Use of Origanum Majorana Extract to Improve some Ram’s Semen Chemical Parameters when Preserved at Cooling and Cryopreservation
Research Article
Use of Origanum Majorana Extract to Improve some Ram’s Semen Chemical Parameters when Preserved at Cooling and Cryopreservation
Mohammed Abdulameer Rashid Al-Sarray1, Hasanain Jihad Neamah2*, Sarah Gatea Fayyadh AL-Omairi1
1Animal Production Department, College of Agriculture, University of Wasit, Iraq; 2Middle Technical University, Al-Kut Technical Institute, Iraq.
Abstract | In this study, we designed this experiment to investigate the effect of adding Origanum Majorana extract (O.M.) in the ram semen at cooling, the experimental groups were divided into four groups (P1=1 mg O.M. raw powder and extender, P2= 2 mg O.M. raw powder and extender, O1 group= 1 mg O.M. oil and extender, O2 group= 2 mg O.M. oil and extender and the control only extender. The sperm motility, pH level, malonaldehyde level (MDA), and total antioxidant capacity (TAC) were investigated post-preservation (5ᵒ C) at 0, 72 hours of cooling, and after thirty days post-cryopreservation in liquid nitrogen. The P2 group showed a high effect (P≤0.01) on the motility of sperm at different times of cooling and post-cryopreservation. The pH level increased significantly in the group O2 compared with other groups. We investigated the MDA level by thiobarbituric acid reaction (TBAR) methods, however, 2, 2 – Diphenyl - 1- picrylhydrazyl (DPPH) was used to estimate the TAC of seminal plasma depending on the absorbance. The P1, P2, O1, and O2 groups recorded a highly significant (P≤0.01) decrease in seminal MDA level. The P1, P2, O1, and O2 groups recorded a highly significant (P≤0.01) decrease in seminal MDA level. On the other hand, the results suggested a higher TAC increase for all groups than the control at different times of cooling and post-cryopreservation. In conclusion, adding 2 mg of O.M. powder extract increases sperm motility at the storage, and the pH level increased by adding 2 mg O.M. oil to the diluted semen, O.M. powder or oil which is added to diluted semen leads to decreased MDA and increased TAC levels.
Keywords | Chemical parameters, Cryopreservation, Origanum majorana, Ram semen, Sperm motility, Cooling storage
Received | April 13, 2024; Accepted | July 10, 2024; Published | August 09, 2024
*Correspondence | Hasanain Jihad Neamah, Middle Technical University–Al-Kut Technical Institute/ Iraq; Email: Hasanain.alkenani@mtu.edu.iq
Citation | Al-Sarray MAR, Neamah HJ, AL-Omairi SGF (2024). Use of origanum majorana extract to improve some ram’s semen chemical parameters when preserved at cooling and cryopreservation. Adv. Anim. Vet. Sci. 12(9): 1768-1774.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.9.1768.1774
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
One of the limitations that affects the characteristics of semen during storage is oxidative stress. The oxidative stress in semen is a big problem, especially for ram semen because the sperm cells are very sensitive to it, many types of research have not resolved this problem completely, which may be related to antioxidant concentrations in the seminal plasma or laboratory manipulation. Although semen has a natural defence line represented by endogenous antioxidants, its effect decreases gradually during storage (Al-Subaihawi et al., 2017). The spermatozoa in all animal species are very sensitive to oxidation (Maxwell and Watson, 1996), and this requires finding an additional source of antioxidants. Wild plants have a high content of natural antioxidants which have an important role in sperm cell viability (AL- Sarray et al., 2019). Many studies are suggesting the benefits of adding certain concentrations of antioxidants to semen extenders (AL-Sarray et al., 2020; Kameni et al., 2021). The strength of plants in resisting oxidative stress is represented by their containing certain concentrations of phenolic compounds, the phenolic compounds can inhibit the effect of free radicals and thus maintain the effectiveness of the sperm cell for a longer storage period (Allverdú-Queralt et al., 2014; Kaliora et al., 2014; Gutiérrez-Grijalva et al., 2017). One such plant that has a detrimental effect on lipid peroxidation is Origanum Majorana (O.M.) (Kaliora et al., 2014). The O.M. from the Lamiaceae family grows in Asia, Africa, and America (Desouky et al., 2015). (Kaliora et al., 2014) reported that Origanum Majorana has more than twenty-seven polyphenols, terpenic acids and flavonoids. This research was distinguished by using Origanum Majorana oil and its powder as additives in the extender, which dilutes the ram’s semen. This study aimed to determine the effect of adding uneven concentrations of Origanum Majorana extract to diluted ram semen as antioxidants and the effect of this on the properties of sperm during storage.
MATERIALS AND METHODS
Animals and Semen Samples Collection
The study was done in the Ruminants Research Station (Researches of Agricultural /Ministry of Agriculture-Iraq). Four healthy local Awassi rams aged 2 - 2.5 years old were selected for this study. This study started on the 12th of Sep. to 28th of Feb. 2021. The semen was taken from each ram by using a R.A.V. (ram artificial vagina), the semen collection procedure was done in a clean place, early in the day, and the semen collected from each ram, and placed in the sterilized graduated glass test tubes and then closed them to prevent contamination, the samples were placed in a container (37 degrees Celsius) until transported to the laboratory, then the tubes placed in a water bath (37 ̊ C). The poor-quality ejaculate was removed and other ejaculates were pooled to remove the individual variations between experimental (we pooled ejaculates that have more than 80 % mass motility) and then kept in a water bath at 37 degrees Celsius, after that, the semen was divided into equally five parts within groups of the experiment. The ejaculate volume was recorded. Sperms count was estimated using the Neubauer Haemocytometer Chamber (Smith and Mayer, 1955). The mass activity was evaluated by putting fresh semen (twenty microliters) on warmed glass slides (35 ºC) and then tested depending on the force of magnification (10x). The mass activity was estimated depending on the wave’s speed and density (Salisbury et al., 1978).
The Preparation of O.M. Powder
The dried O.M. leaves were purchased from a local market in Baghdad. The plant leaves were cleaned, dried, and crushed to a fine powder to a particle diameter (mesh two hundred fifty μm) and placed in the dark bottles and kept (-18 ºC) until used (AL-Dhaheri, 2012). 100 grams of O.M. powder was placed with double distilled water in the volumetric flask (100 ml) and then left overnight in a magnetic stirrer at 40 ̊C, after that the solution was filtered by Whatman’s filter paper (no.1), and centrifuged (2000 rpm), the supernatant was removed and the solution placed in an incubator (40 ̊ C) until dry. The M.O. powder was placed in the dark bottle and stored at 5 ̊ C pending use.
The O.M. Oil Extract
The O.M. powder (20 grams) was weighed and transferred into Soxhlet and extracted with hexane (99%) for eight hours after the extraction, the oil extract was collected and kept in cool storage (5 º C) pending use (Quan et al., 2004).
The Processing and Dilution of Semen
The Tris extender was used to dilute the semen. The Tris extender consists of hydroxy-d-methyl amino-d2-methane (3.63 grams) (Sigma/Germany), Anhydrous citric acid (1.9 grams), dextrose 0.5 grams (Oxoid / England), egg yolk (14 %), glycerin (6 %), and antibiotic (penicillin and streptomycin 100,000 I.U. and 0.1 gram/100 ml respectively (Evans and Maxwell, 1987).
After semen dilution, the semen was added to different levels of O.M. solution. 1 mg O.M. powder /1 ml Tris + semen (P1 group), 2 mg O.M. powder /1 ml Tris + semen (P2 group),1 mg O.M. / 1 ml Tris + semen, (O1 group), and 2 mg O.M. oil /1 ml Tris + semen. Tris + semen without O.M. (control group).
The samples were divided into two parts. The first part is stored at five Celsius degrees for 72 hours. The other part is placed into straws (0.25 ml, I.M.V.), and then, the straws are placed at cooling for 3.5 - 4 hr. for equilibrium with the glycerol. After that, the second group evaporated with nitrogen and plunged into nitrogen liquid at -196 °C for 30 days (Evans and Maxwell, 1987).
The spermatozoa motility was estimated according to the method recommended by (Chemineau et al., 1991) with minor modifications, 20 μL of diluted semen placed on a warmed slide with 2.9 % sodium citrate solution (10 μL) and estimated under the microscope (40x) and estimated depending on the scales recommended by (Walton, 1933). The sperm motility was assessed depending on the progressive motility of the sperm. (Walton,1933) defined the requirements as 0= non-move, 10-20= tail ripple. 20-40= tail ripple with slow forward motion, 40-60= progressive move with a moderate speed, 60-80= fast progressive move, and 80-100= very rapid progression.
The dead spermatozoa percentage estimated carried out the methods reported by (Evans and Maxwell, 1987) The solution of staining was made by dissolving nigrosine (10 grams) and eosin Y (1.67 grams) (Qualikems / India) in distilled water (100 ml), The solution was boiled and left at room temperature leave until cool, then place the diluted semen (20 µl) on a warmed slide with on drop of staining solution and mixed well and examined under microscope (40 x) (Hancock, 1951).
Seminal Plasma Biochemical Parameters
pH assay: Diluted semen pH was measured by a pH meter (Inolab, S, African), which is equipped with a small diameter sensor that can be inserted into the sample tube.
Seminal plasma preparation
The experimental semen samples were centrifuged (Hittich, Germany) in a 5-milliliter test tube at 3000 g for 10 minutes. Then, the supernatants were aspirated and kept at -40 ᵒ C.
Malondialdehyde (MDA Assay)
The MDA is a product of fatty acid peroxidation. MDA was measured depending on a procedure by (Rao et al., 1989), The diluted semen was placed in the centrifuge (4000 rpm/10 min.). The seminal plasma was isolated from platelets (150 μL) mixed with 1 ml of trichloroacetic acid (TCA, 17 % w/v) and 1 ml of thiobarbituric acid (SCR / China) (TBA, 0.6% w/v),
The TCA solution was prepared by adding 17 grams of trichloroacetic acid with 100 ml of distilled water and mixing well. The TBA solution was prepared by adding 0.6 grams of TBA powder to 100 ml of distilled water and mixing well.
1 ml of the solution (17 % TCA and 0.6 % TBA) mixed with 150 μL, the solution tubes were stood in a boiled water bath at 90-100 °C (10 minutes). After cooling, 1 ml of TCA was added. The mix was centrifuged at 1750×g for 10 minutes, and the absorbance was counted by a spectrophotometer (Pye Unicon, England) at 530-540 nm (Rao et al., 1989).
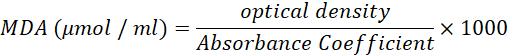
Total Antioxidant Capacity (TAC assay)
The DPPH radicals (2, 2 – Diphenyl - 1- picrylhydrazyl) were used to estimate the TAC of seminal plasma depending on the absorbance at 517 nm by using a spectrophotometer device (Zhang et al., 2013). The DPPH solution was prepared according to a method described by (Kubo, 1984) by adding 0.004 % of DPPH (Sigma-Aldrich) in 100 ml of methanol 99 % (SCR, China). 30 μL of seminal plasma was added to 3 ml of the DPPH solution.
The solution was placed in a dark place for 30 minutes at room temperature. to get stable absorbance values. The higher DPPH values were shown the lower TAC in the DPPH assay. An ascorbic acid curve was established and the results were described by microgram /ml
Preparation of ascorbic acid curve
The ascorbic acid was dissolved (80 mg) in 100 ml of distilled water. the final concentrations of ascorbic acid were 25, 50, 100, 200, and 400 micrograms/ml, 30 μL of it was added to 50 μL of pro-oxidant methanol and the complete the volume to 3 ml of DPPH solution (0.004 %), the absorption was recorded depending on spectrophotometer results at 517 nm (Pisoschi, et al., 2009).
Sample preparation
The seminal plasma (30 μL) was added to 3 ml DPPH solution, the absorption was recorded depending on spectrophotometer results at 517 nm, and the plasma antioxidant activity was calculated by following the equation: (Pisoschi, et al., 2009).
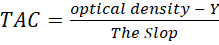
Y = The intersection of the regression line with the y-axis.
The statistical Analysis
The experiment aimed at studying the raw powder effect based on the concentration and oil extract at the time of storage as C.R.D. (completely randomized design) without interaction between storage intervals, Thus, data was analysed by the Statistical analysis system (SAS, 2012). The significant variation between all groups was evaluated based on (Duncan,1955). The statistical model was:
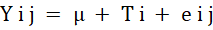
Yij= Variable; j=Observation; i= Treatment; μ= mean, Ti= Treatment effect; eij= The error.
RESULTS AND DISCUSSION
The Sperm Motility
The sperm motility is an indicator of the sperm viability. (Table 1) shows the significance of adding M.O. powder extract to the ram semen. The P2 and P1 groups observed the highest sperm motility percentage at 0 hr. interval at cooling under 5ᵒ C against the control group that recorded a lower percentage, on the other side, O1 and O2 showed non-significant differences from the control. Sperm motility is an indicator of sperm viability. (Table 1) shows the significance of adding M.O. powder extract to the ram semen. The P2 and P1 groups observed the highest sperm motility percentage at 0 hr. interval at cooling under 5ᵒ C against the control group that recorded a lower percentage, On the other hand, O1 and O2 showed non-significant differences from the control.
Table 1: Impact of used O.M. extracts on sperm motility percentage persevered at cooling and cryopreservation (mean ± standard error).
|
TRT. |
Cooling time at 5 ºC (Hours) |
Cryo. Storage |
|
|
0 |
72 |
30 days |
|
|
CO |
75.25±1.01 b |
47.62±1.10 d |
23.00±0.75 c |
|
P1 |
77.75±0.75 a |
54.75±0.92 b |
27.25±1.03 b |
|
P2 |
78.75±0.55 a |
59.12±0.78 a |
30.37±0.80 a |
|
O1 |
76.37±0.98 ab |
48.06±1.09 cd |
23.62±0.56 c |
|
O2 |
77.12±0.83 ab |
50.25±0.95 c |
24.37±0.73 c |
|
P value |
P≤0.01 |
P≤0.01 |
|
The different letters indicate significant differences between treatments. CO: control group; P1: 1 mg / ml O.M. raw powder; P2: 2 mg / ml O.M. raw powder; O1: 1 mg / ml O.M. oil; O2: 2 mg / ml O.M. oil.
The pH Level
The results observed non-significant changes in the pH of diluted semen at a 0-hour storage period between the P1 and P2 groups compared with the control group, However, the other O1 and O2 groups recorded a high increase at the same time (Table 2). After 72 hours of cooling, the results in Table 2 showed highly significant variation in the pH level between the groups, The O2 group recorded a higher increase (P≤0.01) and followed by the control group, non-significant variations were shown between the control and O1 group. The P2 recorded a lower pH level at the same time. After 30 days of freezing storage, (Table 2) recorded a significant increase in pH level in the O2 group compared with all groups, At the same time, the control group recorded a high level of pH compared with the P1, P2, and O1 groups, the P2 group showed a lower level of pH than other groups.
The MDA Concentrations
(Table 3) indicated highly significant variations between the experimental groups and the control group during the first period at cooling at 5ᵒ C (0-hour) in the MDA concentration, The group O2 (58.80±0.42) noticed a higher significant (P≤0.01) decrease than the control, P1, P2, and O1 groups (96.7±0.78, 86.78±1.46, 77.65±0.57, 89.16±0.37) respectively. However, the results refer to a higher decrease (P≤0.01) in the O2 group (63.72 ± 0.54) than the other groups and control groups post 72 hours of cooling (5ᵒ C) and followed by the P1 (113.46 ± 1.57), P2 (99.40 ± 0.42), O1 (119.21 ± 1.36), however, the control group recorded a higher level of MDA127.81±1.72 at 72 hours of cooling. In addition, the storage semen groups recorded significant dissimilarity to the control post 30 days at cryopreservation, while the control group recorded a high concentration of MDA (126.49±0.46) and the O2 group recorded a lower significance (61.27±0.83) compared to other groups and control treatment.
Table 2: Impact of used O.M. extracts on semen pH persevered at cooling and cryopreservation (mean ± standard error).
|
Cooling time at 5 ºC (Hours) |
Cryo. storage |
||
|
TRT. |
0 |
72 |
30 days |
|
CO |
6.68±0.02 bc |
6.51±0.01 b |
6.62±0.01 b |
|
P1 |
6.67±0.02 c |
6.46±0.02 c |
6.54±0.02 c |
|
P2 |
6.72±0.02 b |
6.36±0.01 d |
6.48±0.01 d |
|
O1 |
6.81±0.01 a |
6.48±0.01 bc |
6.51±0.01 cd |
|
O2 |
6.79±0.08 a |
6.56±0.01 a |
6.68±0.01 a |
|
P-value |
P ≤ 0.01 |
P ≤ 0.01 |
P ≤ 0.01 |
The different letters indicate significant differences between treatments. CO: control group; P1: 1 mg / ml O.M. raw powder; P2: 2 mg / ml O.M. raw powder; O1: 1 mg / ml O.M. oil; O2: 2 mg / ml O.M. oil.
Table 3: Impact of used O.M. on seminal plasma MDA (μmol/ml) persevered at cooling and cryopreservation (mean ± standard error).
|
Cooling time at 5 ºC (Hours) |
Cryo. storage |
||
|
TRT. |
0 |
72 |
30 days |
|
CO |
96.70±0.78 a |
127.81±1.72 a |
126.49±0.46 a |
|
P1 |
86.78±1.46 c |
113.46±1.57 c |
111.83±1.24 c |
|
P2 |
77.65±0.57 d |
99.40±0.42 d |
86.61±0.66 d |
|
O1 |
89.16±0.37 b |
119.21±1.36 b |
116.46±1.57 b |
|
O2 |
58.80±0.42 e |
63.72±0.54 e |
61.27±0.83 e |
|
P-value |
P ≤ 0.01 |
P ≤ 0.01 |
P ≤ 0.01 |
The different letters indicate significant differences between treatments. CO: control group; P1: 1 mg / ml O.M. raw powder; P2: 2 mg / ml O.M. raw powder; O1: 1 mg / ml O.M. oil; O2: 2 mg / ml O.M. oil.
The TAC Concentrations
In (Table 4), The results proved a high significant decrease (P≤0.01) in the TAC was recorded by adding 2 mg O.M. powder (O2 group) compared with the control and the other experimental groups. Otherwise, the control group recorded the lowest effectiveness, it was recorded at 14.51±0.78 while the P1, P2, and O1 were recorded at 29.68±0.81, 39.01±0.59, and 31.64±0.61 respectively at zero hours at cooling. Also, the O2 group observed a significant effect (P≤0.01) of the same trails after 72 hours at cooling (92.61±0.42) Followed by P2(62.56±0.68), P1 (48.15±0.84), and O1(46.72±0.73), on the other hand, the control group recorded a higher significant decrease (P≤0.01) in TAC group compared with the other experimental groups post 72-hours at 5ᵒ C. However, the results indicated that highly significant variations were recorded among all groups compared with the control post-freezing – thawing for one month at liquid nitrogen, while the O2 group showed a significant increase (P≤0.01) against the other groups and the control group at the same time of cryopreservation for one month.
Table 4: Impact of used O.M. on seminal plasma TAC persevered at cooling and cryopreservation (mean ± standard error).
|
Cooling time at 5 ºC (Hours) |
Cryo. storage |
||
|
TRT |
0 |
72 |
30 days |
|
CO |
14.51±0.78 e |
25.66±0.82 e |
19.62±0.39 e |
|
P1 |
29.68±0.81 d |
48.15±0.84 c |
36.64±0.31 d |
|
P2 |
39.01±0.59 b |
62.56±0.68 b |
49.72±0.42 b |
|
O1 |
31.64±0.61 c |
46.72±0.73 d |
39.76±0.54 c |
|
O2 |
87.83±0.32 a |
92.61±0.42 a |
83.71±0.63 a |
|
P-value |
P ≤ 0.01 |
P ≤ 0.01 |
P ≤ 0.01 |
The different letters in the means refer to the significant differences. CO: control group; P1: 1 mg / ml O. M. raw powder; P2: 2 mg / ml O.M. raw powder; O1: 1 mg / ml O.M. oil; O2: 2 mg / ml O. M. oil.
This study aims to investigate the role of Origanum Majorana as an antioxidant and its effect on the ram semen quality during storage. Many studies are proving that using plant extracts reduces qualitative destruction of the semen at storage for different times depending on the extract concentration and periods of storage (AL-Sarray et al., 2022).
The Sperm Motility Percentage
The results have clarified that there was a significant improvement in the sperm motility percentage, which was recorded by adding 2 mg O.M. powder (P2 group) at zero time and 72 hours under cooling at 5°C and after freezing for thirty days in liquid nitrogen than other experimental treatments and the control group. The addition (1 mg O.M. powder) enhanced the diluted ram semen under cooling conditions and post-cryopreservation against the control group. However, adding O.M. oil doesn’t improve the semen quality exactly after 30 days of freezing. The increase in sperm motility by adding O.M. powder may be caused by the O.M.’s properties as an antioxidant and antimicrobial (Noh et al., 2020). The O.M. have a powerful activity against oxidation stress and bacteria, which may lead to saving semen quality during storage.
The pH Level
Non-significant effect O.M. The pH level in most groups may be due to a high concentration of antioxidants, which may lead to an imbalance therefore, affecting the system of energy enzymatic in the cell membrane (Dorostkar et al., 2012) which was promoted by our results through the pH value recorded by the O2 group, which indicates a decrease in sugar consumption and its conversion to lactate acid. This result is consistent with (Dorostkar et al., 2012) who state that using a high level of external sources of antioxidants may hurt sperm parameters in rats, humans, cattle, and rams during the semen preparation for freezing storage, (Moghbeli et al., 2016) reported that the sperm cell activity could have a defect consequent from a high antioxidant concentration which leads to the sapping of the tail axis, mitochondria’s membranes, breathing processes and energy production, (Al-Sarray et al., 2022) found that a high concentration of marjoram leads to a decrease in sperm viability, acrosome abnormality percentage under cooling and freezing storage.
The MDA and TAC Concentrations
On the other hand, all O.M. groups showed a significant effect on MDA concentration and TAC especially the O2 group which noticed a highly significant effect (P≤0.01) against the other treatments and control groups post 72 hours at 5ᵒ C. The O.M. was the herb plant known as marjoram, used in old traditional medicine food ingredients and products and for use in cooking (Gutiérrez-Grijalva et al., 2017). It has a good role as an exogenous origin of powerful antioxidants and a high activity to protect cells against polyunsaturated fatty acid oxidation and prevent diseases that arise from producing free radicals in a high concentration within cells. So, it can be added as an active source of antioxidants against phospholipids peroxidation and free radical formation of the sperm cell membrane (Erenler et al., 2016).
Many studies have evaluated the bioactive phenolic compounds of O.M. (Kaliora et al., 2014) found that there are more than twenty-seven polyphenols profiles and terpenic acids of O.m.L including flavonoids (chrysin and Naringenin), hydroxycinnamic acids (p-coumaric acid, ferulic acid, Rosmarinic acid, and Caffeic acid), Terpenoid phenols (Carvacrol and Thymol), and Phenolic acids (three,four-Dihydroxy-phenylacetic acid and Syringic acid), on the other hand, (Vallverdú-Queralt et al., 2014) estimate that the O.M. leave has the highest concentration of rosmarinic acid and protocatechuic acid. while, (Gutiérrez-Grijalva et al., 2017) state that the flavonoids compounds and phenolic acids of O.M. were including so many compounds like catechin, caffeic acid, rosmarinic acid, kaempferol, protocatechuic acid, ferulic acid, p-coumaric acid, Vanillic acid, syringic acid, p-coumaric acid, quercetin, gallic acid, o-coumaric acid, sinapic acid, cinnamic acid, epicatechin, naringenin, kaempferol, and, chrysin. All of these compounds have a good power as antioxidant capacity which leads to protecting mammalian cells against free radical damage.
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, the use of 2 mg/ml O.M. raw powder for the Tris extender procedure has had a good impact on improving some of the ram’s biochemical seminal plasma parameters. These rolls can contribute to reducing some of the factors affecting semen when preserved at 5 C post 72 hours or post-cryopreservation in nitrogen. Also, may contribute to the development of sheep artificial insemination in Iraq.
ACKNOWLEDGMENTS
The authors thank the Ruminants Researches Station/ Baghdad technicians for assisting during the experiments.
AUTHOR’S CONTRIBUTIONS
There was participation from all researchers in writing the research and preparing information related to the additive, preparation of the sample, the statistical analysis and work on the methods associated with the experiment.
Mohammed Abdulameer Rashid Al-Sarray: Collect the data and write the paper.
Hasanain Jihad Neamah: Conceived and designed the analysis.
Sarah Gatea Fayyadh AL-Omairi: performed the analysis and other contributions.
Conflict of Interest
The authors have declared no conflict of interest.
REFERENCES
AL- Sarray MAR, Hobi AA, Al-Ani AAT (2020). Effect of adding Selenium nanoparticles to the Tris extender on Awassi rams semen quality preserved at cooling and freezing storage. Biochem. Cell. Arch., 20(1): 543-548.
AL-Dhaheri SKM (2012). Studying the effect of the addition of Origanum majorana L. (Majoram) and their extracts on some quality characteristics of minced beef meat during frozen storage. M.Sc. thesis,, Coll. Agric. Univ. Baghdad,
Allverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Rinaldi AJF, Leal LN, Lamuela-Raventos RM (2014). A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary thyme oregano cinnamon cumin and bay. Food Chem., 154: 299–307. https://doi.org/10.1016/j.foodchem.2013.12.106
AL-Sarray MAR, Hassan GM, Dheerib GS (2022). Effect of Origanum majorana L. raw powder and oil extract as antioxidant on ram semen extender of some Iraqi Awassi ram’s semen parameters preserved under 5ºC and cryopreservation. Biochem. Cell. Arch., 22: 2667-2672.
AL-Sarray MAR (2012). Effect of freezing Turkish awassi ram semen in sperm acrosome by used different dyes. M.sc. thesis, Coll. Agric. Univ. Baghda,.
AL-Sarray MAR, Hobi AA, Al-Ani AAT (2019). Effect of adding Lycopene to the Tris extender on Awassi rams semen quality persevered at cooling and freezing storage. Plant Arch., 19(2): 3971-3976.
Al-Subaihawi HRB, Al-Saab HKJ, AL-Sarray MAR (2017). Extrication and lyophilization low – density lipoproteins and used it instead of egg yolk in semen extender of awassi rams and their influences in sperm abnormalities store at 5 ° C. The IJAS, 48 (1): 342-349. https://doi.org/10.36103/ijas.v48i1.453
Chemineau PY, Caginie Y, Guerin P, Arguer JC (1991). Vallet. Training Manual on Artificial Insemination in Sheep and Goat. F. A. O. Anim. Prod. Health, Paper No: 83.
Desouky S, Marzouk M, Soliman AM, Sayed AA (2015). Modulatory effect of Origanum majorana extract against cisplatin-induced dyslipidemia in rats. Int. J. Curr. Life Sci., 4(6): 228–234.
Dheerib GS, Banana HJH, Al-Sarray MAR (2020). Effect of Adding Lyophilized Low-Density Lipoproteins (L-LDL) Methionine and Their Combinations on Semen Quality of Holstein Bulls after Cryopreservation. Sys. Rev. Pharm., 11(11): 1813-1817.
Dorostkar KSM, Alavi-Shoushtari A, Mokarizadeh (2012). Effects of in vitro selenium addition to the semen extender on the spermatozoa characteristics before and after freezing in water buffaloes (Bubalus bubalis). Vet. Res. Forum. Fall., 3 (4):263-8. PMID: 25653769; PMCID: PMC4313046.
Duncan D (1955). Multiple Ranges and Multiple F-test. Biometrics, 11:1-24. https://doi.org/10.2307/3001478
Erenler R, Sem O, Aksit H, Demirtas I, Yaglioglu AS (2016). Elmastas M. and Telci İ. Isolation and identification of chemical constituents from Origanum majorana and investigation of anti-proliferative and antioxidant activities. JSFA., 96: 822-836. https://doi.org/10.1002/jsfa.7155
Evans G, Maxwell WMC (1987). Salomon’s Artificial Insemination of Sheep and Goats. Butter worth’s Sydney, pp. 194.
Gutiérrez-Grijalva EP, Picos-Salas MA, Leyva-López N, Criollo-Mendoza MS, Vazquez-Olivo G, Heredia JB (2017). Flavonoids and Phenolic Acids from Oregano: Occurrence Biol. Act. Health Benefits, Plants (Basel Switzerland), 7(1): 2. https://doi.org/10.3390/plants7010002
Hancock JL (1951). A staining technique for the study of temperature shock in semen. Nature (London). 169: 323-326.
Kaliora AC, Kogiannoua DAA, Kefalas P, Papassideri IS, Kalogeropoulos N (2014). Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; balancing delight and chemoprevention. Food Chem., 142: 233–241. https://doi.org/10.1016/j.foodchem.2013.07.056
Kameni S, Meutchieye F, Ngoula F (2021). Liquid Storage of Ram Semen: Associated Damages and Improvement. Open J. Anim. Sci., 11: 473-500. https://doi.org/10.4236/ojas.2021.113033
Kubo K, Yoshitake I, Kumada Y, Shuto Nakamizo KN (1984). Radical scavenging action of flunarizine in rat brain in vitro. Arch. Int. Pharmacodyn., 272: 283–95. PMID: 6525007
Maxwell WMC, Watson PF (1996). Recent Progress in the Preservation of Ram Semen. Anim. Reprod. Sci., 42: 55-65. https://doi.org/10.1016/0378-4320(96)01544-8
Moghbeli M, Kohram H, Zare-Shahaneh A, Zhandi M, Sharideh H, Sharafi M (2016). characteristics and fertilization capacity of rooster sperm frozen in the presence of the antioxidants catalase and vitamin E. Theriogenology, 86(6): 1393-1398. https://doi.org/10.1016/j.theriogenology.2016.03.038
Noh S, Go A, Kim DB, Park M, Jeon HW, Kim B (2020). Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxidants (Basel). Oct 7;9(10):957. doi: 10.3390/antiox9100957. PMID: 33036328; PMCID: PMC7600260.
Pisoschi AM, Cheregi MC, Danet AF (2009). Total antioxidant capacity of some commercial fruit juices: electrochemical and spectrophotometrical approaches. Molecules., (1):480-93. PMID: 19158657; PMCID: PMC6253775. https://doi.org/10.3390/molecules14010480
Quan L, Li S, Tian S, Xu H, Lin A, Gu L (2004). Determination of Organochlorine Pesticides Residue in Ginseng Root by Orthogonal Array Design Soxhlet Extraction and Gas Chromatography. Chromatographia, 59: 89–93. https://doi.org/10.1365/s10337-003-0120-9
Rao B, Souflir JC, Martin M, David G (1989). Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res., 24(2): 127-34. https://doi.org/10.1002/mrd.1120240202
Salisbury GW, Van N, Demark L, Lodge JR (1978). Extenders and extension of unfrozen semen. In: Physiology of Reproduction and Artificial Insemination in Cattle. W. H. Freeman and San Francisco Co. 2nd edn., pp: 442-493.
SAS (2012). SAS / STAT User’s Guide for Personal Computers. Release 9.1 SAS Institute. Inc. Cary N. C. USA.
Smith JT, Mayer DT (1955). Evaluation of sperm concentration by the haemocytometer method. comparison of four counting fluid. Fert. Steril., 6: 271-275. https://doi.org/10.1016/S0015-0282(16)31987-2
Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Alvarenga JFR, Leal, LN, Lamuela-Raventós RM 2014. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chemistry, 154, 299-307. PMid:24518346. http://dx.doi.org/10.1016/j. foodchem.2013.12.106
Walton A (1933). Technique of artificial insemination. mp. Bur. Anim. Genet. Iiius- Edinburgh.p 56.
Zhang W, Chen H, Wang Z, Lan G, Zhang L (2013). Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. J. Food Sci. Technol., 50(6): 1122–1129. https://doi.org/10.1007/s13197-011-0447-4
To share on other social networks, click on any share button. What are these?






