Biology, Histopathology and Treatment Evaluation Against Cryptosporidium meleagirids on Infected Quails
Research Article
Biology, Histopathology and Treatment Evaluation Against Cryptosporidium meleagirids on Infected Quails
Khitam J. Yahya*, Mohammed T. S. Al-Zubaidi
Department of Parasitology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq.
Abstract | Cryptosporidium is a ubiquitous protozoan parasite causing gastrointestinal disorders in various hosts worldwide. The current study was undertaken to study the biology of Cryptosporidium meleagridis and examine the anti-Cryptosporidial efficacy of curcumin in experimentally infected quails compared with that of paromomycin. This study carries out from September 2022 to January 2023 in Baghdad city, Iraq. Oocysts were isolated from naturally infected quails identified as Cryptosporidium meleagridis and used as a strain in experimental infection, about fifty birds were divided into five groups (G1: control negative group (con-), G2: control positive group (con+), G3: infected and treated with paromomycin, G4: infected and treated with curcumin and G5: uninfected and received curcumin). The oocyst shedding was recorded daily for 30 days. At 3,8,14, and 23 days post-infection (DPI), two quails from each group were killed humanely; ileum tissue samples were collected before and processed for histopathological evaluation. Improvement of tissue and the number of oocysts on the villi decreased after treatment. In comparison with G2, oocyst shedding was stopped at the end of the treatment period in both G3 and G4 without recurrence 10 days after drug withdrawal. The pre-patient periods (p.p) was 8-9 DPI. The maximum intensity was 11,000 oocysts per gram of feces. The first clinical sign was detected on 8 th DPI. Top of shedding occurred on 14 DPI, The patient period was 16 DPI. No clinical signs appeared on experimentally infected birds, (incubation period of 8 days). Histopathology examination of the infected ileum showed the development stages of C. meleagridis. There were significant differences between the curcumin (4.33 mg/kg/day) and paromomycin (100 mg/kg/day) treatments. Curcumin was able to treat the infection and needed father evaluation.
Keywords | Cryptosporidium, Biology study, Histopathology examination, Curcumin, Paromomycin, Herbal medicine, Quails
Received | February 25, 2023; Accepted | March 15, 2023; Published | March 25, 2023
*Correspondence | Khitam J. Yahya, Department of Parasitology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq; Email: kitam.jassim@gmail.com
Citation | Yahya KJ, Al-Zubaidi MTS (2023). Biology, histopathology and treatment evaluation against Cryptosporidium meleagirids on infected quails. Adv. Anim. Vet. Sci. 11(5):701-710.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.5.701.710
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Cryptosporidium a genus of Apicomplexan parasitic protozoa is well known as a global protozoan parasite in both humans and a wide range of animals. In domesticated animals, Cryptosporidiosis may lead to severe economic loss due to mortality, weight loss, and medication cost. Quails that are infected with coccidian causes more pathology changes leading to sever bloody interties and economic loss (Al-Zarkoushi and Al-Zubaidi, 2021a). Oocysts that have sporulated and shed in the feces are instantly contagious. They are typically spread by the fecal-oral route, either directly or on food or water that has been contaminated even filtered water (Hadi, 2014). Cryptosporidium oocysts are destroyed by desiccation but can survive for over a year if kept in a cool, wet environment and for at least six months if kept at 4-6 °C (Gerace et al., 2019). Some birds may eventually pass away from the infection if it becomes persistent and all three Cryptosporidium spp. were isolated from birds in Iraq (Faraj, 2014). There is currently no drug that can cure Cryptosporidiosis, so efforts are still needed to develop an effective drug and a wide variety of medications and components have been tested as a potential treatment for the illness (Shahiduzzaman and Daugschies, 2012).
One of the tiniest poultry species, the quail (Coturnix Coturnix), offers more benefits than chickens, including high production rates, lower feed consumption, and resistance to a variety of poultry diseases. They are also distinguished by their initial low expenses, which do not require a large area for farming, profitability, and amusement, therefore it represented a contemporary trend in the poultry sector (Hassan et al., 2020).
Curcumin (Curcuma longa) is a plant extract and is a very safe substance; there is no toxicity even at high doses, and it has anti-proliferative, anti-microbial, anti-inflammatory, antioxidant properties, and anti-protozoal actions against (Plasmodium, Leishmania, Trypanosoma, Schistosoma, Babesia, Giardia, and Coccidia). Our research is an experiment to discover a parasite cure. Numerous drugs and ingredients have been investigated as potential treatments for the condition, but there is presently no pharmaceutical that can cure Cryptosporidiosis, thus further work is required to create a potent therapy.
Materials and Methods
Study area
About 100 birds obtain from Baghdad city, Iraq were examined to look for Cryptosporidium oocysts, positive samples were used for the preparation of infective doses.
Collection and purification of oocysts
The oocysts samples were obtained from infected quails from Baghdad city, Iraq. The direct examination was used to identify the infection, and the modified Kenyon’s acid-fast approach was used to confirm it (Ma and Soave, 1983). The fecal suspension was sieved to remove debris and then washed three times with tap water to isolate the oocysts (2000 rpm, 10 min). The oocysts were then separated and purified using the discontinuous sucrose technique (Arrwood and Sterling, 1987). In a nutshell, Sheathers solution was made by mixing oocysts in 2.5 % aqueous potassium dichromate (K2Cr2O7) at 4 ºC until the experiment (Rekha et al., 2016).
Animal groups and experimental infection
Quails of 2-3 days old were purchased from a nearby market in Baghdad city, their weight and feces were checked daily for five days. Ten quails were housed in a single cage, which had a temperature setting of 25 to 28ºC and provided with food.
Experimental design
Fifty birds without take care about sex were divided into five experimental groups, each consist of ten quails, with an average age of (7-8 days) and a weight of (14-17g).
G1: control negative group (con-) received distilled water, G2: control positive infected with 103 oocysts, G3: infected with 103 and treated by paromomycin, G4: infected with 103 oocysts and treated with curcumin, and G5: uninfected received curcumin only.
Preparation of treatment dose
Curcumin: The recommended therapeutic dose of curcumin (C. longa C1386, Sigma- Aldrish, USA) is 4.33 mg/kg/day was suspended into 50µl of corn oil, as described previously (Cervantes-Valencia et al., 2018).
Paromomycin: The recommended therapeutic dose of Paromomycin (paromomycin sulfate, Sigms-Aldrich, USA) is 100 mg/kg/day was suspended into 100µl of distal water, as described previously (Cervantes-Valencia et al., 2018).
Numerous parameters were evaluated in groups of quails, including; the presence of clinical symptoms of clearance in infected quail; recording the pre-patent period and patint period, droop examination by hemocytometer chamber, determining the top of shedding, measured incubation period, and measuring the impact of drugs.
The drugs impact use in this study was measured according to (Xiao et al., 1999).
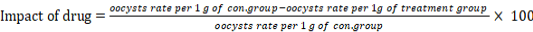
Oocyst shedding
Up to the final of the experiment, 0.5 g of fecal samples were taken daily directly from each quail in the several experiment groups to track oocysts shedding throughout the trial Fecal samples were examined directly under a microscope looking for oocysts and confirmed by a modified Zhen Neelsen stain. Oocysts number was counted every day by using a hemocytometer (×1000) (Ortolani, 2000).
Histopathological analysis
At 3, 8, 14, and 2٣ of treatment days, two quails from each group were slaughtered. This tissue sample was transported to the pathology and poultry diseases laboratory of veterinary medicine at Baghdad University and fixed individually in 10% neutral buffered formalin before being embedded in paraffin after the ileum was removed. Serial 5µm thick sections of ileum tissue blocks were cut out, and they were then stained with hematoxylin and eosin (H & E). The tissue sections were then put on a different slide and inspected under a compound microscope with a 100x (Cardiff et al., 2014). Histopathological lesions were assessed as normal without alterations and without oocysts, mild with few alterations and few oocysts, and severe with severe damage and a high number of oocysts on villi.
Statistical analysis
The statistical analysis system, SAS Program (2018) was used to detect the effect of different factors on study parameters. The Chi-Square test was used to significantly compare percentages (0.05 and 0.01 probability) in this study. In order to study the variance in oocysts shedding among the different groups Kruskal-Wallis was used and the data obtained were shown as mean ± standard error of mean (SEM).
Results and Discussion
Oocysts shedding
All infected and uninfected quails were normal throughout the experiment. There were no dead birds. The disease incidence of quails at 7 days of age was inoculated with 1x103 oocysts/ quail was 100% without death. Macroscopic observation revealed no notable modifications. No animals perished throughout the experiment, as well.
Clinical symptoms, including foamy feces, dullness, and reduced weight gain, while post mortem showed a small intestinal distention with gas and mucus, start to manifest on the eighth day after infection (incubation period). At 8 to 9 days after infection, oocysts were initially noticed in quails droppings (pre-patent period) and maximum oocysts shedding occurred at 14 DPI, experimentally infected birds typically remained asymptomatic or had moderate symptoms, Before the experiment began, the weights of all quail ranged from (17-19) g at 7 days of age. During the infection, exactly after the fifth day of infection, weight gain in the infected group G2 was significantly reduced from (17-19) g to (14-16) g, whereas the weight of both treatment groups was increased to (25-30) g and (70- 75) g in G3, G4, respectively. It differs considerably from the control groups G1 and G5, which weigh respectively (95 – 100) and (98- 120) g.
Anticryptosidial effect of paromomycin and curcumin
When treatment began on the 8 (DPT), the number of oocysts in one gram of drops was (2000, 2000, and 2250) oocysts per gram (OPG) in (G2, G3, and G4), respectively. During the ten days of treatment, the number of oocysts decreased while the number of oocysts in the G2 increased steadily and reach the infection peak between 12 and 14 DPI, which recorded (9375-11000) OPG, at 12-14 DPI, G3 was (875-625) OPG while G4 reaches to (625-250) OPG.
The number of oocysts decreased steadily in G3, G4, with a significant difference between the two groups on all experimental days; G3 reach (375-350) OPG on 16-17 DPI, respectively. The treatment of curcumin was able to cease oocyst shedding without a recurrence 10 days after medication withdrawal, whereas G4 achieved (125-0) OPG during the same periods and vanished after stopping therapy. G2 continuously shedding of oocysts for 16 days reach to 250 OPG at 23 DPI then stopped, while G3 reach to 125 OPG at 20 DPI, and G4 reach to 125 OPG at 16 DPI then stopped Table 1.
Histopathological examination
G2 underwent histopathological analysis at 3 DPI, which revealed moderate intestinal villi shortening, sporadic Cryptosporidium oocysts presence at the apical surface with intact villus tissue without inflammatory cell infiltration (Figure ١).
Beginning at 8 DPI, when oocysts first begin to shed
Histopathology assessment of G2 at 8 DPI showing massive atrophy, localized ulceration, epithelial sloughing, numerous oocysts adhered to the villi, and stunting of intestinal villi (Figure 2) with goblet cell hyperplasia were all visible in the intestinal ileum mucosa (Figure 3).
Table 1: the average number of Cryptosporidium meleagridis oocysts in each group during the 30 day experience.
|
dpi |
dpt |
G2 (Con+) Mean (Mean Rank) |
G3 (paromomycin) Mean (Mean Rank) |
G4 (curcumin) Mean (Mean Rank) |
Chi- square (x2) |
P-value |
|
0-7 |
- |
- |
- |
- |
- |
|
|
8 |
1 |
2000 (39.65+41) |
2000 (125.50+ 29) |
2250 (85.50+ 41) |
147.02 ** |
0.0063 |
|
9 |
2 |
3700 (64.75+33) |
1750 (115.50+ 16) |
1125 (73.35+ 40) |
172.38 ** |
0.0001 |
|
10 |
3 |
5500 (84.85+39) |
1125 (104.70+ 20) |
1000 (67.65+ 25) |
185.62 ** |
0.0001 |
|
11 |
4 |
6500 (103.10+ 33) |
1000 (95.70+ 21) |
750 (54.75+ 22) |
207.75 ** |
0.0001 |
|
12 |
5 |
9375 (133.50)+ 27 |
875(85.95 + 13) |
625 (46.25+ 8) |
186.56 ** |
0.0001 |
|
13 |
6 |
10250 (144.60+ 42) |
750 (75.35+ 12) |
375 (35.20+ 15) |
264.09 ** |
0.0001 |
|
14 |
7 |
11000 (155.40+ 46) |
625 (65.00+ 17) |
250 (21.65+ 12) |
178.41 ** |
0.0001 |
|
15 |
8 |
9000 (128.20+ 27) |
500 (55.40+ 21) |
250 (17.30+ 7) |
248.09 ** |
0.0001 |
|
16 |
9 |
7850 (115.80+ 45) |
375 (41.90+ 15) |
125 (7.85+ 29) |
187.55 ** |
0.0001 |
|
17 |
10 |
6375 (97.90+ 37) |
350 (38.80+ 17) |
- |
179.02 ** |
0.0001 |
|
18 |
5250 (76.15+ 34) |
250 (25.80+ 15) |
- |
186.71 ** |
0.0001 |
|
|
19 |
3500 (51.26+ 34) |
125 (10.95+ 23) |
- |
155.37 ** |
0.0001 |
|
|
20 |
2100 (43.35+ 36) |
125 (10.95+ 23) |
- |
147.07 ** |
0.0001 |
|
|
21 |
1125 (26.50+ 30) |
- |
- |
-- |
-- |
|
|
22 |
750 (16.50+ 24) |
- |
- |
-- |
-- |
|
|
23 |
250 (6.50+ 32) |
- |
- |
-- |
-- |
|
|
24-30 |
- |
- |
- |
-- |
-- |
|
|
Chi-square (x2) |
273.08**(157.40) |
192.66**(126.76) |
117.04**(85.90) |
-- |
-- |
|
|
P-value |
0.0001 |
0.0001 |
0.0001 |
--- |
--- |
|
|
**P≤ 0.01 |
||||||
At the peak of shedding (14 DPI)
Histopathological evaluation of ileum on 14 DPI of (G2) revealed the presence of a development stage at the brush boundary of intestinal villi enterocytes, which were visible as small, round or oval basophilic formations (Figure 4),
G3 (14 DPI) revealed infiltration of several cell types (neutrophils and mononuclear cells) in the lamina propria together with goblet cell hyperplasia and multifocal necrotic findings, Crypt tissue manifestation revealed varying degrees of size reduction, considerable depletion of lymphoid- related tissue, apparent abnormalities of villus tissue linked with villous blunting and diffuse mononuclear cell infiltration, and few to moderate oocysts are identified in the villi surface (Figure 5). G4 (14 dpi) indicated mild to minimal mononuclear infiltration in the mucosal and submucosal tissue, and the parasite was missing from the villi, the intestinal manifestation included indications of some villus and shortening, as well as an increase of the gut–associated lymphoid tissue, along with a partial improvement of the lesion (Figure 6).
At the end of oocysts shedding (23DPI) G2 the intestinal tissue shows extensive mononuclear cell infiltration in the necrotic villi, along signs of various villus abnormalities, with a few Cryptosporidium are present as a rounded basophilic structure on the surface of the remaining villi (Figure 7). At the same period on G3, intestinal tissue displayed mild to moderate goblet cell hyperplasia with no discernible changes in the mucosal and submucosal tissue surrounding it (Figure 8). In contrast, a section from a G4 bird showed mild mucosal mononuclear cell infiltration with normal crypt tissue (Figure 9). During all days of experiment, G1and G5 showed no clear pathological alteration (Figures 10, 11).
Drug effectivness
The result showed no significant differences between curcumin and paromomycin, the effectiveness of curcumin was recorded 91.29% which higher than paraomomycin that recorded 87.29% Table 2.
Table 2: The drug effects of paromomycin and curcumin after ten days of treatment.
|
Drug type |
Drug effectiveness |
|
Curcumin |
91.29% |
|
Paromomycin |
87.29% |
|
Chi-square (x2) |
0.091NS |
|
P-value |
0.764 |
NS: Non- Significant.
During experimental infection of quails by 1x103 oocysts per quail without mortality, This outcome is consistent with Al-Mahmood (2011) finding that 1 x 103 oocysts are able to cause infection with notable oocysts shedding and histopathological alteration, with a 100% infection rate. That gives an indication about the ability of quails to infection in the early days of life that’s due to an incomplete immune system and also due to the ability to infect with first time by high doses. And also agree with (Al-Khayat and Al- Zubaidi, 2015; Kadhim and Al-Zubaidi, 2018; Marwa et al., 2018; El-Shafei et al., 2018).
Because of the preferential side of Cryptosporidium meleagridis inside the intestine, experimentally infected birds typically remained asymptomatic or had moderate symptoms, this result agreement with (Al-Mahmood, 2011; Guechtouli et al., 2022). At 8 to 9 DPI, oocysts were initially noticed in quails droppings (P.P) this is due to the ability of quails for infection and the viability of oocysts to cause infection, and maximum oocysts shedding occurred at 14 DPI, this is in agreement with (Al-Zubadia et al., 2018; Cui et al., 2018; Kopacz et al., 2019; Holubov et al., 2020).
Oocysts shedding varies depending on the species of Cryptosporidium and the condition of the host; C. meleagridis is known for having low shedding, which is consistent with (Lin et al., 2022). On (G2, G3, and G4), the oocysts shed continuously for 16, 13, and 9 days, respectively.
Depending on the species of Cryptosporidium, the condition of test animals, and pathogenicity, different (p.p) exist for different Cryptosporidium species. The p.p in pigs is 10-12 days, in healthy humans 3-4 days, in old chickens 12-16 days, in young chickens 8-9 days, and in C. avium 8-9 days in hens and ducks. Oocysts excretion in feces begins roughly when symptoms begin and may last for a month. Species-specific differences exist in Cryptosporidium spp. preference sites. The development of C. meleagridis, C. gill, and C. bailyia is restricted to the villous epithelium of the terminal third of the small intestine, the proventriculus, and (trachea, bursa, and conjunctiva) respectively (Joker et al., 2021; Altamimi and Al-Zubaidi, 2021).
Before the experiment began, the weights of all quail ranged from (17-19g) at 7 days of age. During the infection, exactly after the fifth day of infection, weight gain in the infected group G2 was significantly reduced from (17-19g) to (14-16g), whereas the weight of both treatment groups was increased to (25-30g) and (70-75g) in G3, G4, respectively. It differs considerably from the control groups G1 and G5, which weigh, respectively (95–100) and (98-120g). that’s due to reduced observation surface which leads to histological alteration that disagrees with Guechtouli et al. (2022) how found that the clinical signs in birds infected with C. baliayi were non-specific signs, normal rales were 28%, cachexia was 22% and diarrhea was 20%.
Anticryptosidial effect of paromomycin and curcumin
When treatment began on the eighth day of infection, the number of oocysts in one gram of drops was (2000, 2000, and 2250) oocysts per gram (OPG) in (G2, G3, and G4), respectively.
The number of oocysts decreased steadily in G3, G4, with a significant difference between the two groups on all experimental days; G3 reach (375-350) OPG on 16-17 DPI, respectively. The treatment of curcumin was able to cease oocyst shedding without a recurrence 10 days after medication withdrawal, whereas G4 achieved (125-0) OPG during the same periods and vanished after stopping therapy. Paromomycin did not completely eradicate the cryptosporidial infection in quails, and there was a strong link between treatment days and a decrease in oocyst shedding in both treatment groups, this result was in agreement with Asadpour et al. (2018) who noted that curcumin anticryptosidial effect was more rapid than paromomycin and required further investigation, different pharmacological qualities including antioxidant, antiprotozoal, and anti-inflammatory activity in conjunction with curcumin, which has aid in the treatment of Cryptosporidiosis and its clinical lesion. In the experiment, oocyst shedding, histopathological alteration, and medication efficacy were used to assess the anti-cryptosporidial potential of curcumin in quails. This finding is in agreement with the earlier studies that demonstrated that paromomycin cannot completely eradicate cryptosporidial infection (Van Zeeland et al., 2008; Obaid et al., 2012). Curcumin’s medicinal properties include its polyphenolic curcuminoid compounds (diferuloylmethane), which have the advantage of remaining inside the gut for an extended period of time. These compounds also have an impact on oocyst viability and sporozoite morphology by affecting gene transcription, and they inhibit intracellular adhesion molecules, which are involved in the sequestration and establishment of aporozoites and agreement with Kadir et al. (2008) how found that the medical plant extracts have an effect on cryptosporidiosis in experimental infected mice.
Drug effectiveness
The result showed no significant differences between curcumin and paromomycin, there are numerous variables that affect a drug’s effectiveness, including an individual’s age, sex, body weight, and general health. The medicine had a considerable impact on clinical symptoms (changed feces to the usual form, decreased the number of days that oocysts shed, improved food intake, raised body weight, and the tissue improvement was evident in the histology analysis).
Histopathological examination
Histological examination changes in this study were agreed with (Al-Mahmood, 2011; Marhoon and Jasim, 2017; Al-Zubaidi et al., 2018; Kadhim and Al-Zubaidi, 2018; Asadpour et al., 2018). The Same result was obtained from Al-Zarkoushi and Al-Zubaidi (2021b) on quails intestinal tissue that was infected with coccidian. Al-Warid et al. (2014) conclouded that lymphocyte and phagocytosis are good indicter to evaluate the cellular immune response in experimentally mice with Cryptosporidium spp. This result disagreed with Cui et al. (2018) who found numbers of C. avium oocysts cover the epithelial cell of the small intestine without any pathological change in the epithelial cell.
When treated to infected quail, paromomycin and curcumin both displayed anticryptosidal activity; however, curcumin was more successful in curing lesions and lowering the number of oocysts on the villi, but paromomycin was unable to stop infection, oocysts shedding, or improve affected tissue (Aydogdu et al., 2018) Contrary to paromomycin, among other things, inhibits protein synthesis by binding to 16 sr RNA, curcumins’ therapeutic effect are also related to changes in the gut microbiota and genes associated with the innate immune system, plant extract have an effective result in the treatment of infection (Hazaa et al., 2016). Paromomycin only affects the parasite and has no effect on the colonization or improvement of tissue (Fuloria et al., 2022).
Conclusions and Recommendations
Our research revealed that curcumin was a reliable option against cryptosporidial infection, as it significantly reduced the infection load in experimentally infected quails and completely eliminated the infection without recurrence after medication removal.
- Further studies are needed to clarify the mechanism by which curcumin induces apoptosis in Cryptosporidium-infected cells.
- Further studies are necessary to examine the therapeutic potential of curcumin in other animal models and also in immunosuppressed individuals.
- More studies on Cryptosporidium spp. subtypes and determination of zoonotic types between humans and chickens and determinants of the epidemiology factor with the mode of transmission.
Acknowledgments
I would like to thank prof. Dr. Mohammed Thabit for his flexibility, professional ideas, and advice throughout my study and to the staff of the Parasitology Department/ College of Vet. Med. Baghdad University.
Novelty Statement
Cryptosporidiosis is a fatal disease in quail and their is no effected drug on it , therefore looking for safety substance, anti-proliferative, anti-microbial, antiinflammatory, antioxidant properties, and anti-protozoal. Numerous drugs and ingredients have been investigated as potential treatments for the condition, but there is presently no pharmaceutical that can cure Cryptosporidiosis, thus further work is required to create a potent therapy. their is no data available about quails cryptosporidiosis in Iraq.
Author’s Contribution
Conceived and planned the experiments contributed to sample preparation. Contributed to the interpretation of the results.
Ethical statement
All animals handled in the current experimental study were used according to the ethics of the local committee of animal care and use at the College of Veterinary Medicine within the University of Baghdad (Number 2569/PG on 12/12/2022).
Conflicts of interest
The authors have declared no conflict of interest.
References
Al-Khayat KKK, Al-Zubaidi MTS (2015). Some epidemiological studies of Cryptosporidium spp. in broiler chickens in some areas of Karbala Province. Iraqi J. Vet. Med., 39(1): 8-5.
Al-Mahmood SS (2011). Experimental histopathological study of chicks infected with Cryptosporidium baileyi isolated from wild pigeons in Mosul. Iraqi J. Vet. Sci., 25(1): 43-49. https://doi.org/10.33899/ijvs.2011.5704
Altamimi MK, Al-Zubaidi MTS (2021). High prevalence of Cryptosporidium meleagridis in domestic pigeons (Columba livia domestica) raises a prospect of zoonotic transmission in Babylon Province, Iraq. Iraqi J. Vet. Med., 44(E0): 7–13. https://doi.org/10.30539/ijvm.v44i(E0).1012
Al-Warid HS, Mahmood SH, Alsaqur IM (2014). Evaluation of cellular immune response provoked by experimental infection with Cryptosporidium spp. in mice. J. Biotechnol. Res. Center, 8(1): 8-13. https://doi.org/10.24126/jobrc.2014.8.1.289
Al-Zarkoushi MM, Al-Zubaidi MTS (2021a). Epidemiological, morphological, and histopathological study of quail coccidiosis in Thi-Qar Province, Iraq. Iraqi J. Vet. Med., 45(1): 69-74. https://doi.org/10.30539/ijvm.v45i1.1045
Al-Zarkoushi MM, Al-Zubaidi MTS (2021b). The prevalence and pathological change of caecal coccidiosis in Japanese quails (Coturnix coturnix japonica) in Thi-Qar Province, Iraq. Ann. Roman. Soc. Cell Biol., 25(6): 782-791.
Al-Zubaidi MTS, Kadhim LI, Ibrahim ZI, Al-Rikabi AS (2018). Incidence and experimental infection of Cryptosporidium baileyi in chicken. Iraqi J. Agric. Sci., 49(2): 269-278. https://doi.org/10.36103/ijas.v49i2.162
Arrowood MJ, Sterling CR (1987). Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol., 73(2): 314-319. https://doi.org/10.2307/3282084
Asadpour M, Namazi F, Razavi SM, Nazifi S (2018). Curcumin: A promising treatment for Cryptosporidium parvum infection in immunosuppressed BALB/c mice. Exp. Parasitol., 195: 59-65. https://doi.org/10.1016/j.exppara.2018.10.008
Aydogdu U, Isik N, Ekici OD, Yildiz R, Sen, I., and Coskun, A. (2018). Comparison of the effectiveness of halofuginone lactate and paromomycin in the treatment of calves naturally infected with Cryptosporidium parvum. Acta Scientiae Veterinariae, 46: 9-9. https://doi.org/10.22456/1679-9216.81809
Cardiff RD, Miller CH, Munn RJ (2014). Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor Protocols, 2014(6): pdb-prot073411. https://doi.org/10.1101/pdb.prot073411
Cervantes-Valencia ME, Alcalá-Canto Y, Sumano-Lopez H, Ducoing-Watty AM, Gutierrez-Olvera L (2018). Effects of Curcuma longa dietary inclusion against Eimeria spp. in naturally-infected lambs. Small Rumin. Res., 136: 27–35. https://doi.org/10.1016/j.smallrumres.2015.12.035
Cui Z, Song D, Qi M, Zhang S, Wang R, Jian F, Zhang L (2018). Revisiting the infectivity and pathogenicity of Cryptosporidium avium provides new information on parasitic sites within the host. Parasit. Vectors, 11(1): 1-7. https://doi.org/10.1186/s13071-018-3088-x
El-Shafei OK, Saad AGE, Harba NM, Sharaf OF, Samaka RM, Farag SA (2018). Therapeutic effect of phenyl vinyl sulfone and nitazoxanide on experimentally infected mice with Cryptosporidiosis. Menoufia Med. J., 31(3): 786.
Faraj AA (2014). Distribution of Cryptosporidium spp. infection in wild pigeons in Baghdad city -Iraq. Basrah J. Vet. Res., 13(2): 48-53. https://doi.org/10.33762/bvetr.2014.98793
Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Subramaniyan V, Chidambaram K, Thangavelu L, Nordin R, Wu YS, Sathasivam KV, Lum PT, Meenakshi DU, Kumarasamy V, Azad AK, Fuloria NK. (2022). A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front. Pharmacol., 13. https://doi.org/10.3389/fphar.2022.820806
Gerace E, Presti VDML, Biondo C (2019). Cryptosporidium infection: Epidemiology, pathogenesis, and differential diagnosis. Eur. J. Microbiol. Immunol., 9(4): 119-123. https://doi.org/10.1556/1886.2019.00019
Guechtouli S, Mimoune N, Messai CR, Salhi O, Kaidi R, Khelef D (2022). Cryptosporidium spp. infection in the broiler chickens and turkeys on farms in north central Algeria. Vet. Stanica, 53(4): 403-418. https://doi.org/10.46419/vs.53.4.5
Hadi AM (2014). Isolation and identification of Cryptosporidium spp. by reverse osmosis system of tap water in Baghdad. Baghdad Sci. J., 11(2): 894-899. https://doi.org/10.21123/bsj.11.2.894-899
Hassan AK, Naeem EV, Soliman MA (2020). Investigation the prevalence of common parasitic infections in farmed quails in upper Egypt. SVU Int. J. Vet. Sci., 3(2): 38-50. https://doi.org/10.21608/svu.2020.31915.1058
Hazaa IKK, Al-Taai NA, Khalil NK, Zakri AMM (2016). Efficacy of garlic and onion oils on murin experimental Cryptosporidium parvum infection. Al-Anbar J. Vet. Sci., 9: 69-74.
Holubová N, Tůmová L, Sak B, Hejzlarová A, Konečný, R., McEvoy, J, and Kváč, M (2020). Description of Cryptosporidium ornithophilus n. sp. (Apicomplexa: Cryptosporidiidae) in farmed ostriches. Parasit. Vectors, 13(1): 1-17. https://doi.org/10.1186/s13071-020-04191-2
Jokar M, Rabiee M, Bokaie S, Rahmanian V, Dehesh P, Hasannejad H, Kiavash H, Hadi K (2021). Prevalence of Cryptosporidiosis in animals in Iran: A systematic review and meta analysis. Asian Pac. J. Trop. Med., 14(3): 99-112. https://doi.org/10.4103/1995-7645.307532
Kadhim AK, Al-Zubaidi MTS (2018). Isolation of Cryptosporidium oocysts from slaughtered broiler chicken and experimental infection in chicks. Basrah J. Vet. Res., 17(3).
Kadir MAA, Al-Alousi TI, Diab A (2008). Comparison between the efficacies of different medical herbs on Cryptosporidium spp. J. Fac. Med. Baghdad, 50(1): 68-76.
Kopacz Ż, Kváč M, Karpiński P, Hendrich AB, Sąsiadek MM, Przemysław L, Bohumil S, John M and Marta K (2019). The first evidence of Cryptosporidium meleagridis infection in a colon adenocarcinoma from an immunocompetent patient. Front. Cell. Infect. Microbiol., 9: 35. https://doi.org/10.3389/fcimb.2019.00035
Lin X, Xin L, Qi M, Hou M, Liao S, Qi N, Sun M (2022). Dominance of the zoonotic pathogen Cryptosporidium meleagridis in broiler chickens in Guangdong, China, reveals evidence of cross-transmission. Parasit. Vectors, 15(1): 1-8. https://doi.org/10.1186/s13071-022-05267-x
Ma P, Soave R (1983). Three-step stool examination for Cryptosporidiosis in 10 homosexual men with protracted watery diarrhea. J. Infect. Dis., 147: 824–828. https://doi.org/10.1093/infdis/147.5.824
Marhoon IA, Jasim GA (2017). Investigation for cryptosporidiosis in some species of wild and domestic birds and study of histopathological changes which combined of it. Al-Qadisiyah J. Pure Sci., 22(4): 67-78.
Marwa MD, Amany F, El-Refai MD, Samar A (2018). Effects of Echinacea purpurea on cryptosporidiosis in immunosuppressed experimentally infected mice. Med. J. Cairo Univ., 86(September): 3209-3222. https://doi.org/10.21608/mjcu.2018.60289
Obiad HM, Al-Alousi TI, Al-Jboori AH (2012). The in vivo effect of some medicinal plant extracts on Cryptosporidium parasite. J. Univ. Anbar Pure Sci., 6: 3. https://doi.org/10.37652/juaps.2012.78251
Ortolani E (2000). Standardization of the modified Ziehl-Neelsen technique to stain oocysts of Cryptosporidium spp. Rev. Bras. Parasitol., 9: 29–31.
Rekha KMH, Puttalakshmamma GC, D’Souza PE (2016). Comparison of different diagnostic techniques for the detection of cryptosporidiosis in bovines. Vet. World, 9(2): 211. https://doi.org/10.14202/vetworld.2016.211-215
SAS, 2018. Statistical analysis system, User & # 39s guide. Statistical. Version 9.4th ed. SAS. Institute. Inc. Cary. N.C. USA.
Shahiduzzaman M, Daugschies A (2012). Therapy and prevention of cryptosporidiosis in animals. Vet. Parasitol., 188: 203–214. https://doi.org/10.1016/j.vetpar.2012.03.052
Van Zeeland Y, Schoemaker N, Kik M, Van Der Giessen J (2008). Upper respiratory tract infection caused by Cryptosporidium baileyi in three mixed-bred falcons (Falco rusticolus× Falco cherrug). Avian Dis., 52: 357–363. https://doi.org/10.1637/8121-100207-Case.1
Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, W Shulaw, RC Thompson, R Fayer, AA Lal. (1999). Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol., 65(8): 3386-3391. https://doi.org/10.1128/AEM.65.8.3386-3391.1999
To share on other social networks, click on any share button. What are these?






