Evaluating the Impact of Various LED Light Spectrums on Dendrobium officinale Tissue Culture Seedlings
Research Article
Evaluating the Impact of Various LED Light Spectrums on Dendrobium officinale Tissue Culture Seedlings
Hafiz Ishtiaq Ahmad1, Jinlong Zhang1, Fuxun Luo1, Owais Iqbal2 and Yuying Wang1*
1College of Landscape and Horticulture, Yunnan Agricultural University, Kunming 650201, China; 2State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan Agricultural University, Kunming 650201, China.
Abstract | Light plays a crucial role in plant growth and development and extends mere illumination it’s a pivotal environmental factor shaping plant physiology and morphology. To gain a comprehensive understanding, an experiment was designed to investigate the impact of various light-emitting diode (LED) spectrums including red, blue, yellow, green, and their composite lights on the plant growth parameters, physiological characteristics, and antioxidant activity of Dendrobium officinale tissue culture seedlings. The fluorescent white light served as control. The tissue culture seedlings were grown in simple light and transplanted about 0.1 cm long into a solid culture medium. The plants were subjected to various LED spectra for a duration of 14 hours each day and continuous subsequent 100 days. The experiment was completely randomized design with nine treatments and each treatment have ten replications. The SPSS statistics software was used to analyses experimental data. In addition, the result of the current study revealed that the plant height, leaf length, leaf width and root number were significantly increased in the red, blue and white (RBW) composite treatment, while composite red, blue and yellow (RBY) subjected seedlings exhibited maximum leaf number, root length, and proliferation rate as compared to the others. Therefore, seedlings exposed to the light combination of red and blue (RB) exhibited the highest chlorophyll a and b, total chlorophyll content, catalase and root activity. The soluble sugar, soluble protein, superoxide dismutase and peroxidase levels increased significantly under compound red, blue and green (RBG) light conditions, while malondialdehyde content was highly decreased in the same treatment (RBG) as compared to control. The study indicated that the various light qualities could enhance the growth parameters of D. officinale seedlings by improving biochemical features and their enzymatic activities.
Received | March 18, 2024; Accepted | April 24, 2024; Published | June 05, 2024
*Correspondence | Yuying Wang, College of Landscape and Horticulture, Yunnan Agricultural University, Kunming 650201, China; Email: wyysxp@126.com
Citation | Ahmad, H.I., J. Zhang, F. Luo, O. Iqbal and Y. Wang. 2024. Evaluating the impact of various LED light spectrums on Dendrobium officinale tissue culture seedlings. Sarhad Journal of Agriculture, 40(2): 615-624.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.2.615.624
Keywords | Dendrobium officinale, Tissue culture seedlings, Light quality, Growth parameters, Antioxidant enzyme, Chlorophyll content
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Dendrobium officinale belong to the family Orchidaceae, is an important medicinal plant, thriving primarily in the moist and cool climates of southeastern and southwestern of China (Mudoi et al., 2023). It plays an important role to boost immune system, control blood pressure, enhance life span and relive from stomach diseases (Chen et al., 2014). The stems of this plant wield a powerful influence, promoting Yin nourishment, lung moisturization for blood glucose reduction, immune system enhancement, relief from throat inflammation, fever reduction, facilitated expectoration, and potent antioxidant effects (Zhang et al., 2006). The convention of international trade in Endangered Species of Wild Fauna and Flora (CITES) included this plant into endanger plant species (Hinsley et al., 2018). The slow growth, low natural reproduction rate by seed, and high market demand, further increase the demand due to uncontrolled collection for medical uses (Jin et al., 2016; Iqbal et al., 2023a). In vitro propagation of D. officinale is pressing need to content the demand of medicinal market and to decrease the pressures on natural populations from wild harvesting (Hou et al., 2012). Previous study demonstrate that in vitro plant propagation technique is most important to promote the rapid growth and reproduction rate of plants (Jin et al., 2009). Tissue culture techniques must be used to cultivate this plant, ensuring its sustainable availability and economic significance. Previous research has predominantly explored artificial cultivation and tissue culture methods for D. officinale. While studies on D. officinale tissue culture have predominantly centered on aspects such as the selection of culture medium, hormone type, explant type, and culture formula (Deng et al., 2013; Khan et al., 2019; Gao et al., 2020; Iqbal et al., 2023b), still now limited attention has been given to quality-related considerations. Consequently, there is an urgent imperative for continued research to promote the quality growth of D. officinale using tissue culture technique. Environmental factors, including light quality, temperature, and humidity, play pivotal roles as key factors in tissue culture which significantly influencing the growth and development of plant tissue culture seedlings (Batista et al., 2018; Yaseen and Hájos, 2021). Light plays an important role on the plant growth and development, as well as effect of plant photosynthesis, propagation, chlorophyll synthesis, photomorphogenesis, metabolism, gene expression, and modulation of antioxidant enzyme activity (Yang et al., 2018). Light quality, intensity, and duration regulate plant physiology, steering adaptive responses to environmental dynamics and influencing biochemical and morphological changes (Wei et al., 2023). In the realm of in vitro culture, the conventional use of fluorescent white light is giving way to the ascendance of light-emitting diodes (LEDs) as a superior energy source. LEDs boast numerous advantages, encompassing a long lifespan, wavelength specificity, small size, precise spectra, durability, and cool emitting surfaces, thereby underlining the increasing preference for LED lighting systems in optimizing plant tissue culture environments (Nardelli et al., 2017).
In the previous study, monochromatic and composite light enhance plant growth and physiological metabolism, and these effects differ based on the plant species and their various growth stages (Wang et al., 2017; Zhao et al., 2020). In addition, previously research reported that due to the red-light effect tissue culture seedling showed maximum growth parameters such as; height of plant, leaf and root length, soluble sugar content, root activity, and decreased MDA content (Kurilčik et al., 2008; Shang et al., 2013; Li et al., 2010; Mengxi et al., 2011; Li et al., 2019). While blue light can enhance the number of leaves (Ramírez-Mosqueda et al., 2017a), synthesis of chlorophyll content (Lim et al., 2023), soluble protein (Liu et al., 2020), and CAT activity (Ye and Shao, 2017). The aim of the present study was to check the effect of different light quality on tissue culture seedling of D. officinale, seeking an optimal light spectrum for enhancing their in vitro growth and development. Utilizing energy-efficient LED lamps, the investigation assessed the impact of diverse light qualities on seedling growth, chlorophyll levels, soluble proteins, soluble sugar, root activity, and antioxidant enzyme activity, aiming to identify the most conducive light conditions for optimal growth and development in tissue-cultured D. officinale seedlings.
Materials and Methods
Plant materials, culture methods and lighting condition
The Dendrobium officinale tissue culture seedlings were grown in the tissue culture Lab of the Institute of Flower, Yunnan Agricultural University, China. Murashige and Skoog medium (1/2 MS) supplemented with indole acetic acid (IAA 0.3 milligram per liter), Thidiazuron TDZ (1.25 mg/l), Potato (50 g/l), Banana (50 g/l), Sucrose (30 g/l), Agar (7 g/l), Huabao (No.1) (1 g/l) and activated charcoal (1 g/l). The pH was modified to 5.8 using NaOH solution and pour into sterilized bottles and autoclaving at 121 °C for 20 min. A tissue culture seedling (approximately 0.1 cm high) was placed on the MS medium with three seedlings per bottle. The experiment was completely randomized design with nine treatment and each treatment have ten replications. The materials were pre-cultured under fluorescent lamps for 7 days. Next, bottles were transferred to culture rack with a consistent light source, maintaining a 14 h/d. The culture room’s relative humidity was adjusted to (75±5%), with temperature (25±2 ℃). The seedlings were grown under nine different light sources (Table 1) for 100 days and the growth parameters, physiological characteristics and antioxidant enzymes activity were recorded.
Growth parameters
Ten seedlings were randomly collected from each treatment and the growth parameters including, seedling height, leaf number, leaf length, leaf width, root number and root length were measured using scale method given by (Huang et al., 2023). The proliferation rate was calculated by counting the number of newly developed buds.
Determination of soluble sugar and protein content
One gram of the samples originating from different light treatments was dipped sterilized water and boil 30 minutes. The extracts were boiled, filtered and adjusted to 100 ml by diluting with sterilized distilled water. 0.3 ml of the extract was transferred to 10 ml test tube containing 0.5 ml anthrone ethyl acetate, and 5 ml H2SO4. The soluble sugar content was measured through anthrone-sulfuric acid method (Hernandez and Hernandez, 1994). For protein content, two-gram of the sample was finely homogenized and centrifuged for 20 minutes at 4 oC and 12,000 rpm. The supernatant was mixed with 5 ml Coomassie Brilliant blue G-250 solution and incubated for 2 minutes. The protein content was calculated with formula given by Ku et al. (2013).
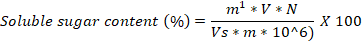
M1= represent of sugar mass, V= represent volume in milliliter, N= represent dilution factor, Vs= represent volume of sample, M= represent tissue mass sample.
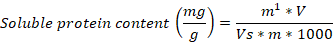
M1= represent of protein mass, V= represent volume in milliliter, Vs= represent sample volume, M= represent tissue mass sample.
Chlorophyll contents and root activity
A 0.1g fresh leaves were chopped and ground with 80% acetone and placed in the experiment room overnight at room temperature. Spectrophotometer was used for measuring the absorbance wavelength. The chlorophyll content was recorded with the following method described by Cao et al. (2007). The roots of the seedlings were cut into small pieces and dipped into 20 ml TTC solution for 24 hours followed by rising in sterilized distilled water and soaked in 95% ethanol solution and then placed in the water bath at 85 oC for 10 minutes to extract the triphenyl methyl hydrazone (TTF). Root activity was recorded using the triphenyl tetrazolium chloride (TTC) staining method (Wang and Huang, 2006). The absorbance was measured at 485 nm, and root activity was quantified as OD g-1 FW.
Table 1: Detail of optical combination system of the light spectrum.
|
PPFD (umol.m-2s-1) |
Total PPFD (umol.m-2s-1) |
(W) |
||||||
|
Treatment |
CK |
R660 |
Y590 |
G520 |
B440 |
W |
50 |
60 |
|
CK |
50 |
-- |
-- |
-- |
-- |
-- |
50 |
60 |
|
R660 |
-- |
50 |
-- |
-- |
-- |
-- |
50 |
60 |
|
Y590 |
-- |
-- |
50 |
-- |
-- |
-- |
50 |
60 |
|
G520 |
-- |
-- |
-- |
50 |
-- |
-- |
50 |
60 |
|
B440 |
-- |
-- |
-- |
-- |
50 |
-- |
50 |
60 |
|
R660B |
-- |
36.49 |
-- |
-- |
13.51 |
-- |
50 |
60 |
|
R660BG |
-- |
33.75 |
-- |
3.75 |
12.5 |
-- |
50 |
60 |
|
R660BY |
-- |
33.75 |
3.75 |
-- |
12.5 |
-- |
50 |
60 |
|
R660BW |
-- |
33.75 |
-- |
-- |
12.5 |
3.75 |
50 |
60 |
CK is the control group, R660 red light, Y590 yellow light, G520 green light, B440 blue light, R660B red and blue combination, R660BG red, blue and green combination, R660BY red, blue and yellow combination, R660BW red, blue and white combination and represent empty.
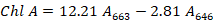
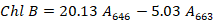
Chl A and Chl B is the concentration of chlorophyll A and B, respectively, mg/l.
Superoxide dismutase, peroxidase, catalase enzyme activity and malondialdehyde content
A 0.2 g leaf sample was ground with a mortar and pestle and homogenized with 2ml phosphate buffer (pH= 7.8). The homogenized sample was centrifuged at 10,000 rpm for 20 min and 20 microliter of the supernatant was mixed with 2.5 ml reaction solution in 5 ml test tube. The mixture was placed in 4,000 Lux illumination for 30 min and the absorbance was measured at 560 nm for Superoxide dismutase (SOD). For Peroxidase (POD), the different reaction solution was used and the absorbance was recorded at 470 nm. A 0.1 ml supernatant was mixed in 5 ml test tube with 2.5 ml of the reaction solution and the CAT absorbance was measured with 240 nm. A malondialdehyde (MDA) content was measured using thiobarbituric acid (TBA). 1 ml supernatant was mixed with 2 ml of TBA 0.6% solution and heat in water bath for 15 min at 80 oC followed by centrifugation at 8,000 rpm for 15 min. The color was measured at 600 nm, 532 nm, and 450 nm wavelengths. The calculation methods of (SOD, POD, CAT and MDA) was described by Wang (2018).
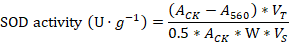
ACK= represents control absorbance; A560= represents sample tube absorbance, VT= represents total volume of extracted enzyme solution, W= sample fresh weight, Vs= volume of enzyme solution.
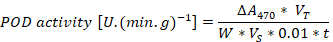
ΔA470= represents change value of absorbance of sample, VT= total volume, W= fresh weight, VS= volume of enzyme solution, t= represent the reaction time.
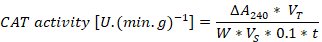
ΔA240= represents change value of absorbance of sample, VT= total volume, W= fresh weight, VS= volume of enzyme solution, t= represent the reaction time.
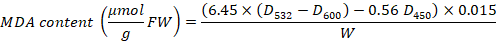
W= represents sample fresh weight, D532= absorbance value of the sample tube at 532 nm; D600= refers to the absorbance value of the sample tube at 600 nm, D450= absorbance value of the sample tube at 450 nm.
Data analysis
Statistical analysis of the data was carried out using the one-way ANOVA and Duncan multiple comparisons. IBM SPSS software version 26.0 (IBM Inc., Chicago, IL, USA), and GraphPad Prism version 8.0.1 was used for graph plotting.
Results and Discussion
Morphological parameters
In the present study, the effects of various light sources, including monochromatic (red, blue, yellow, green), composite lights (RB, RBY, RBG, RBW), and fluorescent white light as a control (CK), were investigated on the growth and chlorophyll content of Dendrobium officinale tissue culture seedlings (Figure 1A-I). The results showed that, maximum plant height was observed in RBW treatment followed by RBY and RB compared treatments and control (Figure 2 A-I). The highest leaf length was recorded in RBW followed by blue and RBY while RBY, yellow and RBW exhibited the higher leaf width. The largest root length was recorded in the RBY treatment followed by RBW and RBG treatment compared with other treatments. The root number significantly increased under RBW, RBG and blue treatments. The proliferation rate was significantly reduced under RBY, blue and yellow treatments (Table 2). Previous study has demostreated that RBW increase the plant height, leaf length and leaf width of lettuce plants (Park et al., 2012). Furthermore, studies have shown that red and combined RB light increase the growth of Chrysanthemum, grapes, Gerbera jamesonii and Anthurium andraeanum plantlets under laboratory condition (Poudel et al., 2008; Chen et al., 2013; Kurilčik et al., 2008; Lim et al., 2023). Previous study demonstrates that the RBY light increase plant weight, root number and root length under laboratory conditions (Li et al., 2018). Additionally, maximum proliferation rate was observed under RBY light and
Table 2: Morphological indexes, including seedling height, leaf length, leaf width, leaf no., root length, root no., and proliferation rate of D. officinale tissue culture seedlings under different light qualities.
|
Seedling height |
Leaf length |
Leaf width |
Leaf number |
Root length |
Root number |
Proliferation rate |
|
|
Red |
2.95 ± 0.25 de |
0.90 ± 0.27 d |
0.24 ± 0.05 b |
18.6 ± 3.23 bc |
1.11 ± 0.22 d |
9.50 ± 2.17 cd |
12.0 ± 3.52 f |
|
Blue |
3.33 ± 0.39 bc |
1.65 ± 0.34 ab |
0.32 ± 0.08 a |
21.4 ± 3.40 ab |
0.79 ± 0.16 e |
12.3 ± 3.05 bc |
34.0 ± 4.73 b |
|
Yellow |
3.23 ± 0.33 cd |
1.27 ± 0.29 c |
0.33 ± 0.06 a |
19.1 ± 3.57 abc |
0.97 ± 0.18 de |
8.90 ± 2.07 d |
28.0 ± 6.41 cd |
|
Green |
3.52 ± 0.37 bc |
1.32 ± 0.26 c |
0.33 ± 0.06 a |
16.7 ± 2.40 cd |
0.85 ± 0.20 e |
10.5 ± 2.22 cd |
24.0 ± 3.74 de |
|
RB |
3.47 ± 0.28 bc |
1.42 ± 0.19 bc |
0.28 ± 0.08 ab |
17.4 ± 2.54 cd |
1.42 ± 0.26 c |
11.1 ± 2.68 cd |
20.0 ± 3.36 e |
|
RBY |
3.64 ± 0.35 b |
1.62 ± 0.22 ab |
0.29 ± 0.08 ab |
21.7 ± 2.26 a |
2.45 ± 0.25 a |
12.1 ± 3.60 bc |
39.0 ± 3.82 a |
|
RBG |
2.82 ± 0.27 e |
1.45 ± 0.21 bc |
0.30 ± 0.08 ab |
14.8 ± 2.48 d |
1.71 ± 0.30 b |
14.5 ± 3.17 b |
15.0 ± 5.20 f |
|
RBW |
4.21 ± 0.39 a |
1.75 ± 0.19 a |
0.34 ± 0.06 a |
20.8 ± 2.20 ab |
2.35 ± 0.31 a |
17.9 ± 2.64 a |
27.0 ± 4.16 cd |
|
CK |
3.23 ± 0.42 cd |
0.99 ± 0.31 d |
0.29 ± 0.03 ab |
18.4 ± 4.45 bc |
1.37 ± 0.18 c |
11.8 ± 3.15 bc |
29.0 ± 6.89 c |
Different letters (a, b, c, d, e, f) in the same column indicate significant differences between treatments at P≤0.05 by using Duncan’s test. Data are the means of ten replicates ± standard deviation.
minimum under red light as compared to CK which is in accordance with the previous finding (Cavallaro et al., 2023). Another study reported a higher proliferation rate under combined red and blue (RB) light (Naznin et al., 2019).
Chlorophyll contents
The effect of different light quality significantly increased chlorophyll (Chl) contents of Dendrobium officinale seedlings. The maximum chlorophyll a, chlorophyll b and total chlorophyll content were recorded in RB treatment, while RBW showed moderate impact followed by RBW and blue light treatments (Figure 3A-C). However, the chlorophyll A/B ratio was higher under RBW treatment followed by red and RBY, respectively (Figure 3D). blue light enhances chlorophyll A/B and total chlorophyll contents, while the combination of RB significantly increases chlorophyll A/B ratio Wang et al. (2017). Similarly, the RB combination has been reported to increased chlorophyll A/B and total chlorophyll content Doritaenopsis, Peony plantlets, G. jamesonii and Stevia rebaudiana tissue culture seedlings (Shin et al., 2008; Pawłowska et al., 2018; Ramírez-Mosqueda et al., 2017). In another study, blue light showed highly chlorophyll A/B and total chlorophyll content of D. officinale tissue culture seedlings (Lin et al., 2011).
Soluble sugar content, soluble protein content, and root activity
The soluble sugar content (SSC), soluble protein content (SPC), and root activity of the D. officinale tissue culture seedlings were significantly affected by light treatments. As shown in Figure 4, the maximum SSC was highly observed in RBG treatment seedlings followed by RBY and red light treated plants (Figure 4A). In addition, the RBY was showed minimum SPC followed RBW compared with other treatments. In comparison, RBG showed highly SPC in seedlings of D. officinale (Figure 4B). These suggest that variations of light could increase the root activity, soluble sugar content and soluble protein contain and the maximum root activity was recorded with RB followed by RBG, treated seedlings (Figure 4C). Previous study in Solanum tuberousm L. reported the highest SSC and SPC under RBG treatment (Ma et al., 2015). According to Shang et al. (2013), the combination of RB light plays a crucial role to enhance the root activity of D. officinale seedlings. According to Zhou et al. (2020), the combined RB light positively impacts and causes an increase in root activity, favorably contributing to the improvement of the root system.
Superoxide dismutase, peroxidase, catalase enzyme activity and malondialdehyde content
Antioxidant enzymes play a crucial role in eliminating reactive oxygen species in plants, and their activity serves as an indicator of both physiological activity and senescence in plants (Molassiotis et al., 2004). Under different light treatments, Superoxide dismutase (SOD), Peroxidase (POD) and Catalase (CAT) significantly increased, while Malondialdehyde (MDA) content decreased. The highest SOD was recorded with RBG treatment followed by RBW and RBY light treated seedlings. In comparison, the yellow light showed least effect on SOD (Figure 5A). Similarly, the RBG light showed maximum effect on tissue culture seedlings and increase the POD activity followed by RB treatment (Figure 5B). Furthermore, CAT was significantly increase in RB light treatment followed by RBG and red treated seedlings (Figure 5C). Previous study showed the maximum SOD and POD activity in RBG light treatment (Mengxi et al., 2011). RB light treatment enhances the CAT activity of D. officinale seedlings (Wang et al., 2017). The RB also enhance tomato plantlets under in vitro condition (Khattak et al., 2007; Naznin et al., 2019). These studies results suggest that different light qualities have significant effect on D. officinale tissue culture seedlings. Moreover, the MDA content was highly decreased in RBG treated seedlings followed by RBW as compared to others. In comparison, the MDA content was significantly increase in red treated seedlings (Figure 5D). Similarly, the red treated seedlings showed minimum MDA content in G. jamesonii under laboratory condition (Meng et al., 2019). Feng et al. (2021) reported that the treated seedling of D. officinale showed highly decreased MDA content as compared to control.
Conclusions and Recommendations
The role of lights on plant growth is vital. It’s a critical environmental factor shaping plant physiology and morphology. Many researchers have made much contribution to achieve adequate plant growth through different lights to control environmental factors. On contrary our research has been much focused on the different light qualities aspects. To gain a comprehensive understanding, an experiment was designed to investigate the impact of various light-emitting diode (LED) spectrums including red, blue, yellow, green, and their composite lights with fluorescent white light as a control on the plant growth parameters, physiological characteristics, and antioxidant activity of Dendrobium officinale tissue culture seedlings. According to our results, we conclude that the combination of Red, Blue and White (RBW) light enhanced the growth parameters of tissue culture seedlings. Also, the combination of Red and Blue (RB) and Red, Blue and Green (RBG) increased SSC, SPC, chlorophyll contents, SOD, POD, CAT, and root activity. It should be possible; therefore, evaluating these different lights in combination could enhance the growth and quality of D. officinale seedlings.
Acknowledgments
The authors thank Professor Wang Yuying for giving us valuable suggestions and supervision throughout the entire research. We also thanks to Dr. Subhan Musa Jibrael to read and improve scientific language of this manuscript.
Novelty Statement
This study checked the effect of different light qualities on Dendrobium officinale seedlings under in vitro condition. As compared to other studies, the current study clearly highlights the effect of different light qualities on D. officinale seedlings under in vitro conditions. The evidence from this study will assist in considering the best light quality for optimum growth and physiological characteristics that will improve the quality and stimulate the growth of D. officinale seedlings.
Author’s Contribution
Hafiz Ishtiaq Ahmad: Conducted research and write original draft of manuscript.
Jinlong Zhang: Re- analyzed data.
Fuxun Luo: Revise and editing part of the manuscript.
Owais Iqbal: Evaluate and revise the final version of manuscript.
Yuying Wang: Supervised, editing and approved manuscript.
Conflicts of interest
The authors have declared no conflict of interest.
References
Batista, D.S., S.H.S. Felipe, T.D. Silva, K.M. de Castro, T.C. Mamedes-Rodrigues, N.A. Miranda and W.C. Otoni. 2018. Light quality in plant tissue culture: Does it matter? In vitro cell. Dev. Biol., 54: 195-215. https://doi.org/10.1007/s11627-018-9902-5
Cao, J.K., W.B. Jiang and Y.M. Zhao. 2007. Experiment guidance of postharvest physiology and biochemistry of fruits and vegetables. China Light Industry Press, Beijing, pp. 84-87.
Cavallaro, V., G. Avola, G. Fascella, A. Pellegrinoand and A. Ierna. 2023. Effects of spectral quality and light quantity of LEDs on in vitro shoot development and proliferation of Ananas comosus L. Merr. Agron., 13(4): 1072. https://doi.org/10.3390/agronomy13041072
Chen, B., S.J. Trueman, J. Li, Q. Li, H. Fan and J. Zhang. 2014. Micropropagation of the endangered medicinal orchid, Dendrobium officinale. Life Sci. J., 11(9): 526-530.
Chen, Y., Z. Wang, S. Ji, S. He and Y. Xia. 2013. Effects of different light quality ratios of light emitting diode (LED) on the growth of Anthurium andraeanum plantlets in vitro. Acta. Agric. Univer. Jiangx., 35(2): 375-380
Deng, R., H. Luo, X. Liu, D. Qiu, L. Zhang and S. Cai. 2013. The tissue culture and rapid propagation of Dendrobium officinale.
Feng, X.Y., D.D. Cui, L.J. Zeng, Z.X. Wu, X.X. Xie, J.S. Zhang and X.Y. Zhang. 2021. Effects of UV-B irradiation alone and in combination with salicylic acid on the growth and active ingredients of Dendrobium officinale. Russ. J. Plant. Physl., 68(3): 483-490. https://doi.org/10.1134/S1021443721030043
Gao, H., D. Xu, H. Zhang, X. Cheng and Q. Yang. 2020. Effects of culture medium composition and PEG on hyperhydricity in Dendrobium officinale. In vitro. Cell. Dev. Biol. Plant, 56: 143-149. https://doi.org/10.1007/s11627-020-10075-y
Hernandez, T. and A. Hernandez. 1994. Available carbohydrates in alfalfa leaf protein concentrates. J. Agric. Fd. Chem., 42(8): 1747-1749. https://doi.org/10.1021/jf00044a033
Hinsley, A., H.J. De Boer, M.F. Fay, S.W. Gale, L.M. Gardiner, R.S. Gunasekara and J. Phelps. 2018. A review of the trade in orchids and its implications for conservation. Bot. J. Linn., 186(4): 435-455. https://doi.org/10.1093/botlinnean/box083
Hou, B., M. Tian, J. Luo, Y. Ji, Q. Xue and X. Ding. 2012. Genetic diversity assessment and ex situ conservation strategy of the endangered Dendrobium officinale (Orchidaceae) using new trinucleotide microsatellite markers. Pl. Syst. Evol., 298: 1483-1491. https://doi.org/10.1007/s00606-012-0651-3
Huang, L., L. Ding, L.W. Wang, L. Zhao, X. Zhao and P. Wang. 2023. Effects of simulated drought stress on the growth and physiological and biochemical parameters of Paspalum wettsteinii. Acta Physiol. Plant., 45(6): 82. https://doi.org/10.1007/s11738-023-03556-1
Iqbal, O., C. Li, and A.M. Lodhi. 2023a. Antagonistic Pseudomonas: Alternative to chemical fungicides for the management of phytopathogens. In: Biofungicides: Eco-safety and future trends. CRC Press. pp. 216-246. https://doi.org/10.1201/9781003287575-9
Iqbal, O., C. Li, N.A. Rajput and A.M. Lodhi. 2023b. Management of phytopathogens by antagonistic Bacillus spp. in tomato crop. Tomato cultivation and consumption-innovation and sustainability. https://doi.org/10.5772/intechopen.112439
Jin, H., Z.X. Xu, J.H. Chen, S.F. Han, S. Ge and Y.B. Luo. 2009. Interaction between tissue-cultured seedlings of Dendrobium officinale and mycorrhizal fungus (Epulorhiza sp.) during symbiotic culture. Chin. J. Plant Ecol., 33(3): 433.
Jin, Q., C. Jiao, S. Sun, C. Song, Y. Cai, Y. Lin and Y. Zhu. 2016. Metabolic analysis of medicinal Dendrobium officinale and Dendrobium huoshanense during different growth years. PLoS One, 11(1): e0146607. https://doi.org/10.1371/journal.pone.0146607
Khan, M.F., M. Mohibullah, M. Iqbal, M. Urooj and U. Arif. 2019. Combining ability analysis for morphological traits in 6× 6 diallel crosses of maize (Zea mays L.) Opvs in Nowshehra (KPK) Pakistan. Sarhad J. Agric., 35(1). https://doi.org/10.17582/journal.sja/2019/35.1.182.186
Khattak, A.M., A. Salam and K. Nawab. 2007. Response of exotic tomato lines to different light intensities. Sarhad J. Agric., 23(4): 927.
Ku, H.K., H.M. Lim, K.H. Oh, H.J. Yang, J.S. Jeong and S.K. Kim. 2013. Interpretation of protein quantitation using the Bradford assay: Comparison with two calculation models. Anal. Biochem., 434(1): 178-180. https://doi.org/10.1016/j.ab.2012.10.045
Kurilčik, A., R. Miklušytė-Čanova, S. Dapkūnienė, S. Žilinskaitė, G. Kurilčik, G. Tamulaitis and A. Žukauskas. 2008. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol., 3: 161-167. https://doi.org/10.2478/s11535-008-0006-9
Li, H., Z. Xu and C. Tang. 2010. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult., 103: 155-163. https://doi.org/10.1007/s11240-010-9763-z
Li, R., W. Huang, X. Wang, X. Liu and Z. Xu. 2018. Effects of yellow, green, and different blue spectra on growth of potato plantlets in vitro. HortScience, 53(4): 541-546. https://doi.org/10.21273/HORTSCI12848-18
Li, R., Z. Zhi-Lin, S. Zheng-Rui, Z. Li-Ying, B. Jian-Kun and W. Yu-Ying. 2019. Effects of different light quality of LED on flowering of Dendrobium officinale Kimura et Migo in vitro. J. South Agric., 50(7): 1550-1556.
Lim, M.J., H.N. Murthy, H.Y. Song, S.Y. Lee and S.Y. Park. 2023. Influence of white, red, blue, and combination of led lights on in vitro multiplication of shoots, rooting, and acclimatization of Gerbera jamesonii cv.‘Shy Pink’Plants. Agron., 13(9): 2216. https://doi.org/10.3390/agronomy13092216
Lin, Y., J. Li, B. Li, T. He and Z. Chun. 2011. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult., 105: 329-335. https://doi.org/10.1007/s11240-010-9871-9
Liu, Y., Y.Y. Wang, X. Zhu and R. Li. 2020. Effect of led light quality on hybrid plantlets growth and physiological characteristics of Cymbidium hybridum’hongjiu’ × Cymbidium tortisepalum’biancaosuhua’.
Ma, X., Y. Wang, M. Liu, J. Xu and Z. Xu. 2015. Effects of green and red lights on the growth and morphogenesis of potato (Solanum tuberosum L.) plantlets in vitro. Sci. Hortic., 190: 104-109. https://doi.org/10.1016/j.scienta.2015.01.006
Meng, X., Z. Wang, S. He, L. Shi, Y. Song, X. Lou and D. He. 2019. LED-supplied red and blue light alters the growth, antioxidant status, and photochemical potential of in vitro-grown Gerbera jamesonii plantlets. Hortic. Sci. Technol., 37(4): 473-489. https://doi.org/10.7235/HORT.20190048
Mengxi, L., X. Zhigang, Y. Yang and F. Yijie. 2011. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult., 106: 1-10. https://doi.org/10.1007/s11240-010-9887-1
Molassiotis, A.N., K. Dimassi, G. Diamantidis and I. Therios. 2004. Changes in peroxidases and catalase activity during in vitro rooting. Biol. Plant., 48: 1-5. https://doi.org/10.1023/B:BIOP.0000024267.68394.96
Mudoi, K.D., P. Borah, D. Gorh, T. Gupta, P. Sarmah, S. Bhattacharjee and S.P. Saikia. 2023. Biotechnological Interventions and Societal Impacts of Some Medicinal Orchids. In: Advances in orchid biology, biotechnology and omics. Singapore: Springer Nature Singapore. pp. 59-144. https://doi.org/10.1007/978-981-99-1079-3_3
Nardelli, A., E. Deuschle, L.D. de Azevedo, J.L.N. Pessoa and E. Ghisi. 2017. Assessment of light emitting diodes technology for general lighting: A critical review. Renew. Sustain. Energy Rev., 75: 368-379. https://doi.org/10.1016/j.rser.2016.11.002
Naznin, M.T., M. Lefsrud, M.O.K. Azad and C.H. Park. 2019. Effect of different combinations of red and blue LED light on growth characteristics and pigment content of in vitro tomato plantlets. Agriculture, 9(9): 196. https://doi.org/10.3390/agriculture9090196
Park, Y.G., J.E. Park, S.J. Hwang and B.R. Jeong. 2012. Light source and CO2 concentration affect growth and anthocyanin content of lettuce under controlled environment. Hortic. Environ. Biotechnol., 53: 460-466. https://doi.org/10.1007/s13580-012-0821-9
Pawłowska, B., M. Żupnik, B. Szewczyk-Taranek and M. Cioć. 2018. Impact of LED light sources on morphogenesis and levels of photosynthetic pigments in Gerbera jamesonii grown in vitro. Hortic. Environ. Biotechnol., 59: 115-123. https://doi.org/10.1007/s13580-018-0012-4
Poudel, P.R., I. Kataoka and R. Mochioka. 2008. Effect of red-and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult., 92: 147-153. https://doi.org/10.1007/s11240-007-9317-1
Ramírez-Mosqueda, M.A., L.G. Iglesias-Andreu and I.J. Luna-Sánchez. 2017a. Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. S. Afr. J. Bot., 109: 288-293. https://doi.org/10.1016/j.sajb.2017.01.205
Ramírez-Mosqueda, M.A., L.G. Iglesias-Andreu and J.R. Bautista-Aguilar. 2017b. The effect of light quality on growth and development of in vitro plantlet of Stevia rebaudiana Bertoni. Sugar Tech., 19: 331-336. https://doi.org/10.1007/s12355-016-0459-5
Shang, W., Z. Wang, J. Hou, W. Zheng and S. He. 2013. Effects of light emitting diode (LED) with different red/blue quality ratios on the growth of Dendrobium officinale plantlets in vitro.
Shin, K.S., H.N. Murthy, J.W. Heo, E.J. Hahn and K.Y. Paek. 2008. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant., 30: 339-343. https://doi.org/10.1007/s11738-007-0128-0
Wang, X.K and J.L. Huang. 2006. Principles and techniques of plant physiological biochemical experiment. Higher Education Pres, Beijing.
Wang, Y., Y. Tong, H. Chu, X. Chen, H. Guo, H. Yuan and B. Zheng. 2017. Effects of different light qualities on seedling growth and chlorophyll fluorescence parameters of Dendrobium officinale. Biologia, 72(7): 735-744. https://doi.org/10.1515/biolog-2017-0081
Wang, Y.J. 2018. Study on population quality and individual physiology function of super high-yielding maize (Zea mays L.). Shandong Agricultural University: Tai’an, China.
Wei, Y., S. Wang and D. Yu. 2023. The role of light quality in regulating early seedling development. Plants, 12(14): 2746. https://doi.org/10.3390/plants12142746
Yang, L., K.S. Wen, X. Ruan, Y.X. Zhao, F. Wei and Q. Wang. 2018. Response of plant secondary metabolites to environmental factors. Molecules, 23(4): 762. https://doi.org/10.3390/molecules23040762
Yaseen, A.A and M.T. Hájos. 2021. The Potential Role of Moringa Leaf Extract as Bio-Stimulant to Improve some Quality Parameters of Different Lettuce (Lactuca sativa L.) Genotypes. Sarhad J. Agric., 37(4). https://doi.org/10.17582/journal.sja/2021/37.4.1107.1119
Ye, S and Q. Shao. 2017. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front. Plant Sci., 8: 266635. https://doi.org/10.3389/fpls.2017.00857
Zhang, Z.G., Q.X. Yu and Z.G. Ye. 2006. A rare Chinese drug-herba Dendrobium officinale (Tiepi Shihu). ShangHai Scientific and Technological Literature, Shanghai, pp. 44-50.
Zhao, J., L.T. Thi, Y.G. Park and B.R. Jeong. 2020. Light quality affects growth and physiology of Carpesium triste Maxim. cultured in vitro. Agriculture, 10(7): 258. https://doi.org/10.3390/agriculture10070258
Zhou, C., Y. Zhang, W. Liu, L. Zha, M. Shao and B. Li. 2020. Light quality affected the growth and root organic carbon and autotoxin secretions of hydroponic lettuce. Plants, 9(11): 1542. https://doi.org/10.3390/plants9111542
To share on other social networks, click on any share button. What are these?








