Effect of Berberine on Some Histopathological, Enrofloxacin Residue and Aniti-Diarrheaginic Escherichia coli in Broilar Chicken
Research Article
Effect of Berberine on Some Histopathological, Enrofloxacin Residue and Aniti-Diarrheaginic Escherichia coli in Broilar Chicken
Raed Hussein Salih Rabee1, Yahya Sabah Abdulameer1, Walla Farhan Obed1, Noor R Abady1, Adnan Mansour Jasim1*, Firas Hussein Albawi1, Mohammed Jasim Jawad2, Ahmed Samir Abukhomra3
1College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq; 2College of Veterinary Medicine, Karbala University, Karbala, Iraq; 3Anesthesia Department Hilla University College Babylon, Iraq.
Abstract | Background Antibiotic residues in poultry have the potential to have a number of harmful effects on health, including the emergence of multidrug-resistant microbial strains, allergic and an alteration of the normal flora in the intestines. The study aimed to evaluate the residue levels of enrofloxacin in chicken meat after administering berberine (BBR). Escherichia coli (E. coli) was isolated from chickens suffering from diarrhea, and its isolation was confirmed by culturing it on MacConkey agar medium. In this study, a total of one hundred chicken chicks were reared, and on the tenth day of the experiment, they were divided into five equal groups:NCG: This group represented the control and was given normal drinking water. T1: The chickens in this group were orally inoculated with E. coli at a dose of 0.5 ml containing 6x108 CFU/ml of E. coli. T2: The chickens in this group were orally inoculated with E. coli at a dose of 0.5 ml containing 6x108 CFU/ml of E. coli and were treated with enrofloxacin at a dose of 12.5 mg/kg orally through drinking water. T3 group: The chickens in this group were orally inoculated with E. coli at a dose of 0.5 ml containing 6x108 CFU/ml of E. coli and were treated with 250 mg/kg BBR through drinking water. T4 group: The chickens in this group were orally inoculated with E. coli at a dose of 0.5 ml containing 6x108 CFU/ml of E. coli and were treated with both combination enrofloxacin and BBR. Conclusion: study showed that berberine increased the residue levels of enrofloxacin in chicken meat, specifically in the thigh and liver. The docking study confirmed inhibitory activity of Cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) an enzyme responsible for the metabolism of antibiotics. Additionally, berberine demonstrated antibacterial effects against E. coli, as well as anti-inflammatory and antioxidant properties. Furthermore, histopathological examination of the intestinal broiler received BBR showed improvements and restore tissue near to normal.
Keywords | Enerofloxacin, Berberine, E. coli, Antibiotic residues, CYP450, Antioxidant, Drug docking
Received | August 28, 2023; Accepted | October 12, 2023; Published | November 23, 2023
*Correspondence | Adnan Mansour Jasim, College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq; Email: adnan.mansouri81@vet.uoqasim.edu.iq
Citation | Rabee RHS, Abdulameer YS, Obed WF, Abady NR, Jasim AM, Albawi FH, Jawad MJ, Abukhomra AS (2023). Effect of berberine on some histopathological, enrofloxacin residue and aniti-diarrheaginic Escherichia coli in broilar chicken. Adv. Anim. Vet. Sci., 11(12):1945-1954.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.12.1945.1954
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Poultry chickens are commercially breed for egg and meat production because they grow quickly and are fairly inexpensive (Atsbeha and Hailu, 2021). In fact, the demand for meat is rising day by day, as various drugs have been used to promote growth and control infection. When antibiotics are introduced to poultry, some of the antibiotic and its derivatives are released into the meat and eggs, and after a few weeks, the level gradually tapers off due to metabolic regression (Yousif and Jwher, 2021). The antibiotic withdrawal period is the time that passes between the final doses of antibiotic given to the chicken and the time that the level of antibiotic residue in the meat passes (Jabber et al., 2022). Prior to withdrawal periods, chicken meat or eggs are not fit for human consumption. Berberine powder is isoquinoline alkaloid has yellow color and bitter taste, commonly isolated from different plant families (Ibrahim et al., 2023). It has been utilized since the 50 years ago as an active medication for the treatment of human and animal gastro-enteritis and secretory type diarrhea (Luo et al., 2013). Additionally, it serves well to avoid and treat diarrhea in animals, including poultry. Moreover, studies have confirmed that berberine support the P-glycoprotein (P-gp) and co-administered with P-gp substrate result in mediate drug–drug interactions (Murakami et al., 2023). Berberine is a substrate of P-gp chicken and down-regulation of P-gp expression in chicken tissues, resulting enhancing the absorption of P-gp substrates as well as berberine boost the bioavailability other substrate specially orally co-administered with other P-gp substrates like enerofloxacin that resulting toxicity and antibiotics accumulation (Zhang et al., 2019). In recent years, berberine as a broad-spectrum antimicrobial activity has attracted more and more attention (Hao et al., 2022). It is known that berberine has wide medical effects in the treatment of many diseases related to humans and animals, such as gastroenteritis, chronic diarrhea, malaria, polycystic ovaries, Alzheimers disease, obesity, diabetes and lowering blood pressure (Imenshahidi and Hosseinzadeh, 2019; Alnuqaydan et al., 2022). Several studies reported the inhibition effect of berberine on biofilm formation by altering the quorum-sensing system in a drug-resistant Escherichia coli confirmed by Sun et al. (2019). Enrofloxacin is fluoroquinolone from second generation, restricted developed for veterinary use only and has excellent potency versus aerobic bacteria from the Gram-negative type (Dai et al., 2023). Enrofloxacin is until now still essential part in the veterinarian’s armamentarium for the treating of colibacillosis in numerous cities of the world (Joosten et al., 2019; Fairbrother and Nadeau, 2019). The study aimed to use the berberine compound in poultry in vivo, as well as the activity of berberine on inhibition of P-gp using drug docking technique and its influence on the ability Escherichia coli to induce pathogenesis in broiler from side and accumulation of enerofloxacin alone or in combination with berberine in thigh and liver to get suitable data about drug-drug interaction of berberine with antibiotic as well as regulate dose of enerofloxacin to reduce accumulation in broiler meat as well as reduce toxicity and enerofloxacin resistance in human consumption.
MATERIALS AND METHODS
Materials
Enrofloxacin having a purity of 99.9% and Berberine 90% were purchased from Adooq (USA) and TCI (USA), respectively. Phosphoric acid was obtained Valsad (India). HPLC grade Acetonitrile was purchased from fisher scientific (UK).
Diagnosis of E. coli
E. coli was isolated from suffering from diarrhea after confirming the isolation by culturing it on macConkey agar medium. Pink colonies appeared, indicating lactose fermentation in MacConkey medium Figure 1.
It was also detected on a selective medium, which is Eosin Methylene Blue (EMB) agar, and gave metallic green luster Figure 2.
After confirming the presence of E. coli bacteria, a series of decimal dilutions of the bacteria was conducted for the purpose of counting the bacteria (Al-Ahbabi et al., 2016). The fifth dilution was taken and a sensitivity test was performed on Mueller-Hinton Agar medium using the drilling method and injecting a ratio of (0.5) into the hole and after incubating in the incubator at 37 degrees Celsius for 24 hours (Figure 3). The test result was read by reading the zoom with a result of 16 mm (Salman et al., 2022; Othman et al., 2023).
Experimental of design
This study was conducted in the animal house of the College of Veterinary Medicine, Babylon Province. The study was carried out in a semi-closed hall divided into four sections and its dimensions were 3 x 2 m² divided by wooden partitions, wire mesh and independent doors. Wood sawdust with a height of 8-10 cm. It is equipped with all breeding requirements such as feeders, manholes and gas incubators. The poultry field was air-conditioned, the ambient temperature stabilized at approximately 25 to 30°C, and the relative humidity was maintained between 44 and 60%. The light cycle was identical to that of a commercial farm (15 hours light/9 hours dark). No clinical signs of disease were noted before beginning the experiment. In this current study, one hundred chicken chicks were reared, and on the tenth day of the experiment, they were divided into five equal groups, as the following division included: The first group represented the control and left the festive river water (NCG), The second group (T1) was inoculated orally with E. coli at dose 0.5 ml involving 6x108 CFU/ml of E. coli (Al-Taii and Yousif, 2019; Mohammad and Al-Mahmood, 2022). The third group (T2), was orally inoculated with E. coli at dose 0.5 ml containing 6x108 CFU/ml of E. coli and given enrofloxacin 12.5 mg / kg orally with river water. The fourth group(T3) was inoculated inoculated with E. coli at dose 0.5 ml has 6x108 CFU/ml of E. coli and treated by berberine extract with drinking water. The last five group (T4) was inoculated with E. coli at dose 0.5 ml with 6x108 CFU/ml of E. coli and treated by berberine extract and enrofloxacin 12.5 mg/kg with drinking water.
The current experiment lasted for five days. During this period, clinical symptoms were recorded on the chicks, including anorexia and diarrhea as well as macroscopically showed congestion of liver, kidney and slightly hemorrhage in small intestine with mortality rate was 5%. On the fifth day, the chicks were sacrificed. Blood samples were collected from heart puncture and serum was isolated to determine immunological parameters. Sections were taken from the intestines for the purpose of histological examination, and a swab was taken from the intestines to find out the effect of the treatment on the formation of bacterial colonies, The specimen 20 from liver were collected from different chicken received enerofloxacin and mixed enerofloxacin with berberine then samples were placed at -20 ºC for HPLC analysis.
High performance liquid chromatography (HPLC) assay
Enrofloxacin residues were determined quantitively by High Performance Liquid Chromatography (HPLC). Enrofloxacin standard. The stock solutions of enrofloxacin was performed at a concentration of 100 μg powdered dissolving in 10 ml working standard solutions of acetonitrile and phosphoric acid to get concentration of 10 μg/mL of antibiotic to get Standard solutions and kept at 4 °C (Aslam et al., 2016; Mahmood et al., 2019).
Apparatus utilize in the study
The analyses were done using shimadzu (Japan) high performance liquid chromatographic system. A separation was perfects using C18 column (4.6 mm × 250 mm, 5μm) for stationary phase. The temperature of column was set at 25 ºC and the injection volume was 20 μL; the mobile part consist of phosphoric acid buffer (0.01 M, pH 3) and acetonitrile and (75:25% v/v) were used with a flow rate of 1.0 mL/min and wave length was 278 nm (Figure 4). The concentration of antibiotic was calculated from Samples preparation for HPLC (Aresta et al., 2019).
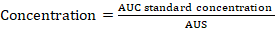
AUC= area under the curve of sample; AUS= area under the curve of standard.
Samples preparation for HPLC
The liver and muscle chicken cold samples were crushed by grinder separately and 5g of each liver and muscle was accurately transferred into a 10 mL glass tube. Then, 5 mL of 25 mM phosphoric acid: acetonitrile (25:75 v/v) solution was transfer to the glass tube and shaken for 5 minutes. The tubes were centrifuged for 8 min at 7000 rpm at room temperature. The supernatant of samples was filtered using a 0.45 μm milipore filter and transferred into second glass tubes to make more suitable for HPLC inspection (Moghadam et al., 2018).
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the data for the presence of normal distribution preconditions. The SPSS 22.0 program (USA) was used for statistical comparisons of parameters between groups. One-way Anova (Posthoc Duncan) was utilized (Nunes et al., 2015).
Histological analysis
Broiler of each group were sacrificed at end of study by decapitation, the small intestine, were isolated and fixed in 10% formalin, dehydrated by ethyl alcohol in ascending grades, cleared in xylol, embedded in paraffin at 56°C and rotator microtome for cutting with size 5μ. The staining was by hematoxylin and eosin (Luna et al., 1972).
RESULTS and Discussion
Drug docking
In the present study azithromycin and berberine have been studied for their potential inhibitory effects on P-glycoprotein (P-gp), which is a protein involved in drug transport and resistance (Figure 7). However, it’s important to note that the research on this topic is still limited, and the precise mechanisms and clinical implications are not fully understood molecules Based on information on the Figure 6.
Antioxidant and anti-inflammatory
The present study showed that serum collected from infected poultry with E. coli and received berberine extract alone or in combination with enrofloxacin 12.5 mg / kg orally with drinking water clear reduction in symptoms of bacteria in broiler Chickens of treated in both enerofloxacin and extract or in combination while the un treated chicks showed sever, dehydration, depression, lowered appetite, cough, and labored breathing and diarrhea. On the other aspect clear reduction in pro-inflammatory cytokines in infected poultry and treated with enerofloxacin when compared with positive control group , as well as excellent reduction was noted in broiler chicken received berberine extract alone or in combination with enrofloxacin to recorded mean value (22.5±1.04, 21.5±0.64 and 21.7±1.65) of serum IL6 for negative control, extract and drugscombination while serum TNFa aclear reduced in this group to recorded mean value (30.5 ±n2.02, 35±0.61 and 32±1.73).
In addition to anti-inflammatory of berberine also noted via a significant reduction of IL6 as compared with (T1) group Figure 10. Serum super oxide dismutase (SOD) broiler chicken showed highly reduced in infected group to (159.2±3.95) as compared with other treated group with extract (173±1.9) and (175±1.19) while poultry infected with E. coli and treated with enerofloxacin showed improvement of SOD but still not a significant as compared with control group Figure 8. Actually, the berberine extract and or enerofloxacin treated the infected E. coli in broiler chicks neutralize the oxidative stress in among groups to recorded mean value for malondialdehyde (MDA) (2.4± 0.14, 2.9±0.13, 2.67±0.19 and 2.72 ±0.20) respectively for control and infected chicken treated with enerofloxacin, extract and their combination Figure 9.
Enerofloxacin liver and muscle residual
The present data in Figure 11 showed that berberine administrated to chicken lead to a significant increase accumulation of enerofloxacin in liver and muscle (tissue residual) as compared with chicken received enerofloxacin alone. Our results demonstrate that berberine could inhibit P-gp expression and activity, that result in change the bioavailability of oral enrofloxacin when it is administered to the broiler’s different organs in chicken. The results of the present study could guide the responsible use of berberine and additives in the poultry industry.
Histopathological study
Inoculated with 6x108CFU/ml of E. coli appear several clinical signs such as fever, emaciation, irregular of feathering with severe diarrhea, thus the macroscopically broiler in T1 group specially noted there is some congestion was seen on liver and small intestine. The current study on histopathology of intestine showed clear restore the tissue to normal state and concord with many studies was done recently. Inoculated broiler with E. coli and then treated with berberine showed hyperplasia of the intestinal associated lymphoid tissues with increase in goblet cells number with normal intestinal villi (Figure 13), thus BBR restore the damage effect result from bacteria and enerofloxacin toxicity Figure 12.
The present study noted that berberine may inhibit P-gp and CP3A4 activity by evaluate drug docking activity of on T137(A) OG1Y247(A) CE2D177(A) OD1 and C442(A) SGF302(A) CBI118(A) CG2 which could have implications for drug interactions and the efficacy of certain medications on this enzyme to alter or increase metabolism of drugs. However, it’s worth mentioning that the evidence regarding the extent and clinical significance of P-gp inhibition by both azithromycin and berberine is still limited and conflicting. Moreover, the inhibitory effects of these compounds may affect according to concentration, cell type, and experimental conditions used in different studies. Our findings proven that berberine elevates the bioavailability of enerofloxacin and that interactions between berberine-enerofloxacin important to reduce dose of P-gp substrates especially narrow therapeutic windows. However, berberine influence on withdrawal period of many drugs that considered P-gp substrates in human and animal (Li et al., 2022).The recent study confirmed by (Mohammad and Al-Mahmood, 2022) showed that chicks oral received of 6x108 CFU/ml will cause the clear pathological lesions in the internal organ specially kidney and intestine, with clear mononuclear penetration of inflammatory cells in hepatic tissue from side and reduce body weight with emaciation of chicks with reduce weight of liver and kidney.
The present study was agreement with recent study reported by Aziza et al. (2019) that noted dietary berberine significantly alleviate the LPS- affect by reduce in the mRNA expression of inducible nitrite synthase, TNF-α, IL-1β, cyclooxygenase-2, nuclear factor-kappa B (NF-κB) in the liver with enhance antioxidant enzyme activity and suppression of inflammatory markers.
Tabatabaei et al. (2015) reported that poultry chicks infected with E. coli significantly serum levels of TNF-α and IL6 NF-κB as well as some liver enzymes including lactate-dehydrogenase (LDH), creating-kinase (CK), alanine-transferase (ALT) and aspartate-transferase (AST) with clear reduction of antioxidant SOD as compared with healthy control group. Berberine (BE) has been appear to have numerous pharmacological activities, including enhance immunity, antibacterial activity, as well as potent antioxidant and anti-inflammatory effects. Dietary supplementation with Forsythia suspense extract (FSE) and berberine (BE) at dose 100 mg/kg, or both can ameliorate the growth performance may be via boost immunity, decrease reactive oxygen species, and intestinal colonization promoting by normal microbiota of broilers (Tabatabaei et al., 2015) (Figure 1). Dehau et al. (2023) study confirmed that supplementation of berberine at a high concentration augment chicken gut morphology toward decreased inflammation and boost villus length and lowering crypt depth and infiltration in the bowels tissue of poultry by CD3+ T-lymphocyte. Berberine also affected the diversity of the microbiota of gut from the jejunum to the colon, both at a compositional and active level, with huge effects showed in the large intestine. The ileal proinflammatory cytokines (IL-1β, IL-6, and TNF-α) were considerably higher in broiler chicks with necrotic enteritis brought on by Clostridium perfringens so treatment with berberine at 100mg/kg lower the number of C. perfringens in the cecum, growth performance was restored to normal state, improved inflammatory cytokines, intestinal morphology, and protein expression of cell tight junction (Yuan et al., 2021).
Table 1: Using the estimated binding affinities, docking scores of berberine against a subset of P-gp substrates and inhibitors were generated.
|
No. |
Protein |
Ligand |
Binding energy (kcal/mol |
Inhibition constant |
Receptor |
No. of H bond |
|
1 |
P-GP |
Berberine |
-8.7 |
33µM |
T137(A)OG1, Y247(A)CE2, D177(A)OD1 |
8 |
|
1 |
CYP3A4 |
Berberine |
-9.9, -9.5 |
21µM |
C442(A)SG, F302(A)CB, I118(A)CG2 |
6 |
|
1 |
P-Gp |
Azithromycin |
-6.9 |
34µM |
T173(A)OG1, R148(A)NH1, N887(A)CB, Y920(A)OH |
3 |
The present study showed that berberine administrated to chicken lead to a significant increase accumulation of enerofloxacin in liver and muscle (tissue residual) as compared with chicken received enerofloxacin alone. The current study was agreement with Li et al. (2022) noted that berberine is a substrate of poultry P-gp and down-regulates P-gp in tissues of chicken, thereby enhancement the absorption of P-gp substrates. Enerofloxacin is one of P-gp substrate in chicken and pig intestines. Therefore, one of the most important lines of evaluation of P-gp is the pharmacokinetic interactions with feed or feed additives and their potential impact on the clinical efficacy of antibiotics (Bhutto et al., 2018). This study confirmed that a substrate of P-gp (beberine) lead to down-regulate (expression) and lower activity of P-gp in birds, thus elevating the bioavailability of P-gp substrates in intestine. The introducer P-gp and P-gp inhibitors, and now many technologies depend on synthesis of drug loaded by delivery systems oral taken that can easily penetrate and inhibit P-gp to accelerate the enteral absorption and improve bioavailability of substrates. Since many drugs are reduced efficacy by P-gp efflux system (Jain et al., 2022). The drugs that cause CYP450 enzymes inducer accelerate drug metabolism, while the inhibition of CYP450 enzymes, which causes a delay in drug metabolism (Zhang et al., 2022). The main enzymes for metabolism of enerofloxacin in microsomal liver chicken CYP3A4 and CYP2A6. Additionally, this study supported that this tilmicosin lead to inhibition was greatly associated with CYP3A4 in animal and in vitro (Zhang et al., 2022). Recent study confirmed that enerofloxacin and ciprofloxacin are tendency to accumulate in liver rather than other organ as skin, muscle and kidney (Xu et al., 2023). Here, we investigated the effects of berberine on the activity of enerofloxacin as well as interactions between berberine and enrofloxacin in liver and muscle residual. Our results demonstrate that berberine could inhibit P-gp expression and activity, that result in change the bioavailability of oral enrofloxacin when it is administered to the broiler’s different organs in chicken. The results of the present study could guide the responsible use of berberine and additives in the poultry industry. Histopathological section of intestine infected broiler and received enerofloxacin appear necrosis of the intestinal villi with inflammatory cells infiltration while berberine ameliorate defect of infection due to antioxidant and antibacterial activity from side and modulate adverse effect of enerofloxacin recording normal intestinal villi with increase in the numbers of the goblet cells. The present data was agreement with Yuan et al. (2021) noted that berberine significantly lowered the number of Clostridium perfringens in the cecum, improved tissue intestinal, lowered inflammatory cytokines, and potentiate immune function of broilers. Our study also agree with Dehau et al. (2023) that noted that berberine feed additive at a high dose improves chicken gut histology and minimize inflammation, with inhibited the growth of many butyrate-producing strains while not influence on Enterobacteriaceae.
CONCLUSION and Recommendations
Berberine has increase enerofloxacin residual in chicken meat of thigh and liver by inhibit activity of CYP450 that responsible of metabolism antibiotic as well as has antibacterial effect on colibacillosis, anti-inflammatory and antioxidant from side and improvement of histopathological of intestinal tissue.
ACKNOWLEDGMENTS
I would like to appreciate the College of Veterinary Medicine at the Iraqi Al-Qassim Green University for their assistance with this project.
Novelty Statement
The special goal and novelty of this investigation is to seek out the berberine antibacterial effects against E. coli, furthermore the anti-inflammatory and antioxidant properties , along with its approachability and accessibility.
AUTHOR’S CONTRIBUTION
Conceived and planned to experiments design of study, contributed to broiler management, drugs and samples preparation. Contributed to the sample analysis and accomplishment of the results.
Ethical approval
The Ethics Commission of Veterinary Medicine has authorized this study under reference number 533FD2. Additionally, Al-Qasim Green University’s institutional animal ethics council approved the procedure.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Al-Ahbabi H, Al-Ahbab HM, Hasson OS, Jassim MA (2016). Antimicrobial activity of aloe vera extract on cases of Keratoconjunctivitisin sheep (in vivo and in vitro study) and compared with penicillin–streptomycin. Basrah J. Vet. Res., 15: 227-245. https://doi.org/10.33762/bvetr.2016.124301
Alnuqaydan AM, Almutary AG, Azam M, Manandhar B, Yin GHS, Yen LL, Madheswaran T, Paudel KR, Hansbro PM, Chellappan DK (2022). Evaluation of the cytotoxic activity and anti-migratory effect of berberine–phytantriol liquid crystalline nanoparticle formulation on non-small-cell lung cancer in vitro. Pharmaceutics, 14: 1119. https://www.mdpi.com/1999-4923/14/6/1119. https://doi.org/10.3390/pharmaceutics14061119
Al-Taii, DHF, Yousif AA (2019). Effects of E. coli O157: H7 experimental infections on rabbits. Iraqi J. Vet. Med., 43: 34-42. https://doi.org/10.30539/iraqijvm.v43i1.468
Aresta A, Cotugno P, Zambonin C (2019). Determination of ciprofloxacin, enrofloxacin, and marbofloxacin in bovine urine, serum, and milk by microextraction by a packed sorbent coupled to ultra-high performance liquid chromatography. Anal. Lett., 52(5): 790-802. https://doi.org/10.1080/00032719.2018.1496093
Aslam B, Kousar N, Javed I, Raza A, Ali A, Khaliq T, Muhammad F, Khan JA (2016). Determination of enrofloxacin residues in commercial broilers using high performance liquid chromatography. Int. J. Food Prop., 19: 2463-2470. https://doi.org/10.1080/10942912.2015.1027922
Atsbeha AT, Hailu TG (2021). The impact of effective microorganisms (EM) on egg quality and laying performance of chickens. Int. J. Food Sci., https://doi.org/10.1155/2021/8895717
Aziza A, Abdelhamid F, Risha E, Elsayed, Awadin W (2019). Influence of Nigella sativa and rosemary oils on growth performance, biochemical, antioxidant and immunological parameters, and pathological changes in Japanese quail challenged with Escherichia coli. J. Anim. Feed Sci., 28: 354-366. https://doi.org/10.22358/jafs/114239/2019
Bhutto ZA, He F, Zloh M, Yang J, Huang J, Guo T, Wang L (2018). Use of quercetin in animal feed: Effects on the P-gp expression and pharmacokinetics of orally administrated enrofloxacin in chicken. Sci. Rep., 8: 1-12. https://doi.org/10.1038/s41598-018-22354-1
Dai J, Wang Y, Lin H, Sun Y, Pan Y, Qiao JQ, Lian HZ, Xu CX (2023). Residue screening and analysis of enrofloxacin and its metabolites in real aquatic products based on ultrahigh-performance liquid chromatography coupled with high resolution mass spectrometry. Food Chem., 404: 134757. https://doi.org/10.1016/j.foodchem.2022.134757
Dehau T, Cherlet M, Croubels S, Van Immerseel F, Goossens, E (2023). A high dose of dietary berberine improves gut wall morphology, despite an expansion of enterobacteriaceae and a reduction in beneficial microbiota in broiler chickens. Msystems, e01239-22. https://doi.org/10.1128/msystems.01239-22
Fairbrother JM, Nadeau É (2019). Colibacillosis. Diseases of Swine, pp. 807-834. https://doi.org/10.1002/9781119350927.ch52
Hao W, Che S, Li J, Luo J, Zhang W, Chen Y, Zhao Z, Wei H, Xie W (2022). Synthesis of berberine and canagliflozin chimera and investigation into new antibacterial activity and mechanisms. Molecules, 27: 2948. https://doi.org/10.3390/molecules27092948
Ibrahim MA, Abdeljawaad KA, Jaragh-Alhadad LA, Oraby HF, Atia MA, Alzahrani OR, Mekhemer GA, Moustafa MF, Shawky AM, Sidhom PA (2023). Potential drug candidates as P-glycoprotein inhibitors to reverse multidrug resistance in cancer: An in-silico drug discovery study. J. Biomol. Struct. Dyn., pp. 1-16. https://doi.org/10.1080/07391102.2023.2176360
Imenshahidi M, Hosseinzadeh H (2019). Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res., 33: 504-523. https://doi.org/10.1002/ptr.6252
Jabber EJ, Alzayd AM, Jawad MJ, Sulbi IM, Hassan SM, Jawad MJ, Jasim AM (2022). Synergistic effect of oxytetracycline as a combination treatment with Carboplatin on MCF-7 breast cancer cell line. Braz. J. Vet. Res. Anim. Sci., 59: e191527-e191527. https://doi.org/10.11606/issn.1678-4456.bjvras.2022.191527
Jain A, Sharma T, Kumar R, Katare O, Singh B (2022). Raloxifene-loaded SLNs with enhanced biopharmaceutical potential: QbD-steered development, in vitro evaluation, in vivo pharmacokinetics, and IVIVC. Drug Delivery Transl. Res., 12: 1136-1160. https://doi.org/10.1007/s13346-021-00990-x
Joosten P, Sarrazin S, Van Gompel L, Luiken RE, Mevius DJ, Wagenaar JA, Heederik DJ, Dewulf J (2019). Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J. Antimicrob. Chemother., 74: 798-806. https://doi.org/10.1093/jac/dky498
Li S, Wang B, Zhang M, Yuan D, Li J, Li X, Liang G (2022). Effects of berberine on the pharmacokinetics of florfenicol and levels of cytochrome P450 3A37, multidrug resistance 1, and chicken xenobiotic-sensing orphan nuclear receptor mRNA expression in broilers. Vet. Med. Sci., 8: 619-625. https://doi.org/10.1002/vms3.660
Luna MA, Bedrossian CW, Lichticer B, Salem PA (1972). Interstitial pneumonitis associated with bleomycin therapy. Am. J. Clin. Pathol., 58(5): 501-510. https://doi.org/10.1093/ajcp/58.5.501
Luo J, Yan, D, Yang M, Dong X, Xiao X (2013). Multicomponent therapeutics of berberine alkaloids. Evid. Based Complement. Altern. Med., https://doi.org/10.1155/2013/545898
Mahmood AR, Al-Haideri HH, Hassan FM (2019). Detection of antibiotics in drinking water treatment plants in Baghdad City, Iraq .Adv. Publ. Health, 10: 1. https://doi.org/10.1155/2019/7851354
Moghadam NR, Arefhosseini SR, Javadi A, Lotfipur F, Ansarin M, Tamizi E, Nemati, M (2018). Determination of enrofloxacin and ciprofloxacin residues in five different kinds of chicken tissues by dispersive liquid–liquid microextraction coupled with HPLC. Iran. J. Pharm. Res., 17: 1182.
Mohammed DA, Al-Mahmood SS (2022). Effects of probchick® on E. coli O157: H7 experimental infection in broilers. Egypt. J. Vet. Sci., 53: 367-379. https://doi.org/10.21608/ejvs.2022.129031.1332
Murakami T, Bodor E, Bodor N (2023). Approaching strategy to increase the oral bioavailability of berberine, a quaternary ammonium isoquinoline alkaloid: Part 1. Physicochemical and pharmacokinetic properties. Exp. Opin. Drug Metab. Toxicol., 19: 129-137. https://doi.org/10.1080/17425255.2023.2203857
Nunes CA, Alvarenga VO, de Souza Sant’Ana A, Santos JS, Granato D (2015). The use of statistical software in food science and technology: Advantages, limitations and misuses. Food Res. Int., 75: 270-280. https://doi.org/10.1016/j.foodres.2015.06.011
Othman SM, Sheet OH, Al-Sanjary R (2023). Phenotypic and genotypic characterizations of Escherichia coli Isolated from veal meats and butchers shops in Mosul city, Iraq. Iraqi J. Vet. Sci., 37: 225-260. https://doi.org/10.33899/ijvs.2022.133819.2306
Salman SAK, Taki MM, Hadi SJ, Jasim AM (2022). Green synthesis and characterization of zinc nanoparticles using herbal plant extracts with their influence on some bacterial infection. Res. J. Pharm. Technol., 15: 3147-3152. https://doi.org/10.52711/0974-360X.2022.00526
Sun T, Li XD, Hong J, Liu C, Zhang XL, Zheng JP, Xu Y, Ou Z, Zheng JL, Yu DJ (2019). Inhibitory effect of two traditional Chinese medicine monomers, berberine and matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli. Front. Microbiol., 10: 2584. https://doi.org/10.3389/fmicb.2019.02584
Tabatabaei SM, Badalzadeh R, Mohammadnezhad GR, Balaei R (2015). Effects of cinnamon extract on biochemical enzymes, TNF-α and NF-κB gene expression levels in liver of broiler chickens inoculated with Escherichia coli. Pesquisa Vet. Brasil., 35: 781-787. https://doi.org/10.1590/S0100-736X2015000900003
Xu N, Sun W, Zhang H, Liu Y, Dong J, Zhou S, Yang Y, Yang Q, Ai X (2023). Plasma and tissue kinetics of enrofloxacin and its metabolite, ciprofloxacin, in yellow catfish (Pelteobagrus fulvidraco) after a single oral administration at different temperatures. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 266: 109554. https://doi.org/10.1016/j.cbpc.2023.109554
Yousif SA, Jwher DM (2021). Detection of multiple presence of antibiotic residues in slaughtered sheep at Duhok abattoir, Iraq. Iraqi J. Vet. Sci., 35: 49-55. https://doi.org/10.33899/ijvs.2019.126259.1276
Yuan L, Li M, Qiao Y, Wang H, Cui L, Wang M (2021). The impact of berberine on intestinal morphology, microbes, and immune function of broilers in response to necrotic enteritis challenge. BioMed. Res. Int., 9: 44-50. https://doi.org/10.1155/2021/1877075
Zhang Y, Guo L, Huang J, Sun Y, He F, Zloh M, Wang L (2019). Inhibitory effect of berberine on broiler P-glycoprotein expression and function: In situ and in vitro studies. Int. J. Mol. Sci., 20: 1966. https://doi.org/10.3390/ijms20081966
Zhang L, Wang X, Wang L, Badawy S, Liu Z, Xie C, Wang X, Tao Y (2022). A new drug-drug interaction-tilmicosin reduces the metabolism of enrofloxacin through CYP3A4. Res. Vet. Sci., 148: 33-41. https://doi.org/10.1016/j.rvsc.2022.05.004
To share on other social networks, click on any share button. What are these?






