Essential Amino Acid, Short Chain Fatty Acid and Omega Content of Skipjack Tuna Offal Meal and its Effects on the Nutrient Digestibility of Local Chicken
Research Article
Essential Amino Acid, Short Chain Fatty Acid and Omega Content of Skipjack Tuna Offal Meal and its Effects on the Nutrient Digestibility of Local Chicken
Srisukmawati Zainudin1,3, Hartutik2, Edhy Sudjarwo2, Osfar Sjofjan2*
1Doctoral Student, Department of Animal Nutrition and Feed Science, Faculty of Animal Science University of Brawjaya, Malang 65145, East Java, Indonesia; 2Faculty of Animal Science, Universitas Brawijaya, Malang 65145, Indonesia; 3Faculty of Animal Science, Universitas Negeri Gorontalo, Gorontalo, Indonesia.
Abstract | In this study, the impact of skipjack tuna offal meal on the nutrient digestibility of local chicken was assessed, with a focus on its short chain fatty acid and omega content. The initial phase involved conducting a skipjack tuna offal meal experiment using a complete randomized design with 3 treatments and 9 replications to examine the short chain fatty acid and omega content. Following this, a second experiment was conducted using 24 male Kampong Unggul Balitnak (KUB) local chickens, employing a completely randomized design with four treatments and six replications. The feed treatments consisted of T0 (basal feed), T1 (basal feed with 10% fresh skipjack tuna offal meal), T2 (basal feed with 10% steamed skipjack tuna offal meal), and T3 (basal feed with 10% fermented skipjack tuna offal meal). Data were collected and analyzed using analysis of variance (ANOVA). The results showed significant differences (p < 0.05) in nutrient digestibility when skipjack tuna offal meal was used as a fish meal replacement. In conclusion, fermented skipjack tuna offal meal was recommended as a source protein for local chickens.
Keywords | Fatty acid, Local chicken, Nutrient digestibility, Skipjack tuna, Omega
Received | February 01, 2024; Accepted | March 03, 2024; Published | March 16, 2024
*Correspondence | Osfar Sjofjan, Faculty of Animal Science, Universitas Brawijaya, Malang 65145, Indonesia; Email: [email protected]
Citation | Zainudin S, Hartutik, Sudjarwo E, Sjofjan O (2024). Essential amino acid, short chain fatty acid and omega content of skipjack tuna offal meal and its effects on the nutrient digestibility of local chicken. Adv. Anim. Vet. Sci., 12(5):942-949.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.5.942.949
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Local chickens are usually bred in the backyards of rural households, often in small quantities of around 24 hens primarily for egg production (Laihad et al., 2019). Some farmers rear native chickens for meat consumption, bartering, or selling, providing an extra income source for the household. Experts note that the population of native chickens in the country remains comparable to that of hybrid or commercially bred stocks (Laihad et al., 2019). Not only breed but also feed, in the poultry industry, feed accounts for the most significant portion (65-75%) of overall production (Sjofjan et al., 2021).
Feed has been recognized as the primary factor contributing to land occupation, primary production use, acidification, climate change, energy consumption, and water dependence (Sjofjan and Adli, 2021). Decreasing the importation of feed ingredients serves as a method to diminish greenhouse gas emissions, with sea freight being a significant contributor to these emissions (Adli et al., 2020; Sohn and Ohshima, 2010). One of the reasons is that breeders still rely on ready-made feed or commercial feed.
One of the ingredients in commercial feed formulations is fish meal which is relied on as a source of animal protein which influences feed quality (Ologhobo et al., 2012). The supply of fish meal is often constrained because the raw materials are still imported, and its use still competes with human needs, so the price of commercial feed becomes expensive (Leke et al., 2015a). The more expensive the price of commercial feed, the greater the production costs. Therefore, efforts are needed to reduce feed costs by using alternative feed ingredients that are of high quality, continuously available and at affordable prices. The extensive use of fishmeal in animal nutrition raises significant environmental concerns. By utilize by-products such as fish trimmings, heads, and viscera can be processed to create fish meal, a valuable protein source for animal feed, and reduce negatively environmental effects. Despite having an extensive coastline, Indonesia faces challenges in producing fish meal for feed locally. In 2021, Indonesia brought in approximately 115.2 thousand tons of fish meal, alongside an import of roughly 268.3 thousand metric tons of fisheries products (Statista, 2023).
One of the local feed ingredients is skipjack tuna innards. Skipjack tuna offal is waste from processing skipjack tuna. Skipjack tuna (Katsuwonus pelamis L), known locally as cakalang, has not been fully utilised but possesses considerable nutritional potential, particularly in its protein content, which is comparable to that of fish meal. Data from the Central Statistics Agency (BPS) for Gorontalo Province in 2020 shows that the production of skipjack tuna catches reached 13,333 tons, previously in 2017 it reached 147.25 tons. The majority of poultry diets include a proportion of fish meal ranging from 2-5% of the total ration. As per Lengkey et al. (2011), mash rations are supplemented with skipjack tuna gill meal at 1.89%, while crumble rations are supplemented at 2.08%. According to Ologhobo et al. (2012) fish innards weigh 10-15% (depending on the species) of fish biomass. Kim et al. (2012a) According to reports, skipjack tuna innards, analyses using the AOAC method, displayed measurements of 55cm in length and weighing 3.5 kg. They were found to comprise 76.80% moisture, 20.20% crude protein, 0.8% crude lipid, and 1.7% ash. Previous reports solely focused on detailing the nutrient content of skipjack tuna fish used in poultry feed and solely discussed the growth performance of the poultry.
As the digestibility of skipjack fatty acid and omega content remains unclear, this study aimed to explore the impact of skipjack tuna offal meal on the nutrient digestibility of local chicken, focusing specifically on its essential amino acid, short-chain fatty acid and omega content.
MATERIALs AND METHODS
Experimental design
Feeding programmed: The feed treatments included T0 (basal feed), T1 (basal feed with 10% fresh skipjack tuna offal meal), T2 (basal feed with 10% steamed skipjack tuna offal meal), and T3 (basal feed with 10% fermented skipjack tuna offal meal). The chicken feed formulation comprised maize, rice bran, soya bean meal, fish meal, premix, and CaCo3 presented on the Table 1.
Table 1: Nutrient Composition of formulated feed during trial.
|
Nutrient composition |
T0 |
T1 |
T2 |
T3 |
|
(%) |
||||
|
Maize |
50.00 |
50.00 |
50.00 |
50.00 |
|
Soya bean meal |
20.00 |
20.00 |
20.00 |
20.00 |
|
Rice bran |
18.00 |
18.00 |
18.00 |
18.00 |
|
Fish meal |
0.00 |
10.00 |
10.00 |
10.00 |
|
Fresh skipjack tuna offal |
0.00 |
10.00 |
0.00 |
0.00 |
|
Steamed skipjack tuna offal meal |
0.00 |
0.00 |
10.00 |
0.00 |
|
Fermented skipjack tuna offal meal |
0.00 |
0.00 |
0.00 |
10.00 |
|
Limestone |
1.00 |
1.00 |
1.00 |
1.00 |
|
Premix |
1.00 |
1.00 |
1.00 |
1.00 |
|
|
100.00 |
100.00 |
100.00 |
100.00 |
|
Calculated composition |
||||
|
CP |
18.24 |
18.24 |
18.24 |
18.24 |
|
Fat |
6.71 |
6.71 |
6.71 |
6.71 |
|
CF |
5.99 |
5.99 |
5.99 |
5.99 |
|
ME (Kcal / Kg) |
2826.80 |
2826.80 |
2826.80 |
2826.80 |
|
Calcium |
0.79 |
0.79 |
0.79 |
0.79 |
|
Phosphorus |
0.29 |
0.29 |
0.29 |
0.29 |
|
Proximate composition |
||||
|
DM |
86.66 |
87.24 |
87.11 |
86.36 |
|
CP |
18.24 |
19.32 |
19.47 |
19.71 |
|
Fat |
6.71 |
7.19 |
7.18 |
7.27 |
|
CF |
5.99 |
6.81 |
6.84 |
6.85 |
|
Ca |
0.79 |
0.75 |
0.78 |
0.66 |
|
P |
0.29 |
0.66 |
0.68 |
0.58 |
|
N in feed |
3.45 |
2.61 |
2.58 |
2.76 |
|
ME (Kcal / Kg) |
2,826.80 |
2,974.89 |
2,970.08 |
2,976.79 |
|
GE (Kcal / Kg) |
5,043.26 |
5,130.82 |
5,127.46 |
5,131.54 |
|
Energy and protein ratio |
154.98 |
153.98 |
152.57 |
151.03 |
CP, crude protein; CF, crude fat; DDGS, distillers dried grains with soluble; DM, dry matter; GE, gross energy; ME, metabolizable energy; N, nitrogen. Nutritional content of ingredients was analyzed at the Animal Feed and Chemistry Laboratory, Faculty of Animal Husbandry, Hasanuddin University, UNHAS, Makasar.
Skipjack tuna offal meal preparation
The processing of skipjack tuna is carried out at the Integrated Laboratory of the Faculty of Agriculture, Gorontalo State University (UNG). The samples were procured from local markets situated in Gorontalo. Specifically, only large, reputable stores were selected for sampling. First, the processing procedure involves cleaning 1 kg fresh skipjack tuna innards with running water, draining them, and then cutting them into small particles.
The innards are subsequently weighed and divided into three treatments. The first treatment (P1) involves drying the skipjack tuna innards under the sun for 2-3 days or an oven at 60-70°C for 8 hours. After drying, they are ground into flour using grinding mill.
Second, the treatment (P2) entails steaming fresh skipjack tuna innards in boiling water (100°C) for 30 minutes, then followed by drying under the sun for 2-3 days. After that, put the samples in an oven at 60-70°C for 8 hours if weather conditions require. After drying, they are ground into flour using grinding mill.
Last, the third treatment (P3) includes grinding skipjack tuna innards and placing them in a plastic drum. A mixture of formic acid and propionic acid (3% per kg of ingredients) is added in a 1:1 ratio to the plastic drum (silo). The mixture is stirred to ensure even distribution, with pH measurements conducted. Stirring is performed 3-4 times daily consecutively, and the silage is stored for approximately 7 days. Once the fermentation process is complete, the fermented offal is weighed, dried in the sun for 2-3 days, or in an oven at 60-70°C for 8 hours, and then ground into flour.
Essential amino acid, short chain fatty acid and omega content determination
The first study involved a skipjack tuna offal meal experiment using a descriptive method, examining the essential amino acid, short chain fatty acid and omega content. The nutritional content of ingredients was analyzed at the Animal Feed and Chemistry Laboratory, Faculty of Animal Husbandry, Hasanuddin University, UNHAS, Makasar. Amino acid content analysis was conducted at the Feed Quality Testing and Certification Center (BPMSP), Jakarta, while fatty acid analysis and omega content was performed by following standard procedures at the Saraswanti Indo Genetech (SIG) Laboratory, Jakarta. The amino acid content was analyzed following the procedure:
Samples (0.1 g) were hydrolyzed using HCl (6N, 10 ml) at 110°C in sealed glass tubes for 24 hours on a multi-place heating mantle. The aliquot containing hydrolyzed amino acids was treated with a redrying solution (methanol 95%: water: triethylamine, 2:2:1 v/v/v), followed by pre-column derivatization of hydrolysable amino acids using phenyl isothiocyanate (PITC, or Edman’s reagent) to form phenylthiocarbamyl (PTC) amino acids. The derivatizing reagent was freshly prepared and composed of methanol 95%: triethylamine: phenylisothiocyanate (20 ml, 7:1:1 v/v/v, prepared by mixing 70 ml methanol, 10 ml distilled water, 10 ml triethylamine, and 10 ml phenyl isothiocyanate). The derivatized sample (PTC derivative, 20 ml) was diluted with sample diluent (20 ml, 5 mM sodium phosphate NaHPO4 buffer, pH 7.4: acetonitrile 95:5 v/v) before being injected into reverse-phase binary gradient HPLC (Waters Corporation, Milford, MA) equipped with a column maintained at 38 ± 18°C in a column oven and detected via UV absorbance (λmax 254 nm) using a 2487 dual λ absorbance detector. A reverse-phase C18 column (dimethyloctadecylsilylbonded amorphous silica; Nova-Pak, 3.9 × 150 mm) was used for amino acid separation. The mobile phase consisted of (A) sodium acetate trihydrate (0.14 M, 940 ml, pH 6.4) containing triethylamine (0.05%), mixed with acetonitrile (60 ml), and (B) acetonitrile: water (3:2, v/v). A gradient elution program with increasing eluent B was applied. Amino acid quantification was performed by comparing the peak area of the sample with the standard (PIERS amino acid standard H; Thermo Scientific), and the amino acid content was expressed as grams per 100 grams of protein.
The sample of skipjack tuna meal, obtained through oven drying and pressing, is then blended. Subsequently, 2.5% NaCl is added to the mixed skipjack tuna, followed by heating at 50°C. The mixture is then separated using a funnel, and the skipjack tuna is extracted. After centrifugation at 7000 rpm for 20 minutes, the obtained skipjack tuna undergoes characterization based on its chemical and physical properties.
Next, 6 g of Skipjack tuna is weighed in a 125 mL Erlenmeyer flask. Distilled water (10 mL), CaCl2 solution (2.5 mL of 0.063 M), Tris-HCl buffer solution (5 mL), and lipase (100 mg) are added, followed by incubation at 37°C for varying durations (up to 10 hours) with periodic shaking. The mixture is then activated with 50 mL of ethanol, transferred to a separating funnel, and allowed to form two distinct layers. The upper layer is extracted, combined with additional ethanol, and evaporated on a water bath.
Subsequently, 25 mg of the skipjack tuna is weighed in a sealed test tube, to which 1 mL of 0.5 N NaOH solution (in methanol) is added and shaken for 1 minute. The tube is heated in a 100°C water bath for 5 minutes, cooled, and then treated with BF3 (1 mL) before another 5-minute heating. After cooling, 1 mL of n-hexane is added and shaken for 30 seconds. The resulting n-hexane layer is separated, and the process is repeated with an additional extraction of the water layer. The combined n-hexane layers are treated with anhydrous Na2SO4 (50 mg), evaporated, and injected (1 μL) for analysis using gas chromatography.
The fatty acid profiles were analysed using a Shimadzu QP 2010 ULTRA chromatography (GC) system with an HP6890 instrument, equipped with a flame ionization detector (FID) and an SPTM2380 column (30 m × 0.25 mm × 0.20 µm). Nitrogen served as the gas carrier, with the column temperature programmed to increase from 140°C to 240°C at a rate of 4°C/min. Fatty acids were identified by comparing their retention times to a chromatographic standard. The deviation value (Δ) was calculated as the absolute difference between the percentage of each class of fatty acids and the ideal value (33.33%). A deviation of 0 indicates good nutritional value, while greater deviations suggest poorer nutritional values.
Nutrient digestibility analysis
A quantity of 24 Kampong Unggul Balitnak (KUB) local chickens aged 60 days were individually and randomly placed into 24 metabolic cages following a complete randomized design featuring 4 treatments and 6 replications. Each cage unit measured 32 cm x 35 cm x 32 cm and was equipped with feeders, drinkers, and a tray placed at the bottom to collect excreta. The experimental animals were maintained for two weeks, with the first seven days for adaptation and the following three days for total excreta collection. The animals were provided with feed and water ad libitum. After the adaptation period, the animals were fasted for 15 hours to neutralize the effects of previous feeding. Following the fasting period, the animals were given experimental feed at a rate of 100 g/head, which was consumed within one hour, and then excreta collection was conducted for 42 hours. During excreta collection, a 5% boric acid solution was sprayed every 3 hours to prevent the nitrogen in the excreta from evaporating. The collected excreta were cleaned of feathers, weighed, and dried either in sunlight or in an oven at 60°C for 24 hours. Once dried, samples of the excreta were weighed for laboratory analysis. The data obtained were used to compute Apparent Metabolizable Energy (AME) and Apparent Metabolizable Energy corrected to zero Nitrogen balance (AMEn) using the following formulas with adapted from Sjofjan et al. (2021) method.
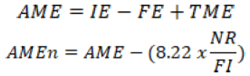
Where; IE represents ingested energy, FE denotes the energy expelled through feces by fed birds, and TME signifies total metabolic energy. AMEn values are calculated after correcting for zero Nitrogen balance, where NR stands for nitrogen retained, and FI represents feed intake. Moreover, the retention of nitrogen and protein digestibility was expressed by using the following formula:

Statistical analysis
The first experiment utilized descriptive analysis, whereas the second experiment focusing on digestibility employed statistical analysis via analysis of variance using GraphPad 9.5.1, with errors depicted as the standard error mean (SEM). Subsequently, probability values underwent the Duncan Multiple Range Test. The applied model is adapted from (Adli et al., 2023).
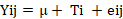
Where; Yij represents the observed parameters, μ is the overall mean, Ti indicates the different of skipjack tuna offal meal effects, and eij is the error term. The treatments are defined as follows: T0 (basal feed), T1 (basal feed with 10% fresh skipjack tuna offal meal), T2 (basal feed with 10% steamed skipjack tuna offal meal), and T3 (basal feed with 10% fermented skipjack tuna offal meal). To compare the means of nutrient digestibility, One-way Analysis of Variance (ANOVA) was utilized, with a significance threshold of p < 0.05. The analysis involved six replications, and significance was determined at the 5% level (p < 0.05). Subsequently, the probability values underwent the Duncan Multiple Range Test (DMRT).
RESULTS AND DISCUSSION
Effects of treatments on the essential amino acid, short chain fatty acid and omega content of skipjack tuna
Table 3 displays the impact of various treatments on the essential amino acid, short-chain fatty acid and omega content of skipjack tuna. The treatments effects on the essential amino acid content of skipjack tuna offal meal showed that lysine had the highest levels in the steamed treatments (3.09%), outperforming other treatments such as 2.16% (T1) and 1.43% (T3) (Table 2). Conversely, for methionine, the fresh treatments exhibited superior results compared to others, with levels at 1.46% (Table 3). Compared with Klomklao and Benjakul (2016) the viscera of skipjack tuna fish meal presented the methionine is superior at the 12.11% and lysine consisted 7.1%. The nutritional quality of any ingredient relies on its protein’s ability to meet organisms’ requirements for essential amino acids (Klomklao and Benjakul, 2016). Therefore, it is conceivable that the protein hydrolysate from skipjack tuna viscera could serve as a dietary supplement to address deficiencies in protein balance within the diet (Klomklao and Benjakul, 2016).
Table 2: Effects of treatments on the essential amino acid, short chain fatty acid and omega content of skipjack tuna offal meal.
|
Parameters (%) |
T1 |
T2 |
T3 |
|
Histidine |
0.96 |
1.44 |
0.91 |
|
Threonine |
2.04 |
2.32 |
1.96 |
|
Arginin |
3.09 |
2.74 |
1.48 |
|
Phenylalanine |
1.60 |
2.19 |
1.16 |
|
Threonine |
2.04 |
2.32 |
1.96 |
|
Lysine |
2.16 |
3.09 |
1.43 |
|
Isoleucine |
2.12 |
2.09 |
1.94 |
|
Leucine |
3.24 |
3.70 |
3.04 |
|
Methionine |
1.46 |
1.33 |
1.15 |
|
Thryptophan |
0.19 |
0.34 |
0.14 |
|
Valine |
3.79 |
2.56 |
3.43 |
|
Myristic acid (C14:0) |
0.15 |
0.64 |
0.36 |
|
Palmitate acid (C16:0) |
2.61 |
4.11 |
3.91 |
|
Arakhidate acid (C20:0) |
1.19 |
1.43 |
1.82 |
|
Omega 3 |
2.81 |
3.89 |
4.59 |
|
Omega 6 |
0.64 |
0.57 |
0.81 |
|
Omega 9 |
1.18 |
1.71 |
1.20 |
T1 (basal feed with 10% fresh skipjack tuna offal meal), T2 (basal feed with 10% steamed skipjack tuna offal meal), and T3 (basal feed with 10% fermented skipjack tuna offal meal). Amino acid content analysis was conducted at the Feed Quality Testing and Certification Center (BPMSP), Jakarta, while fatty acid analysis was performed at the Saraswanti Indo Genetech (SIG) Laboratory, Jakarta.
The steamed skipjack tuna exhibited higher levels of palmitate, stearic, and mysristic acids compared to other treatments (4.11%, 1.43%, 0.64%, respectively) (Table 2). The amount of lauric acid didn’t quite reach 0.1%. Nonetheless, the treatment using fermented skip jack tuna offal meal showed superior results compared to others, with levels of 0.08% as opposed to 0.02% (T2) and 0.05% (T1), respectively (Table 2). In accordance, the arakhidate acid (C20:0) content in the fermented skip jack offal (T3) yielded superior outcomes compared to the other treatments provided (1.82 (T3) as opposed to 1.42 (T2) and 1.19 (T1), respectively) (Table 2). In a separate investigation by Trilaksani et al. (2023), it was discovered that skipjack tuna fish exhibit diverse fatty acid profiles. More precisely, myristic acid (C14:0) levels ranged from 1.70% to 2.90%, while palmitic acid (C16:0) levels varied from 14.41% to 20.40%.
The fermented skipjack tuna, on the other hand, showed elevated levels of palmitate and stearate acids, securing the second position in terms of acid composition. The findings were contrasted with those of Peng et al. (2013), who reported that the short-chain fatty acid content in skipjack tuna fish is approximately 6%. The primary amino acids observed were glutamic acid, aspartic acid, and lysine, accounting for a range of 7.93% to 12.45%. Among these, glutamic acid stood out as the most prevalent, comprising 12.45% and 11.28% of the amino acid composition in the muscle tissues of yellowfin tuna and bigeye tuna, respectively. Apart from glutamic acid, no significant differences were detected in the other amino acids between the two tuna species (P > 0.05) (Peng et al., 2013). Meanwhile, Sutrisno et al. (2023) reported that the extraction yield of oil from Katsuwonus pelamis was 5.60% of the dry weight. The analysis revealed the presence of palmitic acid (42.34%), stearic acid (14.14%), and oleic acid (4.65%).
The percentages of essential amino acids (EAAs) relative to total amino acids (TAAs) in yellowfin tuna and bigeye tuna were 44.95% and 45.64%, respectively (Peng et al., 2013). The silage fermentation process leads to the liquefaction of fish innards, facilitated by naturally occurring enzymes and accelerated through the addition of acids. The combination of formic acid and propionic acid (3%/kg in a 1:1 ratio) contributes to maintaining a suitable low pH, promoting the rapid hydrolysis of proteins into simpler elements like amino acids and peptides. This acid-induced hydrolysis, as suggested by Tomczack-Wandzel and Medrzycka (2013), aids in breaking down proteins into peptides and amino acids, influencing the increase in protein levels observed in the T3 treatment compared to T1.
The acidic environment created by the added organic acids (formic and propionic) also inhibits the growth of proteolytic bacteria, preventing substrate decomposition and protein damage. Higher concentrations of organic acids correlate with a lower degree of change in crude protein, as reported by Batalha et al. (2017). The value of essential amino acid protein in trash fish silage, according to Al-Abri et al. (2014), was lower than that in fermented skipjack tuna offal (T3 treatment). The crude protein (CP) levels in T2 (steamed skipjack viscera) were lower than in T3 (fermented skipjack viscera), attributed to protein coagulation and denaturation during the high-temperature steaming process. Denaturation occurs due to heat, penetrating the offal material and reducing protein functionality, ultimately affecting amino acids and protein availability. The CP content in T2 increased by 2.27% compared to T1 (fresh skipjack viscera). Treatment T1 (fresh skipjack innards) served as a control, lacking steaming or fermentation. The CP level in T1 represents the pure protein value from skipjack tuna viscera.
Additionally, the omega-3 content exhibited the highest value in the fermented skip jack tuna offal meal at 4.59%, surpassing that of fresh or steamed skip jack tuna offal meal (3.89% and 2.81%, respectively). Similarly, both omega-6 and omega-9 levels in the fermented skip jack tuna offal meal outperformed those in the other treatments, with values of 4.59% and 0.81%, respectively (Table 3). Omega-3 fatty acids play a role in lowering cholesterol and triglyceride levels, promoting the reduction of blood biochemistry, lowering high blood pressure, preventing artery hardening, and inhibiting the proliferation of cancer cells (Leke et al., 2015a). Apart from their cardiovascular benefits, they also have a positive impact on immune function and blood lipid profiles. Furthermore, Omega-3 fatty acids are essential for the development of brain tissue and the human retina (Leke et al., 2015b).
Effects of treatments on the nutrient digestibility of local chicken
Table 3 displays the influence of treated skipjack tuna on the nutrient digestibility of local chicken. Moreover, the utilization of treated skipjack tuna (fresh, steamed, and fermented) resulted in significant differences (p < 0.01) in nitrogen retention (Figure 1), crude protein digestibility (Figure 2), apparent metabolizable energy digestibility (AME), and apparent metabolizable energy digestibility-n corrected (AMEn) (Table 3).
Regarding crude protein digestibility, the fermented treatment demonstrated the most favorable outcome compared to the other treatments (80.04% versus 70.63%, 71.21%, and 68.39%, respectively). Similarly, for apparent metabolizable energy digestibility, the fermented treatment showed superior results (2635.39 kcal/kg versus 2606.01 kcal/kg, 2490.61 kcal/kg, and 294.56 kcal/kg, respectively). In contrast to findings by Kim et al. (2012b), which indicated no significant effects of incorporating up to 6% tuna fish silage in the diet and optimal results observed at 4% tuna fish silage, particularly concerning final body weight, carcass percentage, and meat protein conversion in broiler chicken. Lengkey et al. (2011), this study observed notable reductions in cholesterol levels in the carcass and liver of broilers fed with Lactobacillus cultures. However, this effect was not observed in muscle. Furthermore, supplementation of Lactobacillus culture in broiler diets resulted in a significant decrease in fat content in the liver, muscle, and carcass (Abdullah et al., 2006). Fermentation is a secure, eco-friendly, and financially advantageous method that facilitates the extraction of diverse compounds, including bioactive peptides and aromatic substances (Ramírez et al., 2013). Moreover, fermentation enhances the digestibility of proteins by breaking them down into shorter peptides and amino acids, thus offering a valuable means to enhance the nutritional value of fish-based feed, where they serve as a key protein source (Özyurt et al., 2019).
Table 3: Effects of treatments on the nutrient digestibility of local chicken.
|
Parameters |
Treatments |
SEM |
P-Value |
|||
|
T0 |
T1 |
T2 |
T3 |
|||
|
Retention of Nitrogen |
65.48b |
67.28b |
55.44a |
72.05c |
2.20 |
< 0.001 |
|
Crude protein digestibility |
68.39a |
71.21 a |
70.63 a |
80.04 b |
2.80 |
< 0.001 |
|
AME |
2294.56 a |
2490.61 b |
2606.01 b |
2635.39 c |
27.82 |
< 0.001 |
|
AMEn |
2318.19 a |
2519.29 b |
2636.11 b |
2665.95 c |
25.72 |
< 0.001 |
AME, apparent metabolizable energy; AMEn, apparent metabolizable energy corrected to zero Nitrogen balance. T0 (basal feed), T1 (basal feed with 10% fresh skipjack tuna offal meal), T2 (basal feed with 10% steamed skipjack tuna offal meal), and T3 (basal feed with 10% fermented skipjack tuna offal meal).
The similar results found on the Al-Abri et al. (2014) there is no significant effect (p >0.05) in digestibility coefficient of crude protein. In poultry rations, fish meal is commonly included at levels of approximately 2-5% of the total feed. According to Lengkey et al. (2011), mash ration supplemented with skipjack tuna gill meal was at 1.89%, and crumble ration supplemented with skipjack tuna gill meal was at 2.08%. Fish silage, a valuable protein with high biological significance in animal nutrition, can be derived from deceased fish, underutilized species in the fish industry, as well as marine fishing by products, commercial fish waste, and industrial residues (Geron et al., 2007). The digestibility of fish meal in chicken feed is correlated with several factors. One key factor is the processing method used to produce the fish meal. Proper processing techniques can enhance the digestibility of proteins and nutrients in fish meal, making them more readily available for absorption by chickens. Additionally, the quality and freshness of the fish used in the production of fish meal can affect its digestibility. Fish meal made from fresh, high-quality fish is likely to be more digestible than fish meal made from lower-quality or degraded fish. These materials are often deemed low-quality, and their non-utilization may pose environmental, health, and economic concerns (Geron et al., 2007). In a separate investigation conducted by Leke et al. (2015), it was found that incorporating sun-dried, steamed, and boiled skipjack tuna fish into chicken feed, specifically in the form of skipjack gills meal, can serve as a viable substitute for fish meal in chicken diets without causing any negative effects.
CONCLUSIONS and Recommendations
In summary, based on the findings, incorporating skipjack tuna offal meal is advised as a protein source for local chickens, as it proves beneficial without adverse effects. Beyond supplying protein, it also delivers essential amino acids, short-chain fatty acids, and omega content, thereby potentially enhancing the value of local chickens.
ACKNOWLEDGeMENTS
We would like to thank Animal Feed and Chemistry Laboratory, Faculty of Animal Husbandry, Hasanuddin University, UNHAS, Makasar. Amino acid content analysis was conducted at the Feed Quality Testing and Certification Center (BPMSP), Jakarta, while fatty acid analysis was performed at the Saraswanti Indo Genetech (SIG) Laboratory, Jakarta
NOVELTY STATEMENT
The novelty of this research is the use of three treatments of skipjack tuna fish on the local chicken. Since there is lack of information of this fish. The novelty quite applicable among develop countries.
AUTHOR’S CONTRIBUTION
SZ played a role in collecting data, conducting nutritional analysis, analyzing data, and preparing the manuscript. H, ES and OS contributed to the design of the research, provided supervision, and participated in revising the manuscript. All authors have read and approved the final version of the manuscript submitted to the journal.
Ethical approval
Ethical approval for the study was given by the Animal Care and Use Committee, University of Islam Kalimantan Muhammad Arsyad Al Banjary, No. 2-KEP-UNISKA.PPJ-2024. The ethical approval outlined the number of chickens involved in this study, the parameters under observation, and the use of cage pens that do not compromise animal welfare and condition properly.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdullah N, Jalaludin S, Wong MC, Ho YW (2006). Effects of Lactobacillus feed supplementation on cholesterol, fat content and fatty acid composition of the liver, muscle and carcass of broiler chickens. Anim. Res., 55(1): 77-82. https://doi.org/10.1051/animres:2005043
Adli DN, Sadarman S, Irawan A, Jayanegara A, Wardiny TM, Priambodo TR, Nayohan S, Permata D, Sholikin MM, Yekti APA (2023). Effects of oligosaccharides on performance, egg quality, nutrient digestibility, antioxidant status, and immunity of laying hens: A meta-analysis. Ital. J. Anim. Sci., 22(1): 594-604. https://doi.org/10.1080/1828051X.2023.2223211
Adli DN, Sjofjan O, Natsir MH, Nuningtyas YF, Sholikah NU, Marbun AC (2020). The effect of replacing maize with fermented palm kernel meal (FPKM) on broiler performance. Livest. Res. Rur. Dev., 32(7): 1-4.
Al-Abri AS, Mahgoub O, Kadim IT, Al-Marzooqi W, Goddard SJ, Al-Farsi M (2014). Processing and evaluation of nutritive value of fish silage for feeding Omani sheep. J. Appl. Anim. Res., 42(4): 406-413. https://doi.org/10.1080/09712119.2013.875909
AOAC (2005). AOAC: Official Methods of Analysis. USA.
Batalha ODS, Alfaia SS, Cruz FGG, Jesus RSD, Rufino JPF, Costa VR (2017). Digestibility and physico-chemical characteristics of acid silage meal made of pirarucu waste in diets for commercial laying hens. Acta Scient. Anim. Sci., 39(3): 251-257. https://doi.org/10.4025/actascianimsci.v39i3.35112
BPS (2020). Populasi ikan cakalang di Gorontalo. Indonesia.
Geron LJV, Zeoula LM, Vidotti RM, Matsushita M, Kazama R, Neto SFC, Fereli F (2007). Chemical characterization, dry matter and crude protein ruminal degradability and in vitro intestinal digestion of acid and fermented silage from tilapia filleting residue. Anim. Feed Sci. Tech., 136(3-4): 226-239. https://doi.org/10.1016/j.anifeedsci.2006.09.006
Kim HJ, Kim MJ, Kim KH, Ji SJ, Lim KH, Park KH, Kim JS (2012a). Preparation and characterization of canned skipjack tuna Katsuwonus pelamis as a health food. Korean J. Fish. Aquat. Sci., 45(3): 215-223. https://doi.org/10.5657/KFAS.2012.0215
Kim HJ, Kim MJ, Kim KH, Ji SJ, Lim KH, Park KH, Kim JS (2012b). Comparison of food components in various parts of white muscle from cooked skipjack tuna Katsuwonus pelamis as a source of diet foods. Korean J. Fish. Aquat. Sci., 45(4): 307-316. https://doi.org/10.5657/KFAS.2012.0307
Klomklao S, Benjakul S (2017). Utilization of tuna processing byproducts: Protein hydrolysate from skipjack tuna (Katsuwonus pelamis) viscera. J. Food Proc. Preserv., 41(3): 12970. https://doi.org/10.1111/jfpp.12970
Laihad JT, Leke JR, Tinangon RM, Tangkau L, Regar MN, Siahaan R (2019). Production performance and egg quality in native chickens fed diet of Skipjack fish oil. J. Adv. Agr. Tech., 6(1). https://doi.org/10.18178/joaat.6.1.43-47
Leke JR, Widyastuti T, Mandey JS, Najoan M, Laihad J (2015a). Performance of broiler chickens fed skipjack (Katsuwonus pelamis) gills meal as a replacement in several levels and methods for fish meal. Pros. Sem. Nas. Masyarakat Biodiv. Ind., 1(4): 771-775.
Leke JR, Sjofjan O, Najoan M, Mandey JS (2015b). Organoleptic characteristic of eggs laid by local hens fed Skipjack Fish waste as a source of Omega-3 fatty acids in the diets. Livest. Res. Rur. Dev., 27(11).
Lengkey HAW, Bagau B, Adriani L, Ludong M (2011). The effect of various level of skipjack tuna bone meal (Katsuwonus pelamis L.) in ration on broiler carcass tenderness and abdominal fat. Biotech. Anim. Husband., 27(4): 1727-1731. https://doi.org/10.2298/BAH1104727L
Ologhobo AD, Asafa AR, Adejumo IO (2012). Performance characteristics of broiler chicken fed poultry offal meal. Int. J. Agric. Sci., 2(11): 1021-1025.
Özyurt G, Ozogul Y, Kuley-Boga E, Özkütük AS, Durmuş M, Uçar Y, Ozogul F (2019). The effects of fermentation process with acid and lactic acid bacteria strains on the biogenic amine formation of wet and spray-dried fish silages of discards. J. Aqua. Food Prod. Tech., 28(3): 314-328. https://doi.org/10.1080/10498850.2019.1578314
Peng S, Chen C, Shi Z, Wang L (2013). Amino acid and fatty acid composition of the muscle tissue of yellowfin tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus). J. Food Nutr. Res., 1(4): 42-45.
Peng S, Chen L, Qin JG, Hou J, Yu N, Long Z, Sun X (2008). Effects of replacement of dietary fish oil by soybean oil on growth performance and liver biochemical composition in juvenile black seabream, Acanthopagrus schlegeli. Aquaculture, 276(1-4): 154-161. https://doi.org/10.1016/j.aquaculture.2008.01.035
Ramírez JCR, Ibarra JI, Romero FA, Ulloa PR, Ulloa JA, Matsumoto KS, Manzano MÁM (2013). Preparation of biological fish silage and its effect on the performance and meat quality characteristics of quails (Coturnix coturnix japonica). Braz. Arc. Biol. Tech., 56(6): 1002-1010. https://doi.org/10.1590/S1516-89132013000600016
Sjofjan O, Adli DN (2021). The effect of replacing fish meal with fermented sago larvae (FSL) on broiler performance. Livest. Res. Rur. Dev., 33(2): 2-7.
Sjofjan O, Adli DN, Natsir MH, Nuningtyas YF, Bastomi I, Amalia FR (2021). The effect of increasing levels of palm kernel meal containing α-β-mannanase replacing maize to growing-finishing hybrid duck on growth performance, nutrient digestibility, carcass trait, and VFA. J. Ind. Trop. Anim. Agr., 46(1): 29-39. https://doi.org/10.14710/jitaa.46.1.29-39
Sohn JH, Ohshima T (2010). Control of lipid oxidation and meat color deterioration in skipjack tuna muscle during ice storage. Fisheries Sci., 76: 703-710. https://doi.org/10.1007/s12562-010-0248-0
Statista (2023). Import volume of fish meal in Indonesia from 2015 to 2021(in 1,000 metric tons). USA
Sutrisno S, Oktaviana L, Wijaya HW (2023). Characterization of some marine fish oils and identification of their fatty acid content at Indonesia’s East Java oceans. AIP Conf. Proc., 2588(1). https://doi.org/10.1063/5.0111970
Tomczak-Wandzel R, Mędrzycka K (2013). Preparation, composition and properties of fish silage produced with post-coagulation sludge. Environ. Prot. Eng., 39(4): 39-49. https://doi.org/10.37190/epe130404
Trilaksani W, Riyanto B, Ramadhan W, Sinulingga F, Fauziah S (2023). The characteristics of PUFAs-rich virgin fish oil as affected by size of tuna eye. Biodiv. J. Biol. Div., 24(12): 6545-6556.
To share on other social networks, click on any share button. What are these?





