Three Bacteriophages SA, SA2 and SNAF can Control Growth of Milk Isolated Staphylococcal Species
Three Bacteriophages SA, SA2 and SNAF can Control Growth of Milk Isolated Staphylococcal Species
Aiza Tahir, Muhammad Asif, Zaigham Abbas and Shafiq ur Rehman*
Department of Microbiology and Molecular Genetics, University of the Punjab, Quaid-e-Azam Campus, Lahore-54590, Pakistan
ABSTRACT
Staphylococcus aureus along with other coagulase negative Staphylococci are among the major mastitis causing organisms. Consumers of milk and milk products from mastitic animals are at high risk of foodborne infections. Escalating antibiotic resistance strived us to explore the alternate option to control mastitis. In ongoing era, bacteriophages are an alternate possible effective remedy. Therefore, in this study the infection ability of three lytic phages SA, SANF and SA2 was determined against ten isolates of Staphylococci and one Micrococcus, isolated from five raw milk samples. Their morphological, biochemical characterization and 16S rRNA sequencing indicated that two strains were S. aureus, while all others were different coagulase negative Staphylococci. Their sensitivity against three phages indicated broad host range of phages SANF and SA2, and relatively narrow host range of phage SA. Phage SNAF showed an effective growth reduction of S. aureus RP isolate, compared with other bacteriophages. The bacterial challenge test in milk indicated that proliferation of S. aureus was successfully ceased until six hours post infection when applied at and MOI of 100.
Article Information
Received 19 September 2016
Revised 10 November 2016
Accepted 18 November 2016
Available online 08 February 2017
Authors’ Contributions
ZA and SUR has designed the study and analyzed the data. AT has performed the experimental work. MS and AT wrote the article. SUR has supervised and reviewed the article.
Key words
SNAF, SA2, Mastitis, Bacteriophages, Milk
* Corresponding author: Shafiq.mmg@pu.edu.pk
0030-9923/2017/0002-493 $ 8.00/0
Copyright 2016 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.529.533
Introduction
A vast number of microorganisms, including bacteria, fungi and mycoplasmas are responsible for mastitis in dairy animals, and Staphylococcus aureus is the principal causative agent among them, along with other coagulase negative Staphylococci (Buzzola et al., 2001; Thorberg et al., 2009). These organisms reside and propagate on teat skin of dairy animals, which is coated with keratin that prevents microbes to get access towards teat canals (Robinson, 2005). During milking process, they mix in milk and can pose a serious threat of infection in humans on the consumption of contaminated milk (Oliver et al., 2005). S. aureus has been reported for many foodborne outbreaks due to consumption of milk and milk products (Asao et al., 2003; Johler et al., 2015; Ostyn et al., 2010). The emergence of multiple drug resistant S. aureus necessitated search of alternative methods for their elimination (Morandi et al., 2009).
Bacteriophages have an established history as an alternate therapy for bacterial infections. They have been successfully applied to control food contamination due to their host specificity and sparing normal microbial flora in humans (Haq et al., 2012; Hermoso et al., 2007). Many companies in West have prepared phage based products that are applied on ready to eat foodstuffs at any step from processing to consumption (Garcıa et al., 2008; Lu and Breidt, 2015). ListexTMP100 and Eco Shield are being used against Listeria monocytogenes and E.coli O157:H7, respectively in the meat, while Felix-O1 is being used to remove Salmonella from chicken (Garcıa et al., 2008; Sillankorva et al., 2012).
In this study the lytic activity of three bacteriophages have been assessed against mestitic pathogens isolated from milk in vitro. Additionally, a preliminary bacterial challenge test in milk has been assessed to determine the effectiveness of phages in milk.
MATERIALS AND METHODS
Isolation and identification of Staphylococci
Five raw milk samples (approximately 15 mL for each) were collected in sterile falcon tubes from different milk shops in Lahore. They were processed for isolation of Staphylococci. The collected samples were serially diluted in buffer peptone water (Oxoid) at 9:1 ratio and 50µL from serial dilutions were plated on mannitol salt agar (MSA) (Oxoid) followed by incubation for 24 h at 37°C. The isolated colonies on MSA were further streaked on Luria Bertani (LB) agar, followed by incubation for 24 h at 37°C to obtain their pure cultures that were used for biochemical testing. The isolates were identified on the basis of their colony morphology on MSA, gram staining, catalase and coagulase tests (Cappuccino and Sherman, 2008; Cheesbrough, 2006). The isolates were directly sent to Macrogen Korea for sequencing the 16SrRNA fragment for their molecular Identification.
Propagation of bacteriophages and their activity against bacterial isolates
All the bacteriophages used in this study were isolated from sewage exhaust of different hospitals located in Lahore, Pakistan. Currently these bacteriophages are partially characterized (Hamza et al., 2016). The propagation of the bacteriophages (SA, SANF and SA2) and their activity by spot tests against the isolated strains was performed according to already published work (Bibi et al., 2016). Sensitive bacterial isolates 3Y, 2P and RP were selected as the host for the enrichment of phages SANF, SA2 and SA, respectively. Phage titer was determined by counting plaques obtained through a serial dilution of the bacteriophages preparation on double layer agar plates (Bibi et al., 2016). To calculate phage titer, serial dilutions of phage enrichment were prepared followed by incubation for 45 min. After incubation, the mixture was added in 3 mL soft LB agar, poured on the LB agar plates and further incubated for 24 h at 37°C. The plate with countable plaques (3-300 plaques per plate) was observed to calculate the phage titer using the following formula (Obeso et al., 2010).
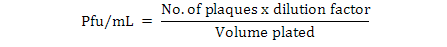
Bacterial growth reduction assay
To determine the lytic activity of phages (SA, SANF and SA2) on the growth rate of host bacterial strain (RP), 24 h old bacterial culture (100 µL) was added in four flasks containing 50 mL of sterilized LB broth. All flasks were inoculated with 50 µL of correspondingly labeled phages except one, which was used as negative control. During incubation of 24 hours at 37°C, optical density (600 nm) was measured at an interval of two hours by taking sample (1 mL) from each flask.
Bacteriophage antibacterial activity in milk
The effect of phage (SA, SANF and SA2) antibacterial activity on the bacterial growth was tested in commercial pasteurized milk at 25°C and 37°C, at multiplicity of infection (MOI; the ratio of phage concentration/bacterial concentration) of 100. Milk (8.6 mL) in sterile tubes was inoculated with diluted overnight cultures of bacterial strain (106 Cfu/mL) and correspondingly labeled phage (108 Pfu/mL). Milk inoculated only with the diluted overnight culture of bacterial strain (106 Cfu/mL) was used as a control. All tubes were incubated at their correspondingly labeled temperature for six hours and from each tube, (1 mL) sample was taken at an interval of two hours and bacterial load was recorded after growth on Chapman agar. The sterilization of pasteurized milk was tested by direct plating (Obeso et al., 2010). The percentage reduction calculated by dividing the decrease in CFU by total number of bacteria in control on a particular time and multiplying by 100.
RESULTS
Majority of milk isolates belong to genus Staphylococcus
Based on cultural and biochemical properties, 11 staphylococci isolates were obtained from 5 raw milk samples. On the basis of 16S rRNA sequencing the isolated strains were identified as different species of the genus Staphylococcus, while one strain (2P) which was identified as Micrococcus caseolyticus (Supplementary Table I).
Bacteriophages SA2 and SNAF showed broad host specificity
Phage SANF showed complete lytic activity against all isolated strains while phage SA2 was also active against all isolates except 3Y and RI. Phage SA infected the four isolates only (Supplementary Table II).
Tested bacteriophage reduced the host bacterial growth
The growth reduction experiment was performed using the S. aureus RP isolates, due to its high susceptibility to all isolated phages. The reduction in growth of S. aureus against each individual phage was measured as optical densities (600 nm) during 24 h of incubation. The results of this in vitro study indicated that phages reduced the growth of target bacteria. There was a common observation that the bacteriophages sustained the growth reduction during the early 8 h of infection. The bacterial cells started to grow after the 8 hours of infection, but interestingly, the bacterial growth started to decrease again after the 16-18 h post infection (Fig. 1).
Bacteriophage antibacterial activity in milk
The antibacterial activity of three phages was also tested in the commercial pasteurized milk against S. aureus RP isolate. The tested phages showed an increased percentage of bacterial load reduction from 4-6 h, post-infection when incubated at 25°C. The three phages also showed antibacterial activity when incubated at 37°C. The activity at 37°C was however, lower compared to that at 25°C. The maximum bacterial growth control was observed by the bacteriophages SANF and SA2 at 25°C temperature, while bacteriophage SA showed reduction in bacterial growth from 99 to 60 percent during transition form 4-6 h post infection. The bacteriophage SANF has not shown bacterial growth control ability when incubated at 37°C. However, the phage SA and SA2 showed a growth reduction up to 65 percent from 2-6 h, post infection (Fig. 2).
DISCUSSION
In the current study, the susceptibility of milk isolated bacterial strains against three phages (SA, SANF and SA2) indicated broad host range of SANF and phage SA2 while SA indicated relatively narrow host range than others. El Haddad et al. (2014) reported the broad host range of three polyvalent phages Team1, phi812 and K. He found 52 out of 57 raw milk isolated strains of of S.aureus isolated from raw milk samples, susceptible to all of three polyvalent phages (El Haddad et al., 2014). The individual phage SANF showed efficient antibacterial activity against Staphylococci isolated from milk as Jia et al. (2015) reported the effective lytic activity of a newly isolated phage JS01 with broad host range against S.aureus. Broad host range of phage vB_SauM_JS25 phage against S.aureus strains was also observed by Zhang et al. (2015).
The three tested phages in this study showed above 90 percent decrease in the growth of target bacteria when incubated at room temperature, while the percentage reduction was reduced to 30-60%, when incubated at physiological temperature. Bacteriophage SA2 showed a considerable potential for growth reduction of target organism in the milk environment. The decrease in the lytic ability of the bacteriophages when applied in the milk environment compared with their activity in broth is similar to previous studies of O’Flaherty et al. (2005) and Gill et al. (2006) who found inactivity of phage K in milk against S. aureus. The presence of milk proteins is mainly responsible for the inactivity of phages in milk. The milk proteins after being adhered to the bacterial cell surfaces change the receptors’ morphology of S. aureus and make it tough for phages to access the host receptors. S. aureus can bind to the broad range of proteins such as immunoglobulin, collagen and fibrinogen (Gill et al., 2006). The presence of IgG and some other unknown components in the lacteal secretion of dairy animals cause aggregation of S. aureus cells in milk. These aggregates also inhibit the absorbance of phages on the bacterial cell receptors (Tanji et al., 2015). O’Flaherty et al. (2005) studied that the immunoglobulins, adhered to the bacterial cells associate them with the fat globules and the coating of fat globules around S. aureus aggregates also hinders the access of phages to S. aureus receptors. The bacterial growth reduction by the tested bacteriophages can be employed for controlling Staphylococcal contamination in raw milk. In future, more detailed studies of bacterial control by bacteriophages might give confidence to dairy industry for their routine use.
Acknowledgements
University of the Punjab, Lahore, Pakistan as annual research grant provided the financial aid for the current work.
Conflict of interest statement
We declare that we have no conflict of interest.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.493.496
REFERENCES
Asao, T., Kumeda, Y., Kawai, T., Shibata, T., Oda, H., Haruki, K. and Kozaki, S., 2003. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect., 130: 33-40. https://doi.org/10.1017/S0950268802007951
Bibi, Z., Abbas, Z. and Rehman, S.U., 2016. The phage P.E1 isolated from hospital sewage reduces the growth of Escherichia coli. Biocont. Sci. Technol., 26: 181-188. https://doi.org/10.1080/09583157.2015.1086311
Buzzola, F.R., Quelle, L., Gomez, M.I., Catalano, M., Steele-Moore, L., Berg, D. and Sordelli, D.O., 2001. Genotypic analysis of Staphylococcus aureus from milk of dairy cows with mastitis in Argentina. Epidemiol. Infect., 126: 445-452. https://doi.org/10.1017/S0950268801005519
Cheesbrough, M., 2006. District laboratory practice in tropical countries part 2, 2nd ed. Cambridge university press, UK. https://doi.org/10.1017/CBO9780511543470
El Haddad, L., Ben Abdallah, N., Plante, P., Dumaresq, J. and Katsarava, R., 2014. Improving the safety of Staphylococcus aureus polyvalent phages by their production on a Staphylococcus xylosus strain. PLoS One, 9: 1-10. https://doi.org/10.1371/journal.pone.0102600
Garcıa, P., Martınez, B., Obeso, J. and Rodrıguez, A., 2008. Bacteriophages and their application in food safety. Lett. appl. Microbiol., 47: 479-485. https://doi.org/10.1111/j.1472-765X.2008.02458.x
Gill, J., Sabour, P., Leslie, K. and Griffiths, M., 2006. Bovine whey proteins inhibit the interaction of Staphylococcus aureus and bacteriophage K. J. appl. Microbiol., 101: 377-386. https://doi.org/10.1111/j.1365-2672.2006.02918.x
Hamza, A., Perveen, S., Abbas, Z. and Rehman, S.U., 2016. The lytic SA phage demonstrate bactericidal activity against mastitis causing Staphylococcus aureus. Open Life Sci., 11: 39-45. https://doi.org/10.1515/biol-2016-0005
Haq, I.U., Chaudhry, W.N., Akhtar, M.N., Andleeb, S. and Qadri, I., 2012. Bacteriophages and their implications on future biotechnology: a review. Virol. J., 9: 9. https://doi.org/10.1186/1743-422X-9-9
Hermoso, J.A., Garcia, J.L. and Garcia, P., 2007. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr. Opin. Microbiol., 10: 461-472. https://doi.org/10.1016/j.mib.2007.08.002
Jia, H., Dong, W., Yuan, L., Ma, J., Bai, Q., Pan, Z. and Yao, H., 2015. Characterization and complete genome sequence analysis of bacteriophage JS01. Virus Genes, 2: 345-348. https://doi.org/10.1007/s11262-015-1168-y
Johler, S., Weder, D., Bridy, C., Huguenin, M.C., Robert, L., Hummerjohann, J. and Stephan, R., 2015. Outbreak of staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. J. Dairy Sci., 98: 2944-2948. https://doi.org/10.3168/jds.2014-9123
Lu, Z. and Breidt, F., 2015. Escherichia coli O157: H7 bacteriophage 241 isolated from an industrial cucumber fermentation at high acidity and salinity. Front. Microbiol., 6: 1-10. https://doi.org/10.3389/fmicb.2015.00067
Morandi, S., Brasca, M., Andrighetto, C., Lombardi, A. and Lodi, R., 2009. Phenotypic and genotypic characterization of Staphylococcus aureus strains from Italian dairy products. Int. J. Microbiol., 2009: 501362. https://doi.org/10.1155/2009/501362
O’Flaherty, S., Coffey, A., Meaney, W., Fitzgerald, G. and Ross, R., 2005. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. appl. Microbiol., 41: 274-279. https://doi.org/10.1111/j.1472-765X.2005.01762.x
Obeso, J.M., García, P., Martínez, B., Arroyo-López, F.N., Garrido-Fernández, A. and Rodriguez, A., 2010. Use of logistic regression for prediction of the fate of Staphylococcus aureus in pasteurized milk in the presence of two lytic phages. Appl. environ. Microbiol., 76: 6038-6046. https://doi.org/10.1128/AEM.00613-10
Oliver, S.P., Jayarao, B.M. and Almeida, R.A., 2005. Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodbour. Pathog. Dis., 2: 115-129. https://doi.org/10.1089/fpd.2005.2.115
Robinson, R.K., 2005. Dairy microbiology handbook: the microbiology of milk and milk products. John Wiley & Sons, USA.
Sillankorva, S.M., Oliveira, H. and Azeredo, J., 2012. Bacteriophages and their role in food safety. Int. J. Microbiol., 2012: 1-13. https://doi.org/10.1155/2012/863945
Tanji, Y., Tanaka, A., Tani, K., Kurimoto, M. and Miyanaga, K., 2015. IgG-dependent aggregation of Staphylococcus aureus inhibits bacteriophage attack. Biochem. Engin. J., 97: 17-24. https://doi.org/10.1016/j.bej.2015.01.007
Thorberg, B.M., Danielsson-Tham, M.L., Emanuelson, U. and Waller, K.P., 2009. Bovine subclinical mastitis caused by different types of coagulase-negative Staphylococci. J. Dairy Sci., 92: 4962-4970. https://doi.org/10.3168/jds.2009-2184
Zhang, L., Bao, H., Wei, C., Zhang, H., Zhou, Y. and Wang, R., 2015. Characterization and partial genomic analysis of a lytic Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis in Mid-east of China. Virus Genes, 50: 111-117. https://doi.org/10.1007/s11262-014-1130-4
To share on other social networks, click on any share button. What are these?










