1987). However, unlike when semen is stored at low temperatures, where egg yolk plays a crucial role in preventing cold shock, when semen is maintained at temperatures above 15 °C, the addition of egg yolk is unnecessary (Paulenz et al., 2005).
Recent studies on semen extenders have investigated the use of organic antioxidants, such as vitamins derived from fruits, to enhance their preservation capabilities. For instance, the addition of fig fruit filtrate (Ficus carica Rob) to the tris-egg yolk extender has been extensively studied for its effects on spermatozoa motility and viability (Zenuri et al., 2013). The incorporation of 0.04 g/100 mL fig filtrate (Ficus carica Linn) in Tris aminomenthan-egg yolk-based extender has also demonstrated a positive impact in maintaining membrane integity and intact acrosomes (44.8±5.4 and 21.6±3.1, respectively) of Kacang buck spermatozoa, 96 hours post extended and stored at 5oC (Zaenuri et al., 2017). Additionally, research has been conducted on the supplementation of extenders with dragon fruit (Lukman et al., 2023), tomato juice (Rosmaridar et al., 2013), lecithin (Saaban et al., 2019), guava (Sumadiasa et al., 2015), and geen tea extract (Susilawati et al., 2021). All of these studies utilized extenders based on tris-egg yolk.
Energy and other nutrients are essential for meeting the needs of spermatozoa, along with a buffer to stabilize pH, a cryoprotectant to shield spermatozoa from damage, an antimicrobial agent, antioxidants, non-toxicity, and isotonicity. These components are critical for an effective semen extender (Handoko et al., 2018; Rizal and Riyadhi, 2016; Swari et al., 2019). Various extender formulations have been researched to preserve both liquid and frozen semen.
The extenders commonly used today include sodium citrate or tris (hydroxymethyl) aminomethane combined with 2.5% egg yolk (v/v) (Evan and Maxwell, 1987; Sumadiasa, 2018). Although albumin has not been widely utilized, Fu et al. (2017) indicate that it is particularly effective in maintaining the integity of semen proteins by protecting the plasma membrane and acrosome of spermatozoa in Boars. Perumal et al. (2015) attribute albumin’s ability to reduce hydroxyl radicals (OH-) to its role in the transfer of Fe2+ and Cu+, making it a highly significant antioxidant. Liu et al. (2015) emphasize that the amino acid composition of albumin is crucial for its antioxidant function. Additionally, Guha et al. (2018) and Benede and Molina (2020) note that albumin contains up to 78 different proteins, including glycoproteins such as lysozyme, cystatin, ovalbumin, ovotransferrin, ovomucoid, and ovomucin.
Spermatozoa metabolic activity can produce free radicals during the storage of liquid semen, potentially leading to a decrease in spermatozoa motility and viability. Organic antioxidants can be added to the extender to protect spermatozoa from damage caused by these free radicals (Trilaksana et al., 2015). A fruit that is a good source of organic antioxidants is the fig (Ficus carica). The antioxidant content in figs ranges from 9% to 10% (Siswoyo, 2007). Furthermore, Vincon (1999) reports that figs contain 2, 3, 5, and 7 times the amount of vitamin C (ascorbic acid) compared to dates, oranges, grapes, and bananas, respectively. According to the United States Department of Agriculture (2009), the concentrations of vitamins C, E, K, and B16 per 100 g are 2 mg, 0.11 mg, 4.7 mg, and 0.113 mg, respectively. Figs also have a relatively high polyphenol concentration of 1.09–1.10 mg/100g of fresh figs, as noted by Lukitasari et al. (2014). The phenolic content in dried figs can enhance plasma lipoprotein levels and protect spermatozoa from oxidation.
Vitamin C also plays a crucial role in recycling vitamin E (Sies et al., 1992). Additionally, vitamin C is also known to increase the number of spermatozoa in vivo in infertile men when administered orally at doses ranging from 200 to 1000 mg per day (Dawson et al., 1987). The combination of flavonoids and vitamin C enhances the effectiveness of both compounds (Mathiesen et al., 1996). Furthermore, flavonoids and vitamin C help protect α-tocopherol levels in low-density lipoproteins and delay the onset of lipid peroxidation (de Whallye et al., 1990). Vitamin E plays a significant role in safeguarding cell membranes by neutralizing hydrogen peroxide (H2O2) and protecting plasma membranes from lipid peroxidation. A previous study demonstrated that the oral administration of 600 mg of vitamin E per day improved the quality of spermatozoa (Kessopoulou et al., 1995).
Better semen preservation techniques are essential for enhancing reproduction through artificial insemination methods. Numerous studies have investigated semen cryopreservation using various extenders and techniques, yielding diverse results. By contrast, the demand for artificial insemination continues to grow. Consequently, this research is crucial for exploring alternative preservation methods, specifically evaluating the effect of adding fig fruit filtrate to a Tris-albumin-based extender on the motility, viability, and morphology of Boer buck spermatozoa. The findings of this study aim to provide valuable references and comprehensive insights for developing an extender formula that can maintain spermatozoa quality over an extended period and achieve high pregnancy rates.
MATERIALS AND METHODS
Fig filtrate (FF) and treatment extender preparation
A buffered experimental extender was prepared by dissolving 3.634 g of tris (hydroxymethyl) aminomethane (Sigma, USA), 2.17 g of citric acid monohydrate (Merck, Germany), 0.50 g of fructose (Merck, Germany), 0.06 g of sodium penicillin G (Wonder, Japan), and 0.1 g of streptomycin (Wonder, Japan) in 100 ml of distilled water at pH 7.0, as described by Evans and Maxell (1987).
Fig filtrate was prepared as described by Zaenuri et al. (2014b). Ripe fig fruit was sliced and mixed with distilled water in a 1:1 (w/w) ratio, blended for 3 to 4 minutes. The resulting fig fruit slurry was centrifuged at 3,500 rpm for 30 minutes. Finally, the supernatant was filtered through a 0.20 μm miniphore filter and stored in an Evendorf tube, then pasteurized for 2 minutes at 70ºC. It should be kept in the refrigerator at 5ºC until use.
The volume of FF in each extender is determined by the concentration of vitamin C. The following calculation is used to obtain the FF concentration for extender treatments: Figs have an average water content of 35.91 mL per 100 g and an average vitamin C concentration of 18.15 mg per 1000 mg, which is equivalent to 1.815 mg per 100 g of fresh fig fruit (Zaenuri, 2014a). After juicing and diluting with distilled water in a 1:1 ratio, extension with distilled water, the vitamin C concentration is calculated as follows: = x 1,815 mg/100 g = 0,9075 mg/71.815 mL = 0.1264 mg/100 mL (the water content of the fresh fruit accounts for 71.815 or 2×35.91). 500 mg of vitamin C per 100 milliliters of extender, the volume of FF added is (500/0.1264 mg)/100 mL extender = 3.955 mL/100 mL, which is rounded to 4 mL per 100 mL. Therefore, the quantity of vitamin C in FF is 0.1264 mg per 100 mL, and the amount of FF added was calculated as (500/0.1264 mg)/100 mL extender, which was then converted to 4 mL/100 mL of extender (Zaenuri et al., 2023).
The preparation of treatment extender is as follows: The first extender (T0) consisted of Tris + 2.5% albumin (v/v), followed by T1 (T0 + 4 mL FF/100 mL), T2 (T0 + 6 mL FF/100 mL), and T3 (T0 + 8 mL FF/100 mL). All treatment extenders were homogenized using a magnetic stierrer. To minimoze osmotic shock, only half of the required extender should be added initially, the remaining extender is added after 20 minutes (Mollineou et al., 2011) and homogeneous by mixed gently by shaking. Each experimental extender tube for each ejaculate was divided into six equal parts, corresponding to the frequency of assessments. The samples were stored in a water bath, with the temperature calibrated to 27 oC.
Semen collection and evaluation
Semen was collected from a 4-years old, 85 kg, healthy, trained, and verified fertile Boer buck maintained by the Faculty of Animal Science at Mataram University. Three types of fresh legume tree leaves Sesbania glandiflora, Tarramba (Leucaena leucocephala cv. Tarramba), and Gliricidia sepium were mixed and provided at a minimum of 5% of the buck’s body weight three times a day for four weeks prior to semen collection. The buck had unlimited access to water and was free from both internal and external parasites.
Ten semen samples were collected using an artificial vagina every four days over a period of ten consecutive days. The samples were promptly transported to the laboratory for an initial assessment, which included the evaluation of semen volume, pH, and spermatozoa concentration. Fresh semen should be evaluated directly, as it can be maintained at room temperature for up to 10 minutes without compromising its overall quality (Hahn et al., 2019). According to the Indonesian National Standard, each ejaculation must have a volume greater than 0.8 ml, with at least 70% motility to qualify for AI (SNI4869.3, 2014). Additionally, a spermatozoa concentration of 2.5 to 3 × 109 per milliliter is necessary for subsequent processing (Evans and Maxwell, 1987; Susilawati, 2011). Only semen that meets these criteria is suitable for further processing.
Re-concentration and storage
Spermatozoa were re-concentrated to a concentration of 75 × 106 per 0.25 ml for each treatment extender. The extended frequencies were calculated as follows:
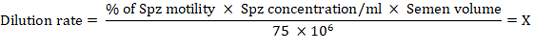
If the volume of fresh semen is 1 ml, then 1/X = x ml (fresh semen) is diluted in 1 ml of extender, resulting in a spermatozoa concentration of 75 × 106 per 1 ml of extender (Zaenuri et al., 2013). To minimize osmotic shock, only half of the required extender should be added initially; the remaining extender should be added after 20 minutes (Mollineou et al., 2011) and mixed thoroughly by gently shaking. Each experimental extender tube was divided into six equal portions, corresponding to the frequency of assessments. The samples were stored in a water bath, with the temperature maintained at 27 °C.
Progesively motile spermatozoa
Spermatozoa exhibiting progressive motility were quantified as a percentage. A single drop of specimen from each treatment tube was placed onto a glass slide, covered with a coverslip, and maintained at 27 °C. The sample was examined using a phase-contrast microscope (Olympus CX 43, Optical Co., Ltd., Tokyo, Japan) connected to a monitor. Two hundred spermatozoa were evaluated in some field of view. The percentage of progressively motile spermatozoa was calculated by dividing the number of spermatozoa that moved forward by 200 and then multiplying by 100 (Ibrahim et al., 2018; Kusumawati et al., 2017; Manehat et al., 2021).
Viability and morfology
The viability percentage refers to the proportion of live spermatozoa observed using the differentiation staining technique with eosin-nigrosine-stained smear preparations (Evans and Maxwell, 1987; Kusumawati et al., 2017). A drop of semen sample is mixed with two drops of nigrosin-eosin and left for 30 seconds. Subsequently, the sample is spread and allowed to dry on a warm glass slide. Live and dead spermatozoa are counted from a total of 200 spermatozoa across several fields of view using a phase contrast microscope (Olympus CX 43, Optical Co., Ltd., Tokyo, Japan) at 400× magnification, which is connected to a monitor. The percentage of live spermatozoa is calculated by dividing the number of spermatozoa that do not absorb the dye by the total number of spermatozoa counted, then multiplying by 100 (Evans and Maxwell, 1987; Susulawati, 2011; Zaenuri et al., 2023a, b). Abnormal spermatozoa are identified by counting those with no head, double heads, no tail, or looped tails (Evans and Maxwell, 1987; Ibrahim et al., 2021).
Data analysis
The statistical significance of the results was assessed using a one-way analysis of variance (ANOVA). significantly different results were further tested with Ducan’s test with SPSS version16.0 software. The data are presented as Mean ± SD. A probability value of P< 0.05 was considered statistically significant (Sawyer, 2009).
RESULTS AND DISCUSSION
Fresh semen: Volume, pH, spermatozoa concentration
The results of the fresh semen examination indicated a volume, pH, and concentration of spermatozoa per milliliter of 2.16±0.65, 7.1±0.02, and 2.8×109, respectively. The semen volume observed in this study is higher compared to previous studies on Boer cross Buck, which reported volumes of 1.54±0.32 ml (Ramadanthi, 2020), 0.96±0.06 ml (Putra et al., 2012), and 1.33±0.2 ml (Putri et al., 2019). Zaenuri et al. (2021), reported semen volumes for Boer, Etawah cross, and Kacang bucks as 1.1±0.5 ml, 1.0±0.2 ml, and 0.7±0.2 ml, respectively. Similarity, the semen volume of Kacang Bucks was 0.82 ± 0.26 ml before they were given a sesbania glandiflora tablet (SGtab), which increased to 1.12±0.37 ml after administration of the SGtab (Zaenuri et al., 2023). Variations in semen volume may be influenced not only by diet but also by several factors, including the type of goat, age, body weight, testicular volume, frequency of semen collection, and the overall health of the goat (Kusumawati et al., 2017). The relatively high semen volume obtained in this study could be attributed to the bucks being well-trained and provided with high-quality feed from tree legumes with elevated protein content. Dahlanuddin et al. (2001) reported that the protein supply from legume tree leaves Sesbania glandiflora, Leucaena leucocephala, and Gliricidia sepium was 979.3±222.8 mg/W 0.75, 835.5±298.6 mg/W 0.75, and 824.3±89.7 mg/W 0.75, respectively.
The pH value of semen was measured in the laboratory immediately after collection using a digital pH meter. The average acidity level observed in this study was 7.1±0.02, which is slightly higher than the findings of Sekosi et al. (2016), who reported a range of 6.4 to 6.8. However, a pH of 7.1 falls within the neutral range, as indicated by Kusumawati et al. (2017), who stated that a normal pH for goat semen is between 6.8 and 7.4. The degree of acidity is a crucial factor that affects semen quality. Deviations in pH can lead to a rapid decline in spermatozoa viability (Manehat et al., 2021). Unfortunately, the pH of liquid semen in this study was not evaluated regularly.
The concentration of spermatozoa in Boer bucks, as determined by this study, was found to be 2.8 × 109 per ml. This concentration is comparable to that of Kacang bucks, which was measured before and after the administration of Sesbania Glandiflora Tablets (SGtab), yielding values of 2,807±417.40 × 106 and 2,955±384.11 × 106, respectively (Zaenuri et al., 2023b). Other studies have reported the spermatozoa concentrations in Boer, Etawah cross, and Kacang bucks as 3,604±867 × 106, 2,660±1,193 × 106, and 3,556 ± 846 × 106, respectively (Zaenuri et al., 2021). Based on these three indicators of fresh semen quality from this research, the semen can be processed further.
Treatments semen
Progressive motility of spermatozoa
The results of this study indicated that six hours after preservation at 27°C, the percentage of progressive motility of spermatozoa in FF3 was statistically significantly higher (66±2.53; P < 0.05) compared to the progressive motility in treatments FF0, FF1, and FF2, which were 59±4.1, 60±2.74, and 62±2.24, respectively. A similar trend was observed at eight and ten hours after dilution and storage at 27°C (Table 1). This decline may be attributed to the temperature of 27°C, at which spermatozoa metabolism accelerates more rapidly than at 5°C. Consequently, on one hand the supply of fructose, an energy source for spermatozoa, depletes quickly, while on in the other hand lactic acid, pH levels, and free radicals also increase (Bearden and Fuquay, 2000). This study found that an additional 8% FF in a Tris-albumin based extender had a significant impact (P < r0.05) in maintaining progessive motility (Table 1) for up to 10 hours compared to the other treatments. The progressive motility of spermatozoa is a critical factor for the success of artificial insemination (AI). Lower progressive motility indicates a reduced ability to fertilize an ovum, leading to decreased pregnancy rates. Therefore, spermatozoa motility serves as the primary indicator for assessing semen quality (Garner and Hafez, 2008; Sumadiasa, 2018).
Table 1: The effect of preservation and fig fruit filtrate supplementation in a Tris albumin-based extender on the average percentage of progressive motility of spermatozoa at 27°C.
|
Preservation (hours) |
Treatments |
|||
|
FFO |
FF1 |
FF2 |
FF3 |
|
|
0 |
80.05±1.02a |
81.03±1.03a |
80.13±2.04a |
81.14±2.24a |
|
2 |
76.11±2.24b |
75.04±1.21b |
77.21±2.74ab |
79.34±1.24a |
|
4 |
66.21±3.53ab |
66.14±3.53b |
70.41±2.74a |
68.05±3.53ab |
|
6 |
59.13±4.12a |
60.04±2.74a |
62.03±2.24aa |
66.81±2.53b |
|
8 |
54.06±2.74a |
54.21±4.18a |
57.01±2.50a |
62.97±2.74b |
|
45.04±2.74a |
43.04±4.18a |
47.05±1.74a |
53.8±3.74b |
|
a, ab, b Different superscripts in the same rows indicate significant differences (P<0.05).
Previous researchers reported that the motility of individual spermatozoa supplemented with 4% FF (equivalent to 0.5 g/100 ml of vitamin C in a Tris-egg yolk-based extender) was significantly higher (P < 0.05) than that of spermatozoa not supplemented with FF, specifically 45.8±7.64% compared to 35.0±8.4% at 4 days post-extension when stored at 5°C (Zaenuri et al., 2023b). Lubis et al. (2013) found that the addition of 0.2 grams of vitamin C to the Tris-egg yolk extender maintained the individual motility of Boer goat spermatozoa at 40.20±3.35% when stored at 5°C for 4 days. In contrast, Pahlevy et al. (2022) reported that the inclusion of 0.2 grams of vitamin C preserved individual spermatozoa motility at 52.44±4.17% in Sapera bucks, evaluated 3 days after dilution and storage at 5°C. Additionally, Lestari et al. (2018) indicated that the percentage of individual motility of Boer goat spermatozoa diluted with the commercial extender Andromed was 40 ± 4.08%, assessed 300 minutes after dilution and stored at room temperature.
There are two types of antioxidants: Non-enzymatic antioxidants, such as vitamins E, C, and A, which are highly effective against free radicals, and enzymatic antioxidants, including superoxide dismutase (SOD), catalase, glutathione, and alpha-lipoic acid. For this study, the standard concentration of FF was calculated based on its vitamin C content, a non-enzymatic antioxidant. Vitamin C, in the form of L-ascorbic acid, is classified as a reducing agent due to its low redox potential (Ibrahim et al., 2008). Fig fruit filtrate may help prolong the quality of sperm preserved in tris albumin-based extenders because it contains a high concentration of exogenous antioxidants, fructose, and microminerals. There are 0.81, 20.40, 266.34, 181.50, and 0.46 mg of β-carotene, vitamin C, and total tocopherol, and total tocopherol per 100 grams of fresh fig fruit, respectively. Additionally, fig fruits are rich in minerals (mg/1000 g), including Cu (0.15), and energy sources (g/100 g) such as protein (1.26 g), carbohydrate (24.27 g), glucose (17.58 g), fructose (18.20 g), along with Ca (0.044 g), and Mg (223.53 g) (Zaenuri, 2014a). Spermatozoa require each of these microelements to counteract the harmful effects of free radicals generated by their own metabolism (Zaenuri et al., 2014b). Furthermore, these antioxidants are effective against oxidizing agents by disrupting radical reactions produced by lipid peroxidation (Padayatti et al., 2003).
As shown in Table 1, the FF concentrations that affected the extender’s ability to maintain spermatozoa motility were observed in the FF2 and FF3 treatments. Antioxidants in the extender can inhibit the chain reactions that lead to the formation of free radicals by acting as electron acceptors (Funahashi and Sano, 2005). According to Kulaksiz and Daskin (2010), oxidative molecules can exacerbate the effects of oxidative stress, while antioxidant compounds play a crucial role in protecting cells from the harmful effects of reactive oxygen spesies, thereby allowing spermatozoa motility to be sustained for a longer duration. During the extending and storage processes, a reaction occurs between spermatozoa and oxygen, leading to the formation of free radicals. These free radicals can trigger the peroxidation of membrane lipids, which ultimately reducing the viability and motility of spermatozoa (Saraswat et al., 2012; Siahaan et al., 2012).
There were four studies that utilized organic antioxidants derived from natural sources as additional extenders to protect spermatozoa from cellular damage caused by free radicals, thereby allowing them to maintain motility for a longer duration. Benede and Moline (2020) explained that antioxidant peptides function in various of ways to prevent oxidative damage, including chelating pro-oxidative transition metal ions, reducing hydroperoxides, inactivating reactive oxygen species, and scavenging free radicals. Dasrul and Lubis (2013) found that an extender composition consisting of 20% sodium citrate, 40% tomato filtrate, and 20% egg yolk was able to sustain spermatozoa motility at 30.40±9.89, 72 hours after dilution and storage at 5°C. Saaban et al. (2018) reported that the addition of 2% lecithin (v/v) to the Tris extender was effectively maintained a spermatozoa motility percentage of 52.73±27.51. Similarly, additionally, a 20% guava filtrate in the CEP-2 extender preserved the percentage of progressive motility of goat spermatozoa at 45.7±1.7, eight days after dilution and storage at 5°C (Sumadiasa et al., 2015). The addition of 0.10 mg green tea extract per 100 mL of skim milk–egg yolk extender was able to maintain viability, motility, and IPM of 43.10±0.27, 39.75±0.22, and 36.06±0.09, respectively, when stored at cold temperatures for five days (Susilawati et al., 2021).
The use of FF in Tris-albumin is a quite effective in maintaining spermatozoa motility and is comparable to the Tris-egg yolk extender. This study found that the percentages motility of Boer Buck spermatozoa in Tris-albumin supplemented with 8% (v/v) was 53.8±3.74 at 27°C for 10 hours. The percentage motility of Buck spermatozoa preserved in a Tris -egg yolk extender at 27°C for 9 hours was reported to be 67 ± 1.58 (Kusumawati et al., 2017). The motility percentage of goat spermatozoa in a Tris-egg yolk extender supplemented with 4% and 6% FF, evaluated 144 hours after dilution and stored at 5°C, was maintained at 39.0±2.33 and 38.0±2.49, respectively (Zaenuri et al., 2013). Furthermore, supplementation with 0.04 and 0.06 g of FF was able to sustain the percentage of intact acrosome spermatozoa in Kacang bucks at 24.2±2.4 and 22.6±2.8, respectively, when evaluated 72 hours after dilution and storage at 5°C (Zaenuri et al., 2017). The addition of 4 mL of FF per 100 mL of Tris-egg yolk extender maintained the percentage of progressive motility of Boer goat spermatozoa at 45.0±10.2 and 35.0±8.4, which is relatively higher than the addition of 4 mL of FF per 100 mL of guava filtrate, yielding 43.0±11.4 and 31.4±8.26, respectively, when evaluated 4 days after dilution and storage at 5°C (Zaenuri et al., 2023a).
Spermatozoa viability
The viability of Boer goat spermatozoa in this study demonstrated that 8 to 10 hours after dilution and storage at 27°C, spermatozoa viability in Tris-albumin supplemented with FF consistently remained significantly higher (P < 0.05) compared to Tris-albumin without FF (Table 2). The factors that most significantly influence spermatozoa viability include the physical and chemical properties of the extender, the concentration of spermatozoa per milliliter of diluent, the temperature during treatment and storage, the pH of the semen and diluent, as well as the osmotic pressure and the content of electrolytes and non-electrolytes in the extender (Garner and Hafez, 2008).
The addition of 8% (v/v) FF to the tris-albumin extender in this study represents the optimal concentration for maintaining spermatozoa viability. This effectiveness may be attributed to the synergistic action of albumin in the extender, the proteins present in the semen (Fu et al., 2017), and the supplementation of FF, all of which enhance the extender’s ability to protect not only viability but also the plasma membrane and acrosome (Zaenuri et al., 2017). Perumal et al. (2015) noted that albumin serves as a crucial antioxidant due to its capacity to transport Fe²⁺ and Cu⁺ ions, which can reduce hydroxyl radicals (OH⁻). Additionally, Liu et al. (2015) highlighted that the amino acids leucine (Leu), aspartic acid (Asp), serine (Ser), glutamic acid (Glu), and lysine (Lys) found in albumin play significant roles in antioxidant activity. The total amino acid content in chicken egg albumin is 43.23% (Benede and Moliana, 2020; Sun et al., 2019). Albumin protein primarily consists of ovalbumin (54%), ovotransferrin (12%), lysozyme (3.4%), and ovomucin (3.5%) (Liu et al., 2015).
Table 2: The effect of preservation and fig fruit filtrate supplementation in a tris albumin-based extender on the average percentage of spermatozoa viability at 27°C.
|
Treatments |
||||
|
FF0 |
FF1 |
FF2 |
FF3 |
|
|
0 |
81.28 ± 1.14a |
87.45±2.61b |
88.34±1.52b |
88.96±2.24b |
|
2 |
80.63±2.16a |
80.37±1.4a |
83.65±1.95ab |
84.04±0.7b |
|
4 |
79.31±2.30a |
77.26±2.28a |
79.10±2.39a |
79.85±2.05a |
|
6 |
71.00±3.08a |
70.03±3.53a |
71.81±3.27a |
75.54±2.70b |
|
8 |
57.09±5.15a |
60.52±4.50a |
62.12±3.83a |
68.88±5.85b |
|
48.09±3.15a |
50.52±2.50a |
50.12±2.83a |
57.88±3.85b |
|
a, ab, b Different superscripts in the same rows indicate significant differences (P<0.05).
The results of this study on the viability percentage of Boer buck spermatozoa diluted with a Tris-albumin + 8% FF (v/v) extender were comparable to those reported by Lestari et al. (2018), who used andromed, a commercial diluent. This study found a viability percentage of 57.88±3.85 (Table 2) at 10 hours post-dilution when stored at 27°C, compared to 57.94±1.71 evaluated 300 hours after dilution at room temperature (Lestari et al., 2018). Additionally, Kusumawati et al. (2017) reported significantly higher results, with the viability percentage of goat spermatozoa diluted with Tris aminomethane-egg yolk and stored for 9 hours at room temperature remaining notably high at 90.8±0.8.
Although the extender used in this research has not showed optimal protective ability compared to previous studies, the viability percentage data shows FF3 is a suitable diluent for artificial insemination (AI) for up to 10 hours after dilution and storage at 27°C. This aligns with the findings of Kusumawati et al. (2016), who asserts that semen suitable for AI must contain at least 50% live and motile spermatozoa.
Spermatozoa morphology
Data from this study on spermatozoa abnormalities indicates that a higher concentration of FF in the Tris-albumin extender correlates with a lower percentage of morphological abnormalities in goat spermatozoa (Table 3). As shown in Table 3, the percentages of abnormal sperm at ten hours post-extention and storage at 27ᵒC were significantly higher (P<0.05) in FF3 compared to F1 and F0, respectively. The addition of FF to the Tris-albumin extender has a positive impact on reducing the rate of spermatozoa exhibiting morphological abnormalities. However, the types of spermatozoa abnormalities observed in this study were not specifically categorized and were classified as abnormal in general.
Table 3: The effect of preservation and fig fruit filtrate supplementation in a tris albumin-based extender on the average percentage of abnormal spermatozoa morphology at 27°C.
|
Preservation (hours) |
Treatments |
|||
|
FF0 |
FF1 |
FF2 |
FF3 |
|
|
0 |
14.21±6.78a |
11.03±2.00b |
9.11±2.6c |
11.04±2.61b |
|
2 |
11.03±5.54a |
11.16±5.86a |
11.02±4.97a |
11.06±2.49a |
|
4 |
10.21±2.92a |
11±3.94a |
11±2.07a |
11±2.74a |
|
6 |
10.10±2.28b |
8.14±2.41c |
10.12±3.91b |
12.03±2.35a |
|
8 |
10.91±3.36b |
9.21±2.86c |
11.32±1.41a |
11.11±1.92a |
|
15.21±3.26bc |
14.15±2.16ac |
13.03±1.11ad |
12.12±1.22d |
|
a, ab, b Different superscripts in the same rows indicate significant differences (P<0.05).
There are two types of abnormalities in spermatozoa morphology, each with distinct causes. According to Afiati et al. (2015) and Susilawati (2011), primary abnormalities arise from issues within the seminiferous tubules and the process of spermatogenesis. Moreover, Gardner and Hafez (2008) explained that these include conditions such as microcephalus (small heads), macrocephalus (large heads), wide heads, short heads, and double tails. Secondary abnormalities, on the other hand, are characterized by the presence of cytoplasmic droplets, which result from abnormal testicular temperatures due to excessively high environmental temperatures. These abnormalities may manifest as proximal or distal cytoplasmic granules, folded tails, acrosomal sheaths that are detached from the head, and severed tails.
This study found that the percentage of abnormal spermatozoa in all treatments was higher than the results of previous studies. Kusumawati et al. (2017) reported that the percentage of an abnormal spermatozoa in a Tris aminomethane-egg yolk extender, stored for 9 hours at room temperature, was recorded as 3.4±1.14. Similarly, Lestari et al. (2018) reported that the percentage of an abnormal spermatozoa in Boer buck using the Andromed extender, evaluated 300 hours after dilution and storage at room temperature, was recorded as 13.20±0.20. However, if the percentage of an abnormal spermatozoa ranges between 8% and 10% (Bearden and Fuquary, 2000) or 5%-29% (Garner and Hafez, 2008), which does not significantly affect fertility. It is only when the percentages exceed 25% that fertility may decline. Sekosi et al. (2016) suggested that for artificial insemination (AI), the percentage of abnormal spermatozoa should not exceed 20%. In this study, the percentage of an abnormal spermatozoa remains suitable for AI up to 10 hours after dilution and storage at 27°C, with levels still below the recommended threshold.
CONCLUSIONS AND RECOMMENDATIONS
The preservation efficacy of Tris-albumin diluent was found to be significantly improved with the supplementation of FF in comparison to the diluent without FF. A Tris-albumin diluent supplemented with 8% FF (v/v) was determined to be suitable for artificial insemination for up to 10 hours after dilution when stored at 27°C. However, it would be beneficial to conduct further comprehensive studies to investigate the impact of varying concentrations of FF in different storage conditions such as room temperature, refrigeration, and freezing on semen preservation. By doing so, the most effective methods of semen preservation can be determined.
ACKNOWLEDGMENT
The authors are thankful to the Research Project Unit of the Mataram University who has financed this research.
NOVELTY STATEMENT
This study examined the effectiveness of using a Tris-albumin based extender supplemented with fig fruit filtrate, a subject that has not been extensively studied. The results demonstrate that the combination of Tris-albumin and organic antioxidants derived from fig fruit filtrate is capable of maintaining the quality of liquid semen for up to 10 hours at a storage temperature of 27°C.
AUTHOR’S CONTRIBUTION
Every step of the research process, from the proposal draft to the final report and the paper to be submitted to the journal, involves the writers.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Afiati F, Yulnawati M, Riyadi R, dan Arifiantini RI (2015). Abnormalitas Spermatozoa Domba dengan Frekuensi Penampungan Berbeda. Pros Sem Nas Masy Biodiv Indon., 1(4): 930-934. https://doi.org/10.13057/psnmbi/m010449
Agarwal A, Kiran PN, Shyam SRA, Tamer MS (2004). Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod. Biomed. Online, 8(6): 616-627. https://doi.org/10.1016/S1472-6483(10)61641-0
Bearden IJ, Fuquay JW (2000). Applied animal reproduction. 5th ed. Missisippi State University: pp. 24-143.
Benede S, Moliana S (2020). Chicken egg proteins dan derived peptides with antioxidant properties. Foods MDPI, 9(6): 735. https://doi.org/10.3390/foods9060735
Chunrong LV, Guoquan W, Qionghua H, Guobo Q (2019). Spermatozoa cryopreservation: State of Art and Future in Small Ruminants. Biopreserv. Biobank., 17(2): 171-182. https://doi.org/10.1089/bio.2018.0113
Dahlanuddin, Zaenuri LA, Mashur, dan Muzani M (2001). Optimalisasi Penggunaan Daun Turi (Sesbania gandiflora) sebagai Pakan Ternak Kambing. Laporan Penelitian Kerjasama Fakultas Peternakan Universitas Mataram dan Balai Pengkajian Teknologi Pertanian NTB.
Dasrul R, Lubis TM (2013). Pengaruh penambahan sari buah tomat dalam media pengencer terhadap motilitas dan viabilitas spermatozoa kambing boer yang disimpan pada suhu 3–5°C. J. Ilmiah Petern. 1(1): 7-17.
Dawson EB, Harris WA, Rankin WE, Charpentier LA, McGanity WJ (1987). Effect of ascorbic acid on male fertility. Ann. N. Y. Acad. Sci., 498: 312–323. https://doi.org/10.1111/j.1749-6632.1987.tb23770.x
deWhalley CV, Rankin SH, Hoult JR, Jessup W, Leake DS (1990). Flavonoids inhibit the oxidative modification of low-density lipoproteins by macrophages. Biochem. Pharmacol., 39: 1743–1750. https://doi.org/10.1016/0006-2952(90)90120-A
Evans G, Maxwell WMC (1987). Frozen storage of semen: In: Salamon’s artificial insemination of sheep and goats. Edit by Butterworths, Sidney, pp. 22-141.
Fu J, Li Y, Wang L, Zhen L, Yang Q, Li P, Li X (2017). Bovine serum albumin and skim-milk improve boar sperm motility by enhancing energy metabolism and protein modifications during liquid storage at 17 °C. Theriogenology, 102: 87–97. https://doi.org/10.1016/j.theriogenology.2017.07.020
Funahashi H. and T. Sano. 2005. Select Antioxidant Improve the Functiom of Extended Boar Semen Stored at 10 degrees C. Theriogenology, 63(6): 1605:1616. DOI: 10.1016/j.theriogenology.2004.06.016
Garner DL, Hafez ESE (2008). Spermatozoa and plasma semen. In reproduction in farm animal (eds. Hafez ESE and B Hafez). 7th ed. Lippincott and Williams. Baltimore, Marryland, USA.
Guha SK, Majumder Y, Mine (2018). Egg Proteins. Reference Module in Food Science: 1-12.
Hahn K, Failing K, Wehrend A (2019). Effect of temperature and time after collection on buck sperm quality. BMC Vet. Res., 15: 355. https://doi.org/10.1186/s12917-019-2135-y
Handoko KJ, Ducha N, Purnomo T (2018). Effect of different extender on motility of common carp (Cyprinus carpio) Spermatozoa during short-term preservation. LenteraBio 7(1): 92-98. http://ejournal.unesa.ac.id/index.php/lenterabio
Ibrahim SF, Osman K, Das S, Othman AM, Majid NA, Rahman MPA (2008). A study of the antioxidant effect of alpha lipoicacid on spermatozoa quality. Clinics (Sao Paulo), 63(4): 545-550. https://doi.org/10.1590/S1807-59322008000400022
Kessopoulou E, Powers HJ, Sharma KK, Pearson JK, Russell JM, Cooke ID, Barratt CL (1995). A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil. Steril., 64: 825–831. https://doi.org/10.1016/S0015-0282(16)57861-3
Kulaksiz., R. dan A. Daskin. 2010. The Protective effect of egg yolk from different avian species during the crypopservation of karayaka ram semen. J. Small Rumin. Res. 88:12-15. DOI:10.1016/j.smallrumres.2009.11.014
Kusumawati ED, Leondro H, dan Krisnaningsih ATN (2016). Pengaruh suhu dan lama simpan semen segar terhadap motilitas dan abnormalitas spermatozoa kambing peranakan etawa (PE). Seminar Nasional Hasil Penelitian. Universitas Brawijaya. Malang.
Kusumawati ED, Utomo KN, Krisnaningsih ATN, dan Rahadi S (2017). Kualitas semen kambing kacang dengan lama simpan yang berbeda pada suhu ruang menggnakan pengencer tris aminomethan kuning telur. Jitro, 4(3). https://doi.org/10.33772/jitro.v4i3.3894
Lestari TPS, Ihsan MN, Isnaini N (2018). Pengaruh waktu simpan semen segar dengan pengencer andromed pada suhu ruang terhadap kualitas semen kambing boer. J. Ternak Tropika, 15(1): 43-50. https://doi.org/10.21776/ub.jtapro.2014.015.02.1
Liu J, Jin Y, Lin S, Jones GS, Chen F (2015). Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem., 175: 258-266. https://doi.org/10.1016/j.foodchem.2014.11.142
Lubis TM, Rosmaridar CN, Thasmi dan Akbar (2013). Efektivitas penambahan vitamin C dalam pengencer susu skim kuning telur terhadap kualitas spermatozoa kambing boer setelah penyimpanan dingin. J. S. Pertan., 3(1): 347-361.
Lukitasari NR, Ratnawai, Lyrawati D (2014). Polifenol buah tin (Ficus carica Linn) menghambat peningkatan kadar MCP-1 pada tikus dengan diet tinggi lemak. J. Kedokteran Brawijaya, 28(1). https://doi.org/10.21776/ub.jkb.2014.028.01.1
Lukman HY, Sumadiasa IWL, Yuliani E, Zaenuri LA, Rodiah R (2023). Dragon fruit extract (Hylocereus Undatus) in yolk tris dilution to maintain Kacang goat spermatozoa quality at 5°C. Int. J. Novel Res. Dev., 9(8): c499-c510. https://ijnrd.org/papers/IJNRD2408252.pdf
Manehat, FX, Dethan AA, Tahuk PK (2021). Motility, viability, spermatozoa abnormality and Ph of Bali cattle semen in another-yellow water driller stored in a different time. J. Trop. Anim. Sci. Technol., 3(2): 76-90. https://doi.org/10.32938/jtast.v3i2.1032
Mathiesen L, Wang S, Halvorsen B, Malterud KE, Sund RB (1996). Inhibition of lipid peroxidation in low-density lipoprotein by the flavonoid myrigalone B and ascorbic acid. Biochem. Pharmacol., 51: 1719–1725. https://doi.org/10.1016/0006-2952(96)00107-4
Mollineau WM, Adogwa AO, Garcia GW (2011). Liquid and frozen storage of Agouti (Dasyprocta leporine) semen extender with UHT milk, unpasteurized coconut water, and pasteurized coconut water. Vet. Med. Int., 1(5): 23-27. https://doi.org/10.4061/2011/702635
Padayatty SJ, Katz A, Wang Y (2003). Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr., 22: 18-35. https://doi.org/10.1080/07315724.2003.10719272
Pahlevy JR, Ratnani H, Restiadi TI, Fikri F, Saputro AL, Agustono B (2022). The addition of vitamin C in tris–egg yolk extender maintained Sapera goat semen quality in 5 °C storage. Ovozoa: J. Anim. Reprod., 11(1): 1-8. https://doi.org/10.20473/ovz.v11i1.2022.1-8
Paulenz H, Soltun K, Ådnøy T, Andersen Berg K, Söderquis L (2005). Effect of different extenders on sperm viability of buck semen stored at room temperature. Small Rumin. Res., 59(1): 89-94. https://doi.org/10.1016/j.smallrumres.2004.11.010
Perumal P, Nahak AK, Vupru K, Balamurugan K, Krupakaran (2015). Effect of addition of bovine serum albumin on the liquid storage (5°C) of Mithun (Bos frontalis) semen. J. Cell Tissue Res., 15(1): 4795-4800. https://doi.org/10.18805/ijar.7048
Putra RP, Wahyuningsih S, dan Ciptadi G (2012). Uji kualitas spermatozoa kambing boer yang dibekukan dengan Alat Mr. frosty menggunakan pengencer andromed pada suhu penyimpanan -45 °C. Bagian Produksi Ternak. Fakultas Peternakan. Universitas Brawijaya. Malang.
Putri RF, Hermawan DH, dan Suyadi (2019). Kualitas semen cair kambing boer selama penyimpanan suhu ruang dengan penambahan ekstrak daun kemangi (ocimum sanctum). Pros. Semin. Nasional Teknol. Petern. Vet., pp. 334-344.
Ramadanthi E (2020). Morfologi dan Integitas Plasma Membran Spermatozoa Kambing Boer Simpan Dingin dalam Pengencer Tris Kuning Telur yang disuplementasi Filtrat Buah Jambu Biji dan Filtrat Buah Tin. Skripsi. Universitas Mataram.
Rizal M, dan Riyadi M (2016). Fertility of Swamp buffalo semen (Bubalus bubalis Carabanensis) diluted with sugar palm juice extender. J. Vet., 17(3): 457-467. https://doi.org/10.19087/jveteriner.2016.17.3.457
Saaban N, Arman C, Yuliani E, dan Maskur (2019). Subtitusi kuning telur dengan lesitin kedelai sebagai pengencer semen dalam mempertahankan kualitas spermatozoa keraroma penyimpanan 5 oC. J. Ilmu Teknol. Terapan Petern. Trop., 6(3): 367-374. https://doi.org/10.33772/jitro.v6i3.8173
Saraswat S, Priyadharsini R, Jindal SK, Yadav S, Ramachandran N, Kharche SD, Goel AK (2012). Effect of antioxidants supplementation at refrigeration temperature on sperm motion characteristics and membran integity of Sirohi buck semen. J. Physiol. Pharm. Adv., 2(1): 77-86.
Sawyer S (2009). Analysis of variance: The fundamental concepts. J. Manual Manipul. Ther., 17(2): 27E-38E. https://doi.org/10.1179/jmt.2009.17.2.27E
Sekosi PPP, Kusumawati ED, dan Krisnaningsih ATN (2016). Motilitas dan viabilitas semen segar kambing peranakan ettawa (PE) dengan menggunakan pengencer cauda epididymal plasma (CEP-2) pada Lama dan Suhu Simpan yang Berbeda. J. Sains Petern., 4(1): 34-49. https://doi.org/10.33772/jitro.v4i3.3894
Siahaan EA, Laksmi DNDI, Bebas W (2012). Efektivitas penambahan berbagai konsentrasi b-karoten terhadap motilitas dan daya hidup spermatozoa sapi bali post thawing. Indonesia Med. Vet., 1(2): 239-251.
Sies H, Stahl W, Sundquist AR (1992). Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann. N.Y. Acad. Sci., 669: 7–20. https://doi.org/10.1111/j.1749-6632.1992.tb17085.x
Siswoyo B (2007). Buah tin tingkatkan daya tahan tubuh. Hasil penelusuran, http://www.TribunTimur.com.
Sriana D (2018). Kualitas semen beku kambing peranakan etawah (Pe) setelah Pencucian dengan Metode Sentrifugasi. Skripsi. Uiversitas Hasanuddin Makasar.
Standar Nasional Indonesia (SNI) 4869.3. (2014). Semen beku–Bagian 3: Kambing dan domba. Badan Standardisasi Nasional Indonesia. Retrieved from: https://www.scribd.com/document/392812442/SNI-4869-3-2014-Semen-Beku-Bagian-3-Kambing-Dan-Domba-01
Sumadiasa IWL, Susilawati T, Ciptadi G dan Isnaini N (2015). The potency of guava filtrat (Psidium Guava Linn) for Preservation of Bali Bull Spermatozoa. IOSR JAVS, 8(5): 51-57.
Sumadiasa IWL (2018). Pengelolaan sperma pada ternak. Mataram University Press. Mataram.
Sun C, Liu J, Yang N, Xu G (2019). Egg quality and egg albumen property of domestic chicken, duck, goose, turkey, quail, and pigeon. Poult. Sci., 98(10): 4516-4521. https://doi.org/10.3382/ps/pez259
Susilawati T (2011). Tingkat keberhasilan inseminasi buatan dengan kualitas dan deposisi semen yang berbeda pada sapi peranakan ongole. J. Ternak Tropika, 12(2): 15-24. https://ternaktropika.ub.ac.id/index.php/tropika/article/view/109
Susilowati T, Mustofa I, Wurlina W, Hernawati T, Oktanella Y (2021). Maintaining the quality of kacang buck semen in chilled storage with the addition of green tea extract in extender. Trop. Anim. Sci. J., 44(4): 408-414. https://doi.org/10.5398/tasj.2021.44.4.408
Swari WR, Sabdoningrum EK, Wurlina, Susilowati S, Kurnijasanti R, Safitri E. (2019). Pengaruh Penambahan Ekstrak Teh Hijau (Camelia sinensis) dalam Bahan Pengencer Susu Skim Kuning Telur terhadap Kualitas Spermatozoa Domba Sampudi yang Disimpan pada Suhu Dingin. Ovozoa 8 (2): 122-126. https://doi.org/10.20473/ovz.v8i2.2019.127-131
Trilaksana IGNB, Ndun RN, dan Bebas W (2015). Penambahan vitamin C pada pengencer fosfat kuning telur semen kalkun yang disimpan pada suhu 50C. Bull. Vet. Udayana, 7(2): 186-193. Available at: https://ojs.unud.ac.id/index.php/buletinvet/article/view/19661
United States Dept of Agriculture (USDA) (2009). National Nutrient Database for Standard. Release 22. http://www.nal.usda.gov/fnic/foodcomp/cgibin/list_nut_edit.pl. Accessed October 15, 2023
Vinson J (1999). The functional food properties of fig. Am. Assoc. Cereal Chem., 44(2): 82-87.
Zaenuri LA, Lukman HY, Yanuarianto O, Sumadiasa IWL, Rodiah R (2017). Additional freezed drying fig fruit (Ficus carica L) filtrate into tris egg yolk extender and its effect on sperm membrane integity and acrosome of Kacang buck. Anim. Prod., 19(3): 161-166. https://doi.org/10.20884/1.jap.2017.19.3.647
Zaenuri LA, Rodiah R, Dradjat AS, Yanuarianto O, Sumadiasa IWL, Lukman HY (2023b). Supplementing basal diet with sesbania gandiflora tablet (SGtab) and its effect on semen quality of kacang buck. Adv. Anim. Vet. Sci., 11(5): 843-851. https://doi.org/10.17582/journal.aavs/2023/11.5.843.851
Zaenuri LA, Rodiah R, Drajat AS, Sumadiasa IWL (2021). Komparasi biometri semen dan morfometri spermatozoa Kambing Kacang, peranakan Ettawa dan Boer (Determine the Biometric and Morphometric Differences of Kacang, Ettawa and Boer Buck Spermatozoa). J. Ilmu dan Teknol. Petern. Indonesia, 7(1): 19–28. https://doi.org/10.29303/jitpi.v7i1.85
Zaenuri LA, Rodiah R, Lukman HY, Dradjat AS, Yuliani E (2023a). Spermatozoa quality of liquid boer goat semen in tris egg yolk extender enriched with non-enzymatic antioxidants. J. Kedokteran Hewan, 17(2): 55-61. https://doi.org/10.21157/j.ked.hewan.v17i2.26913
Zaenuri LA, Rodiah R (2016). Effectiveness of dry and fresh progesterone as an oestrus stimulation on does. J. Ilmu Teknol. Petern. Indonesia, 2(1): 129-133. https://doi.org/10.29303/jitpi.v2i1.23
Zaenuri LA, Susilawai T, Sumitro SB, Wahyuningsih S (2013). The prospect of local fig fruit (Ficus glumerata Rob) extract to preserve goat sperm motility. J. Kedokteran Hewan, 7(1): 26-28. https://doi.org/10.21157/j.ked.hewan.v7i1.560
Zaenuri LA (2014a). Penambahan filtrat buah tin (Ficus carica Lin) pada pengencer berbasis tris kuning telur untuk mempertahankan kualitas spermatozoa kambing. Disertasi. Universitas Brawijaya.
Zaenuri LA, Susilawati T, Wahyuningsih S, Sumitro SB (2014b). Effects of additional crude extract of fig fruit (Ficus carica L) into tris egg yolk based extender on quality of buck semen. J. Biol. Agic. Healthc., 4(9): 21-27.
Zaenuri LA, Sumadiasa IWL, Rodiah R (2022). Efforts to increase goat productivity through crossing local buck with Boer buck in Cendi Manik Village, Sekotong Tengah District. J. Abdi Insani, 9(2): 618-626. https://doi.org/10.29303/abdiinsani.v9i2.545
To share on other social networks, click on any share button. What are these?






