Epoxidation of Pumpkin Seed Oil (Curcurbita Maxima) using Acidic Ion Exchange Resin and its Kinetic and Thermodynamic Study
Research Article
Epoxidation of Pumpkin Seed Oil (Curcurbita Maxima) using Acidic Ion Exchange Resin and its Kinetic and Thermodynamic Study
Qamar Javed Iqbal1*, Zainab Masood2, Asad Abbas3 and Sidra Munir4
1Department of Chemistry, University of Engineering and Technology Lahore, Pakistan; 2Department of Zoology, Lahore College for Women University Lahore, Pakistan; 3School of Chemistry, University of Punjab Lahore, Pakistan; 4Department of Zoology, Gulab Devi Educational Complex Lahore, Pakistan.
Abstract | The kinetic properties of epoxidized pumpkin seed oil were examined under different parameters. The iodine number of pumpkin seed oil was found to be 108 g I2/100g oil. This oil was epoxidized by 30% H2O2 which acts as oxygen donor and glacial acetic acid which acts as oxygen carrier using inert solvent (benzene) in the presence of acidic ion exchange resin (Amberlite IR-120) as catalyst. The effect of different parameters such as catalyst loading, temperature, stirring speed, molar ratios of H2O2 and acetic acid to ethylenic unsaturated double bond were studied. It was found that maximum Relative epoxy yield (REY) was 68% at temperature 60oC (333 K) with molar ratio of Double bond: Acetic Acid: Hydrogen peroxide (D: A: H) 1.0:0.5:1.5 at stirring speed of 1000 rpm. The kinetics of epoxide formation was studied under different ranges of temperatures i.e. 50oC, 60oC and 70oC.The Activation Energy of reaction was found to be 23.64kj/mol with the help of Arrhenius Equation (ln k = ln ko – Ea/RT). The purpose of this study is to produce highly epoxidized pumpkin oil which can be further utilized as raw material in polymer industry and to discuss its kinetic and thermodynamic properties. The characterization of epoxidized pumpkin seed oil was accomplished by FTIR analysis which shows peak of epoxy group at 845cm-1.
Received | May 09, 2022; Accepted | August 01, 2022; Published | October 25, 2022
*Correspondence | Qamar Javed Iqbal, Department of Chemistry, University of Engineering and Technology Lahore, Pakistan; Email: qamarjavediqbal16@gmail.com
Citation | Iqbal, Q.J., Z. Masood, A. Abbas and S. Munir. 2022. Epoxidation of pumpkin seed oil (Curcurbita Maxima) using acidic ion exchange resin and its kinetic and thermodynamic study. Journal of Innovative Sciences, 8(2): 202-211.
DOI | https://dx.doi.org/10.17582/journal.jis/2022/8.2.202.211
Keywords | Iodine number, Relative epoxy yield, Thermodynamic properties, Epoxidized pumpkin oil, Ethylenic double bond, Activation energy
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
As the demand of energy is increasing day by day and natural resources are limited so there is an increased interest in utilization of renewable resources as an alternate of naturally occurring petroleum based polymers (Biermann et al., 2011). Therefore, much attention has been diverted to the development of polymeric products obtained from vegetable oils. Oils extracted from plants and vegetables are essential raw material sources for many chemical reactions in industry. These vegetable oils are desirable in chemical industries because they are readily available from renewable resources (Belgacem and Gandini, 2011). These vegetable oils are used as raw material for thermosetting polymers (Galià et al., 2010). The oils extracted from plants and vegetables contain unsaturated triglycerides of fatty acid which can undergo many chemical changes and be modified into various forms. That’s why; these oils have wide applications in chemical industry (Gunstone, 2011). The unsaturated double bond in oils majorly consists of triglycerides of three fatty acids i-e linoleic acid, oleic acid and linolenic acid (Petrović, 2008). These unsaturated double bond in oil are highly reactive and easily be converted to epoxide ring by simple oxidation mechanism. Theses oils can be modified into polyamides (Stemmelen et al., 2011) glycols, hydroxyesters, mercaptoalcohols, alkanolamines, N-hydroxyalkylamines, alcohols, polymers such as polyesters, epoxy resin, and polyols due to reactive epoxy group. In addition, they can also be used as polymer stabilizers and plasticizers, coating and paint components (Erhan, 2005). Many epoxy resins are also used to modify epoxidized vegetable oil i.e soybean oil (Gupta et al., 2011). They not only increase the elasticity of material but are also used in packing material such as wrapping foils. Moreover, the long unsaturated fatty acid chains impart flexibility to some hard resins such as polyester, polyurethane and epoxy resin (Milchert and Smagowicz, 2009).
The most common method used in chemical industry to epoxidize these unsaturated fatty acids is by in situ epoxidation using acidic cation exchange resin as catalyst (Goud et al., 2007). In this method percarboxylic acid (oxygen carrier) and hydrogen peroxide are used as raw materials (Jiang et al., 2012). This method has got commercial importance because of resumablity of catalyst. The reaction mechanism generally consists of two steps (Milchert et al., 2010) i.e., first step involves formation of peroxyacid and second step involves reaction of peroxyacid with carbon-carbon double bond in oil to produce epoxy group. During the course of reaction, peroxyacid formed by reaction of carboxylic acid with hydrogen peroxide migrates into oily layer and then reacts with unsaturated double bond of oil to produce epoxide. The carboxylic acid produced in second step again reacts with hydrogen peroxide and again produces per acetic acid.
Although numerous methods exist in literature which are concerned with the epoxidation of vegetable oils such as rapeseed oil, jatropha oil, soybean oil, mahua oil, almond oil, castor oil, cotton seed oil, karanja oil and canola oil, but only a few of them deal with the kinetic and thermodynamic study of these oils (Abdullah and Jumat, 2010). Epoxidized soyabean oil and linseed oil are also used to synthesise glycidyl esters (Wang and Schuman, 2013). During the formation of epoxy ring, many side reactions also occurred due to the high reactivity of epoxide ring (Petrović et al., 2002). The reaction is highly exothermic and large amount of energy is released; so a limited amount of hydrogen peroxide must be added with the help of dropping funnel. It should be added drop wise with carboxylic acid in the reaction mixture which contains vegetable oil and catalyst. The reaction requires 10 to 15 hours for complete epoxidation within the maintained temperature range from 50oC to 70oC. Initially the rate of reaction was very fast and temperature rises suddenly but with the controlled addition of hydrogen peroxide the rate of reaction became slow and temperature decreases.
Previous literature (Cai et al., 2008; Mungroo et al., 2008) shows some work on different oils in the same manner as ours so it gives us a strong logical approach to further identify epoxidation of pumpkin seed oil in our local settings. Pumpkin seed oil contains high amount of natural unsaturated fatty acids (Younis et al., 2000) which can be epoxidized with the help of acetic ion exchange resin as catalyst in the presence of inert solvent benzene.
The research aimed at studying the influence of some reaction variables on the epoxidation process. Another objective was to propose a mathematical model for the reaction system and the estimation of the kinetic parameters of the model. Therefore, this oil is a good choice for the production of epoxide.
2. Materials and Methods
Pumpkin oil was purchased from local market (Lahore, Pakistan). Glacial acetic acid (>99.5% AR Grade), Aqueous Hydrogen peroxide (30% wt), Benzene, diethyl ether, Potassium Iodide, Sodium thiosulphate, Chloroform, Iodine Monochloride (Hanus Solution), and Acidic Ion Exchange Resin (Amberlite IR-120) were obtained from Abkari Road (Lahore, Pakistan). HBr in acetic was obtained from University of Engineering and Technology (Lahore, Pakistan) and then diluted with acetic acid (>99.5% AR Grade) to prepare standard HBr-acetic acid 0.1N solution.
2.1 Procedure
The epoxidation of pumpkin oil was carried out with peracetic acid formed in situ as described in the previous literature. The calculated quantity of pumpkin oil was kept in 500 mL round bottom flask immersed in water bath, the oil was mixed with equal mass of benzene. Then, calculated amount of glacial acetic acid (0.5-1.0 mol) was added followed by acidic ion exchange resin (Amberlite IR-120) as a catalyst. The whole mixture was stirred for about 30 minutes. Subsequently, the required quantity of H2O2 solution (30% wt) was mixed drop wise at a constant rate so that the procedure was accomplished in one hour. During H2O2 addition, the temperature of mixture was maintained at 30°C and after the addition of hydrogen peroxide the temperature was raised to the desired value. During the operation, the reaction mixture was kept safe and at constant stirring with utmost care. The reaction mixture was stirred at 500-1500 rpm to get a fine dispersion of oil. Samples were taken out at definite time intervals at the rate of 10 mL per sample considering that the addition of H2O2 as initial time. After complete stirring and quenching, oil and aqueous phase were separated. Extraction of samples was obtained with diethyl ether before analysis; the oil samples were filtered and extracted with diethyl ether by separating funnel, flushed with water to remove free acid and then dried by adding anhydrous sodium sulphate so that the final product had pH value 7. All the collected samples were analyzed for Iodine Number and oxirane oxygen value (OOexpt). The Iodine value of fats or oils is used to determine the number of carbon-carbon double bond present in them. The iodine value is determined by number of grams of iodine that reacts with 100g of fats or oils. The percentage of oxygen content (OOexpt) was determined by standard HBr-acetic acid method under which oxygen is directly titrated with HBr solution (Swern et al., 1947). From (OOexpt) theoretical oxirane oxygen value can be calculated as discussed below.
3. Results and Discussion
The following terms were used in:
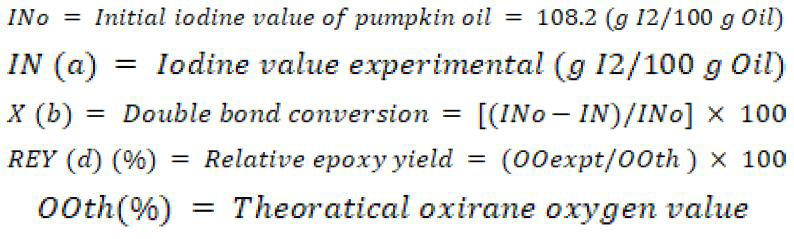
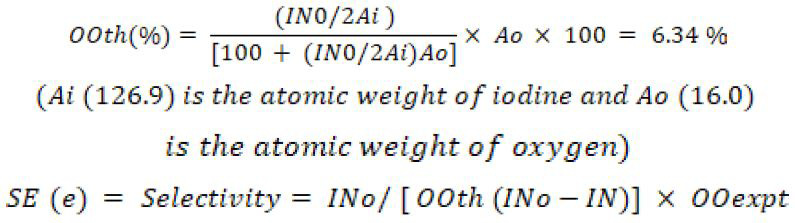
3.1 Effect of hydrogen peroxide loading on oxirane value and iodine number
The effect of hydrogen peroxide to double bond mole ratio was carried out at different mole ratios ranging from 0.6, 1.1 and 1.5 moles of hydrogen peroxide to unsaturated double bond. So the mole ratios were 0.6:1.0, 1.1:1.0 and 1.5:1.0. It was noticed that with increasing concentration of hydrogen peroxide the iodine value is decreased and corresponding oxirane value is incresed. during the addition of hydrogen peroxide organic acid is converted into peroxyacid and then it is regenerated again. Approximately 50% hydrogen peroxide is consumed in the epoxidation process. It was found that the maximum conversion to oxirane oxygen value was found when 1.5 moles of hydrogen peroxide were used against 1.0 mole of unsaturated double bonds. It was also found that the oxirane oxygen content was increased progressively as hydrogen peroxide ratio was raised from 0.6 to 1.5 moles. Details are given in Table 1.
Reaction conditions: CH3COOH per mole unsaturation= 0.5; Amberlite IR-120 Loading= 15%; Temperature= 60oC; Ethylenic double bond= 1.0; Stirring speed= 1000 rpm.
Table 1: Effect of different molar ratios of hydrogen peroxide.
|
Particulars |
Hydrogen peroxide molar ratios |
|||||
|
0.6 |
1.1 |
1.5 |
||||
|
Time (Hrs) |
5 |
10 |
5 |
10 |
5 |
10 |
|
IN (a) |
53 |
32 |
48 |
27 |
43 |
21 |
|
X (b) (%) |
51 |
70.42 |
55.63 |
75.04 |
60.02 |
80.59 |
|
OOexpt(c) (%) |
2.73 |
3.92 |
2.98 |
4.05 |
3.32 |
4.25 |
|
REY (d) (%) |
43.05 |
61.82 |
47 |
63.88 |
52.36 |
67.03 |
|
SE (e) |
0.82 |
0.86 |
0.84 |
0.85 |
0.86 |
0.84 |
3.2 Effect of catalyst loading on oxirane value and iodine number
The effect of cationic ion exchange resin was carried out at three different catalyst concentrations including 5%, 10% and 15%wt of Amberlite IR-120. The molar ratio of double bond: acetic acid: hydrogen peroxide (D: A: H) was 1.0:0.5: 1.5. the temperature of reaction mixture was maintained at 600C. It was found that by increasing the amount of catalyst the rate of reaction is increased and corresponding increase in the oxirane oxygen value. As catalyst loading is increased the time interval of reaching maximum REY was shortened. The use of this catalyst reduces the side reactions e.g., epoxide ring opening and also increases the rate of reaction. It was also found that by increasing catalyst concentration iodine value decreased and double bond conversion is increased which also increases the epoxy yield. The maximum oxirane oxygen content was observed when 15% wt. catalyst was added to reaction mixture and also minimum iodine value was observed. The reaction of epoxidation was completed in maximum 7 hours when 15% Amberlite was used because the active moieties present in catalyst which provide large surface area for reagents to absorb in this. Details are given in Table 2.
Reaction conditions: CH3COOH per mole unsaturation= 0.5; H2O2 per mole unsaturation= 1.5; Temperature= 60oC; Ethylenic double bond= 1.0; Stirring speed = 1000 rpm
Table 2: Effect of different percentages of catalyst.
|
Particulars |
Percentage of catalyst (Amberlite IR-120) used |
|||||
|
5% |
10% |
15% |
||||
|
Time (Hrs) |
5 |
10 |
5 |
10 |
3 |
7 |
|
IN (a) |
48 |
26 |
43 |
21 |
36 |
14 |
|
X (b) (%) |
55.63 |
75.97 |
60.25 |
80 |
66.72 |
87.06 |
|
OOexpt(c)(%) |
2.98 |
4.13 |
3.32 |
4.25 |
3.74 |
4.32 |
|
REY (d)(%) |
47 |
65 |
52 |
67 |
58 |
68 |
|
SE(e) |
0.84 |
0.85 |
0.86 |
0.83 |
0.88 |
0.78 |
3.3 Effect of temperature on oxirane value and iodine number
The effect of temperature on pumpkin oil was carried out at three different temperatures as shown below in table. After the addition of hydrogen peroxide the reaction temperature was raised to 70oC while the addition of hydrogen peroxide was carried out at low temperature so that rate of epoxidation increases slowly. The effect of inert solvent (benzene) was studied at these temperature ranges. The presence of inert solvent decreases the side reactions and increases the stability of oxirane oxygen. This is because of that the inert solvent remains in the oil phase during the course of epoxidation and has no influence on peroxyacid formation. As the solvent remains in oil phase the rate of reaction is very fast but when the organic phase is diluted then the rate of reaction decrease due to cleavage of oxirane ring and many side reactions occur instead of epoxidation i.e., formation of glycol, ester etc. the main reaction and side change reactions increases by increasing the temperature. During the course of reaction, it was found that the maximum conversion to oxirane was found at temperature 700C after 8 hours. Also, the highest iodine value was found at 50oC after 5 hours. Details are given in Table 3.
Reaction conditions: CH3COOH per mole unsaturation= 0.5; H2O2 per mole unsaturation= 1.5; Catalyst loading = 15%; Ethylenic double bond= 1.0; Stirring speed = 1000 rpm.
Table 3: Effect of different temperatures.
|
Particulars |
Temperatures |
|||||
|
50oC |
60oC |
70oC |
||||
|
Time (Hrs) |
5 |
10 |
5 |
10 |
4 |
8 |
|
IN (a) |
52 |
32 |
46 |
27 |
43 |
21 |
|
X (b) (%) |
51.94 |
70.42 |
57.48 |
75.04 |
60.25 |
80.6 |
|
OOexpt(c) (%) |
2.71 |
3.92 |
3.12 |
4.05 |
3.32 |
4.19 |
|
REY (d) (%) |
42.74 |
61.82 |
49.21 |
63.88 |
52.36 |
66.08 |
|
SE (e) |
0.82 |
0.87 |
0.85 |
0.85 |
0.86 |
0.82 |
3.4. Effect of stirring speed on oxiane value and iodine number
The process of epoxidation of pumpkin oil was carried out at three different stirritng speeds ranging from 500 to 1500 rpm as shown in table below. The reaction was studied out under triphase conditions including solid catalyst + pumpkin oil + aqueous hydrogen peroxide. It was observed the oxirane oxygen content, double bond conversion and relative epoxy yield was incresd with increasing stirring speed from 500 to 1500 rpm. There was no appreciable epoxidation observed above 1500 rpm. The molar ratio of double bond: acetic acid: hydrogen peroxide (D: A: H) was 1.0:0.5: 1.5. Details are given in Table 4.
Table 4: Effect of different stirring speeds.
|
Particulars |
Stirring speeds |
|||||
|
500 rpm |
1000 rpm |
1500 rpm |
||||
|
Time (Hrs) |
5 |
10 |
5 |
10 |
5 |
10 |
|
IN (a) |
52 |
33 |
43 |
21 |
39 |
15 |
|
X (b) (%) |
51.94 |
69.50 |
60.25 |
80.6 |
63.95 |
86.13 |
|
OOexpt(c) (%) |
2.71 |
3.79 |
3.32 |
4.25 |
3.69 |
4.30 |
|
REY (d) (%) |
42.74 |
59.77 |
52.36 |
67.03 |
58.20 |
67.82 |
|
SE (e) |
0.82 |
0.86 |
0.86 |
0.83 |
0.91 |
0.78 |
Reaction conditions: Double Bond per mole per mole unsaturation = 1.0; CH3COOH per mole unsaturation= 0.5; Hydrogen peroxide per mole unsaturation = 1.5; Amberlite IR-120 loading = 15%; Temperature = 60oC.
3.5 Effect of acetic acid on oxirane value and iodine number
Hydrogen peroxide and oil do not react of their own significantly. For this purpose acetic acid is used which is further converted into peracetic acid and act as oxygen carrier for epoxidation of unsaturated double bond. The epoxidation of pumpkin oil was carried out in different molar ratios ranging from 0.3 to 1.0 moles of acetic acid per mole of ethylenic double bond. The effect of glacial acetic acid concentration to relative conversion to oxirane oxygen was carried out at 60oC with or without the inert solvent (benzene). Acetic acid acts as catalyst in epoxidation reaction and increase the rate of reaction. This effect becomes more prominent when the temperature is increased to 60oC and the inert solvent stabilizes the oxirane oxygen from decomposition. So the temperature and acetic acid concentration should be balanced to stabilize the epoxidation reaction. At temperature 60oC the conversion to oxirane was maximum with mole ratio 1.0-1.0 was used. At this molar ratio oxirane oxygen had maximum value and iodine value had minimum value. Details are given in Table 5.
Reaction conditions: Temperature = 60oC; Double Bond per mole per mole unsaturation = 1.0; Hydrogen peroxide per mole unsaturation = 1.5; Amberlite IR-120 loading = 15%; Stirring speed = 1000 rpm.
Table 5: Effect of different molar ratios of acetic acid.
|
Particulars |
Acetic acid molar ratios |
|||||
|
0.3 |
0.5 |
1.0 |
||||
|
Time (Hrs) |
5 |
10 |
5 |
10 |
5 |
10 |
|
IN (a) |
50 |
31 |
43 |
21 |
40 |
15 |
|
X (b) (%) |
53.78 |
71.34 |
60.18 |
80.6 |
63.03 |
86.13 |
|
OOexpt(c) (%) |
2.82 |
4.01 |
3.32 |
4.25 |
3.58 |
4.30 |
|
REY (d) (%) |
44.47 |
63.24 |
52.36 |
67.03 |
56.46 |
67.82 |
|
SE (e) |
0.82 |
0.88 |
0.86 |
0.83 |
0.89 |
0.78 |
4. Characterization of pumpkin seed oil and epoxidized pumpkin seed oil
4.1 Fourier transforms infrared spectroscopy of pumpkin seed oil and epoxidized pumpkin seed oil
To support the formation of epoxy group in pumpkin seed oil the samples were analyzed by FTIR spectroscopy. The FTIR used was 4100typeA having scanning speed of 2mm/sec. The wave number range of FTIR was 4000 cm-1 to 400 cm-1 and transmittance was 60% to 100%. Two FTIR spectra were observed for this purpose (a) Raw pumpkin seed oil (b) Epoxidized pumpkin seed oil as shown in Figure 1 below. In sample (a) three peaks were observed at 3458 cm-1, 1640 cm-1 and 1384 cm-1. The peak at 3458 cm-1 shows the stretching vibrations of O-H present in –COOH, the peak at 1640 cm-1represents stretching vibration of -C= C- present in oil and the peak at 1384 cm-1 represents the symmetric deformation of –CH3 group. These peaks are shown in Figure 2. After the epoxidation of pumpkin seed oil peaks appear at 3475cm-1 which represents stretching vibration of –OH group derived from ring opening of epoxy group, other appears at 1741cm-1 due to stretching vibration of –C=O present in ester and the main and most representative peak appears at 845cm-1 due to stretching vibration of C-O-C represents the formation of epoxy ring.The disappearance of peak at 1640 cm-1 and appearance of peak at 845cm-1 shows the contribution of -C=C- double bond and its conversion to C-O-C group. Beside these some peaks also appears at 2926 cm-1and 2859 cm-1. These peaks represent anti symmetric and symmetric stretching vibrations of –CH3 as discussed in Figures 1 and 2.
5. Reaction kinetics of epoxidized pumpkin seed oil
The kinetic study of epoxidation of pumpkin seed oil revealed that the rate of reaction was found to be pseudo first order with respect to ethylenic unsaturated double bond (Sinadinović-Fišer et al., 2012). Both steps of epoxidation mechanism can be written in terms of molar concentration.
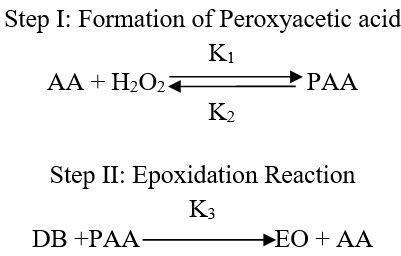
Where;
AA= Molar concentration of Acetic acid; EO= Molar concentration of epoxidized pumpkin oil; DB= Molar concentration of ethylenic unsaturated double bond; PAA= Molar concentration of per acetic acid; K= rate constant; n1= reaction order with respect to double bond concentration; n2= reaction order with respect to per acetic acid concentration.
The following rate equation can be applied by assuming per acetic acid concentration constant.
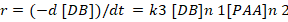
r = rate of disappearance of carbon-carbon double bond.
As the reaction is pseudo first order with respect to double bond concentration so the above equation can be simplified as:
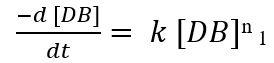
Here; k= k3 [PAA]
The rate equation data for epoxidation of pumpkin oil can be fitted in the above equation. By using the approach of double bond conversion X(b), the concentration of ethylenic unsaturated double [DB] can be defined as following:
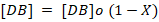
Where;
[DB]o = Initial double bond concentration; X(b) = Double bond conversion.
As the double bond concentration changes with time continuously so the above equation can be simplified and written as change in concentration of double bond with respect to time.
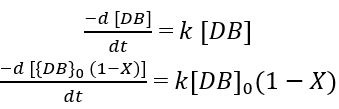
By rearranging the above equation:
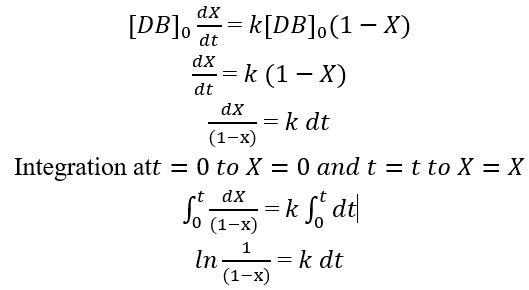
So, by using the above equation the rate constant (k) for each temperature can be calculated by plotting graph between 1/(1-X) vs reaction time (t).
5.1 Determination of reaction kinetics at 50oC
The reaction kinetics was studied at 50oC with respect to time. It was found that conversion of double bond increased as time increased. Conversion of double bond X(b) with respect to time given in Table 6.
Table 6: Conversion of double bond X(b) with time at 50oC.
|
T(Hr) |
X(b) |
1/(1-X) |
ln[1/(1-X)] |
|
0 |
0 |
0 |
0 |
|
1 |
0.14 |
1.15 |
0.15 |
|
2 |
0.22 |
1.30 |
0.25 |
|
3 |
0.35 |
1.55 |
0.44 |
|
4 |
0.47 |
1.90 |
0.64 |
|
5 |
0.52 |
2.08 |
0.73 |
|
6 |
0.56 |
2.31 |
0.84 |
|
7 |
0.59 |
2.46 |
0.90 |
|
8 |
0.64 |
2.74 |
1.01 |
|
9 |
0.68 |
3.18 |
1.16 |
|
10 |
0.70 |
3.38 |
1.22 |
From the above data a graph can be drawn, from which value of reaction constant k can be calculated at 50oC as described in Figure 3.
5.2 Determination of reaction kinetics at 60oC
The reaction kinetics was studied at 60oC with respect to time. It was found that conversion of double bond increased as time increased. Conversion of double bond X (b) with respect to time given in Table 7.
Table 7: Conversion of double bond X(b) with time at 60oC.
|
T(Hr) |
X(b) |
1/(1-X) |
ln[1/(1-X)] |
|
0 |
0 |
0 |
0 |
|
1 |
0.16 |
1.19 |
0.18 |
|
2 |
0.26 |
1.35 |
0.30 |
|
3 |
0.39 |
1.63 |
0.49 |
|
4 |
0.49 |
1.97 |
0.68 |
|
5 |
0.57 |
2.35 |
0.85 |
|
6 |
0.59 |
2.46 |
0.90 |
|
7 |
0.61 |
2.59 |
0.95 |
|
8 |
0.69 |
3.21 |
1.17 |
|
9 |
0.72 |
3.63 |
1.29 |
|
10 |
0.75 |
4.01 |
1.39 |
From the above data a graph can be drawn, from which value of reaction constant k can be calculated at 60oC as described in Figure 4.
5.3 Determination of reaction kinetics at 70oC
The reaction kinetics was studied at 70oC with respect to time. It was found that conversion of double bond increased as time increased. Conversion of double bond X(b) with respect to time given in Table 8.
Table 8: Conversion of double bond X(b) with time at 70oC.
|
T(Hr) |
X(b) |
1/(1-X) |
ln[1/(1-X)] |
|
0 |
0 |
0 |
0 |
|
1 |
0.19 |
1.23 |
0.20 |
|
2 |
0.29 |
1.40 |
0.34 |
|
3 |
0.43 |
1.76 |
0.56 |
|
4 |
0.60 |
2.51 |
0.92 |
|
5 |
0.64 |
2.78 |
1.02 |
|
6 |
0.69 |
3.24 |
1.17 |
|
7 |
0.76 |
4.18 |
1.43 |
|
8 |
0.80 |
5.15 |
1.64 |
From the above data a graph can be drawn, from which value of reaction constant k can be calculated at 70oC as described in Figure 5.
6. Determination of activation energy
The activation energy of the epoxidation reaction can be calculated from rate constant k by using Arhenius equation (Himmelblau and Riggs, 2004) at different temperatures by plotting graph between lnk and 1/T.
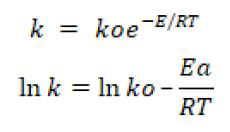
By drawing graph between 1/T and ln k we can find the value of slope and activation energy. Further details are discussed in graph given below.
From the graph slope is calculated below:
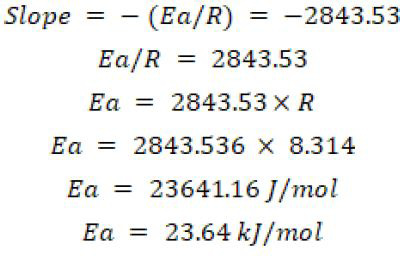
Hence, the activation energy of the reaction is 23.64 kj/mol.
Table 9: Rate constant(k) at different temperatures.
|
T (k) |
1/T |
K (hr-1) |
ln k |
|
323 |
0.00309 |
0.13 |
-2.09 |
|
333 |
0.00300 |
0.14 |
-1.99 |
|
343 |
0.00291 |
0.21 |
-1.58 |
Conclusions and Recommendations
The kinetics of epoxidized pumpkin seed oil was studied in the presence of acidic ion exchange resin (Amberlit IR-120) under different ranges of temperature i.e., 323, 333, 343 K. The result of present research shows that pumpkin seed oil can be successfully utilized for epoxidation using Amberlite IR-120 as catalyst. The use of acidic ion exchange resin (Amberlite IR-120) minimized the side reactions during the epoxidation process. The presence of inert solvent (Benzene) and moderate temperature (333 K) increased the rate of epoxidation. Optimum conditions for epoxide formation included the mole ratios of ethylenic double: Acetic acid: H2O2 (D: A: H) were 1.0: 0.5: 1.5 with catalyst loading of 15% and stirring speed of 1500 rpm.
A mathematical model that described the kinetics of the reaction system forin situ epoxidation of unsaturated fatty acids or triglycerides with organic peroxyacids, catalysed by acidic ion exchange resin, was proposed.Smallvalues of kinetic rate constants for both the side reactions indicated that the ring opening reactions were relatively much slower. The activation energy for the epoxidation reaction was determined to be 23.64 kJ/mol.
Novelty Statement
The epoxidation of pumpkin seed oil is the area which has not been discussed yet. This paper includes the synthesis of epoxidized pumpkin seed oil in the presence of acidic ion exchange resin (Amberlite IR-120) as catalyst. Epoxidized pumpkin seed oil can be used as stabilizer and plasticizer in PVC and its copolymers. This polymer has widespread use in gramophone recorders, floor covering, pipes etc.
Authors Contributions
Qamar Javed Iqbal: Experimental work.
Zainab Masood: Supervised the experimental work.
Asad Abbas and Sidra Munir: Helped in writing research article.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdullah, B.M., and Jumat, S., 2010. Epoxidation of vegetable oils and fatty acids: Catalysts, methods and advantages.
Belgacem, M.N. and Gandini, A., 2011. Monomers, polymers and composites from renewable resources, Elsevier.
Biermann, U., Bornscheuer, U., Meier, M.A., Metzger, J.O. and Schäfer, H.J., 2011. Oils and fats as renewable raw materials in chemistry. Angewandte Chemie International Edition, 50: 3854-3871. https://doi.org/10.1002/anie.201002767
Cai, C., Dai, H., Chen, R., Su, C., Xu, X., Zhang, S. and Yang, L., 2008. Studies on the kinetics of in situ epoxidation of vegetable oils. European Journal of Lipid Science and Technology, 110: 341-346. https://doi.org/10.1002/ejlt.200700104
Deka, D.C., and Basumatary, S., 2011. High quality biodiesel from yellow oleander (Thevetia peruviana) seed oil. Biomass and Bioenergy, 35: 1797-1803. https://doi.org/10.1016/j.biombioe.2011.01.007
Erhan, S.Z., 2005. Industrial uses of vegetable oils, AOCS Press Champaign. https://doi.org/10.1201/9781439822388
Galià, M., De Espinosa, L.M., Ronda, J.C., Lligadas, G., and Cádiz, V., 2010. Vegetable oil-based thermosetting polymers. European Journal of Lipid Science and Technology, 112: 87-96. https://doi.org/10.1002/ejlt.200900096
Goud, V.V., Patwardhan, A.V., Dinda, S. and Pradhan, N.C., 2007. Epoxidation of karanja (Pongamia glabra) oil catalysed by acidic ion exchange resin. European Journal of Lipid Science and Technology, 109: 575-584. https://doi.org/10.1002/ejlt.200600298
Gunstone, F., 2011. Vegetable oils in food technology: Composition, properties and uses, John Wiley and Sons. https://doi.org/10.1002/9781444339925
Gupta, A., Ahmad, S. and Dev, A., 2011. Modification of novel bio-based resin-epoxidized soybean oil by conventional epoxy resin. Polymer Engineering and Science, 51: 1087-1091. https://doi.org/10.1002/pen.21791
Himmelblau, D.M. and Riggs, J.B., 2004. Basic principles and calculations in chemical engineering, Prentice Hall PTR Upper Saddle River.
Jiang, J., Zhang, Y., Yan, L. and Jiang, P., 2012. Epoxidation of soybean oil catalyzed by peroxo phosphotungstic acid supported on modified halloysite nanotubes. Applied Surface Science, 258: 6637-6642. https://doi.org/10.1016/j.apsusc.2012.03.095
Milchert, E., and Smagowicz, A., 2009. The influence of reaction parameters on the epoxidation of rapeseed oil with peracetic acid. Journal of the American Oil Chemists’ Society, 86: 1227-1233. https://doi.org/10.1007/s11746-009-1455-7
Milchert, E., Smagowicz, A. and Lewandowski, G., 2010. Optimization of the epoxidation of rapeseed oil with peracetic acid. Organic Process Research and Development, 14: 1094-1101. https://doi.org/10.1021/op900240p
Mungroo, R., Pradhan, N.C., Goud, V.V. and Dalai, A.K., 2008. Epoxidation of canola oil with hydrogen peroxide catalyzed by acidic ion exchange resin. Journal of the American Oil Chemists’ Society, 85: 887-896. https://doi.org/10.1007/s11746-008-1277-z
Petrović, Z.S., 2008. Polyurethanes from vegetable oils. Polymer Reviews, 48: 109-155. https://doi.org/10.1080/15583720701834224
Petrović, Z.S., Zlatanić, A., Lava, C.C., and Sinadinović-Fišer, S., 2002. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids kinetics and side reactions. European Journal of Lipid Science and Technology, 104: 293-299. https://doi.org/10.1002/1438-9312(200205)104:5<293::AID-EJLT293>3.0.CO;2-W
Sinadinović-Fišer, S., Janković, M., and Borota, O., 2012. Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chemical Engineering and Processing: Process Intensification, 62: 106-113. https://doi.org/10.1016/j.cep.2012.08.005
Stemmelen, M., Pessel, F., Lapinte, V., Caillol, S., Habas, J.P. and Robin, J.J., 2011. A fully biobased epoxy resin from vegetable oils: From the synthesis of the precursors by thiol-ene reaction to the study of the final material. Journal of Polymer Science Part A: Polymer Chemistry, 49: 2434-2444. https://doi.org/10.1002/pola.24674
Swern, D., Findley, T.W., Billen, G.N. and Scanlan, J.T., 1947. Determination of oxirane oxygen. Analytical Chemistry, 19(6): 414-415. https://doi.org/10.1021/ac60006a018
Tan, S., and Chow, W., 2010. Biobased epoxidized vegetable oils and its greener epoxy blends: A review. Polymer-Plastics Technology and Engineering, 49: 1581-1590. https://doi.org/10.1080/03602559.2010.512338
Wang, R. And Schuman, T.P., 2013. Vegetable oil-derived epoxy monomers and polymer blends: a comparative study with review. Express Polym. Lett., 7: 272-292. https://doi.org/10.3144/expresspolymlett.2013.25
Younis, Y., Ghirmay, S. and Al-Shihry, S., 2000. African Cucurbita pepo L.: Properties of seed and variability in fatty acid composition of seed oil. Phytochemistry, 54: 71-75. https://doi.org/10.1016/S0031-9422(99)00610-X
To share on other social networks, click on any share button. What are these?





