The Relevance of Two-Doses of Foot-And-Mouth Disease (FMD) Virus Vaccine on Immune Response in Beef Cattle
Short Communication
The Relevance of Two-Doses of Foot-And-Mouth Disease (FMD) Virus Vaccine on Immune Response in Beef Cattle
Esdinawan Carakantara Satrija1, Sri Murtini2, I Wayan Teguh Wibawan2, Okti Nadia Poetri2*
1Study Program of Medical Microbiology, IPB Postgraduate School, IPB University, Jln Agatis, Kampus IPB Dramaga, Bogor, Indonesia; 2Division of Medical Microbiology, School of Veterinary Medicine and Biomedical Science, IPB University, Jln Agatis, Kampus IPB Dramaga, Bogor, Indonesia.
Abstract | The re-emergence of foot-and-mouth disease (FMD) in Indonesia in 2022 prompted the government to implement control measures, including mass vaccination. The recommended protocol involves two vaccine doses with a 4–5-week interval and a booster every 6 months. This protocol is effective for long-lifespan dairy cattle but may not be ideal for fattening cattle, which are reared for a shorter period (90–150 days). The study aimed to assess the immune response in fattening cattle following two-dose FMD vaccination, specifically the dynamics of white blood cell (WBC) and FMD antibody titer. Thirty healthy fattening cattle were divided into two groups: one receiving two vaccine doses and the other receiving a single dose. The targeted indicators were assessed using hematology analyzer dan enzyme-linked immunosorbent assay (ELISA).At 60 days post-first vaccination (DPFV), there were no significant differences in WBC profiles between the two groups. While antibody titers were slightly higher in the two-dose group at 30 and 60 DPFV, the difference was not statistically significant. Nevertheless, higher variability in antibody titers was observed in the single-dose group, indicating the potential issue of non-uniform herd immunity. The study suggests that a single-dose FMD vaccination may be sufficient to stimulate an immune response comparable to that of the two-dose regimen in fattening cattle. However, it should be implemented alongside stringent biosecurity measures to reduce the risk of FMD virus introduction.
Keywords | Foot-and-mouth disease (FMD), Vaccination, Immune response, Fattening cattle
Received | September 12, 2024; Accepted | October 26, 2024; Published | December 27, 2024
*Correspondence | Okti Nadia Poetri, Division of Medical Microbiology, School of Veterinary Medicine and Biomedical Science, IPB University, Jln Agatis, Kampus IPB Dramaga, Bogor, Indonesia; Email: dia_poetri@apps.ipb.ac.id
Citation | Satrija EC, Murtini S, Wibawan IWT, Poetri ON (2025). The relevance of two-doses of foot-and-mouth disease (FMD) virus vaccine on immune response in beef cattle. Adv. Anim. Vet. Sci. 13(1): 103-107.
DOI | https://dx.doi.org/10.17582/journal.aavs/2025/13.1.103.107
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Foot-and-mouth disease (FMD) is a significant economic disease caused by the foot-and-mouth disease virus (FMDV), an RNA virus from the Aphthovirus genus in the Picornaviridae family (Li et al., 2021). The infection can impair animals’ ability to eat, swallow, and move, leading to reduced milk production, weight loss, and decreased reproductive function (Ekanayaka et al., 2022; Aslam and Alkheraije, 2023; Ozturk et al., 2020; Ismail et al., 2023; Raina et al., 2023).
In response to the re-emergence of FMD in 2022, the Indonesian government implemented control measures, including biosecurity protocols, treatment, testing, culling, and mass vaccination (Antari et al., 2024). The FMD vaccination program, as per Decree No. 510/KPTS/PK.300/M/6/2022, mandates a minimum of three doses for FMD-susceptible animals, with the first two doses 4–5 weeks apart and a booster every six months. Such vaccination regimen is important for dairy cattle which have a relatively long lifespan. Moreover, the higher fat metabolism and mobilization rate in dairy cattle could potentially induce the disruption of neutrophil and mononuclear immune cells activities, especially as antigen-presenting cells crucial for early immune recognition and response (Sordillo 2016; Urrutia et al., 2019; Habel and Sundrum, 2023). On the other hand, fattening cattle are typically reared for a short period of 90–150 days before slaughter. Additionally, these cattle mainly focused on muscle growth by emphasizing the production and conservation of energy. Therefore, more energy reserve is available for other body systems, including immune system (Terry et al., 2021; Rivera-Villegas et al., 2024).
These factors, along with the use of commercially available killed FMD vaccine in the vaccination program, strongly suggested that the need for multiple doses and booster vaccinations may be less critical for fattening cattle. Previous studies have demonstrated that a single dose of the FMD vaccine can stimulate antibody production within four days, providing immunity for up to six months (Golde et al., 2005; Biogénesis, 2022). Therefore, the current vaccination protocol for fattening cattle, as mandated by the government, may need reconsideration. This study aims to evaluate the necessity of two-dose FMD vaccination in fattening cattle by assessing white blood cell profiles and FMD antibody titers.
MATERIALS AND METHODS
This longitudinal study, conducted from November 2023 to January 2024 and involved imported Australian fattening cattle (Bos indicus, Brahman Cross/BX breed, aged 1–2 years) from a single importation shipment reared at PT Rumpinary Agro Industry in Bogor Regency, West Java. As part of the farm standard procedures, all cattle in this shipment were physically examined by the company’s private veterinarian upon their arrival on the farm to ensure their health, and also under its supervision during the study period. These cattle were imported from Australia which have “FMD-free without vaccination” status. The number of samples was determined using the minimum sample number formula by Arifin and Zahiruddin (2017):
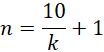
n is the minimum sample number and k is the number of groups. This formula results in a minimum sample number of 6. As such, during the earlier planning stage, 15 cattle were allocated for this study. However, an additional 15 cattle from the same population pool were available from the 30 days post-first vaccination (DPFV), increasing the allocated cattle to 30 head. These cattle were then divided into two groups of 15. The first group received two doses of the FMD vaccine (on arrival and 30 DPFV), while the second group received only one dose on arrival.
Blood samples were collected at 0, 14, 30, and 60 DPFV and analyzed at IPB University for white blood cell (WBC) profiles and FMD antibody titers. WBC profiling was done using the Vetscan® HM5 Hematology Analyzer, and antibody presence was assessed using the ID Screen® FMD Type O Competition ELISA and Foot and Mouth Disease Virus Non-Structural Protein (NSP) Antibodies ELISA Kit. Antibody titers were calculated from optical density (OD) values and compared against a threshold to determine their levels. The first group was evaluated for antibodies at 0, 14, 30, and 60 DPFV, while the second group was assessed at 30 and 60 DPFV. Data were analyzed using descriptive and inferential statistics, with ANOVA used to compare the groups.
RESULTS AND DISCUSSION
In this study, lymphocyte, monocyte, neutrophil, eosinophil, and basophil counts were measured at 0 and 60 days post-first vaccination (DPFV) for both groups. An increase in white blood cell (WBC) counts was observed in both groups at 60 DPFV, with no significant differences between the two-dose and single-dose groups (p > 0.05). All WBC values remained within the normal physiological range as referred from Quishpe et al. (2020), indicating no excessive immune response towards FMD vaccination (Table 1). Our results align with those of Yang et al. (2019), who found that vaccination does not always lead to a significant increase in WBC counts. This may be attributed to the type of FMD vaccine used for disease control. In Indonesia, we used an adjuvanted inactivated FMD vaccine, which is known for its good safety profile but may not mimic natural infections as effectively as live attenuated vaccines. The inactivated vaccine likely induces primarily a humoral antibody response rather than cellular immunity (Barteling, 2002; Diaz-San Segundo et al., 2016; Rodríguez-Habibe et al., 2020).
Table 1: White blood cell profile of two-doses and single-dose FMD vaccination group.
|
WBC type |
Normal Threshold (103/μL)a |
Mean±SD/SE (103/μL) |
p-values |
||
|
0 DPFV |
60 DPFV |
||||
|
Two- Dose |
Single -Dose |
||||
|
Lymphocytes |
2,5 – 7,5 |
5,15±1,26 |
5,93±1,57 |
5,25±1,44 |
0,225 |
|
Monocytes |
0,025 – 0,84 |
0,33±0,28 |
0,29±0,31 |
0,35±0,42 |
0,695 |
|
Neutrophils |
0,6 – 6,7 |
3,38±1,12 |
4,89±2,56 |
4,04±1,19 |
0,251 |
|
Eosinophils |
0 – 2,4 |
0,20±0,23 |
0,35±0,20 |
0,27±0,19 |
0,243 |
|
Basophils |
0 – 0,2 |
0,04±0,04 |
0,05±0,05 |
0,07±0,05 |
0,516 |
WBC: white blood cell; DPFV: days post first vaccination; SD/SE: standard deviation/ standard error; p-values are from the ANOVA test of two-doses and single-dose group at day 60 DPFV; a: Referred from Quishpe et al. (2020).
FMD antibody titers were measured using competitive ELISA, showing slightly higher titers in the two-dose group, but again, there was no significant difference between groups (Table 2). Additionally, there was no health problem encountered among involved cattle during the study period, indicating positive biological responses towards both vaccination regimens. These findings support the possibility that a single-dose vaccination provides sufficient immunity, in line with previous studies (Biogénesis, 2022; Ayele et al., 2023). The negative results for FMD NSP antibodies confirmed that the antibodies detected were from vaccination, not natural infection.
Table 2: FMD antibody titre of two-doses and single-dose FMD vaccination group.
|
Group |
Mean antibody titre ±SD/SE (CV) |
|||
|
0 DPFV |
14 DPFV |
30 DPFV |
60 DPFV |
|
|
Two-dose |
0,00 |
39,90± 41,41 (1,04) |
84,70±26,16 (0,31) |
94,50±0,00 (0,00) |
|
Single dose |
72,10±21,66 (0,30) |
80,00±23,50 (0,29) |
||
|
p values |
0,303 |
0,07 |
||
DPFV: days post first vaccination; SD/SE: standard deviation/ standard error; CV: coefficient of variance; p-values are from the ANOVA test of two-doses and single-dose group at day 30 and 60 DPFV.
The study highlights the sustained increase in antibody titers in both groups, attributed to the slow release of antigens due to the oil-based adjuvant used in the inactivated FMD vaccine (Xiao et al., 2007; Bazid et al., 2023). This ‘depot effect’ achieved through adjuvant-stimulated granuloma formation in antigen injection site which releases antigens slowly, resulting in a delayed B cell response (Tizard, 2021). The delayed cell B response is associated with the slow progress of germinal center (GC) reactions in secondary lymphoid organs due to the small amount of antigen present in these organs early during the post-vaccination period (Pedersen et al., 2020). Conversely, prolonged antigen exposure extends the GC reaction period, leading to increased antibody production (Cirelli et al., 2019). Thus, the combination of these factors contributes to a delayed peak in antibody production and higher titer values with adjuvanted vaccines.
However, higher variability in antibody titers was observed in the single-dose group, emphasizing the role of boosters in reducing titer variation and promoting herd immunity, as previous showed by other studies (Sharma et al., 2017; Gunasekera et al., 2022). Herd immunity could be described as the resistance shown by the proportionate amount of population, i.e., with the ability to produce sufficient amount of antibody. Ideally, such immunity covered at least 80% of the population to protect the said population from the designated disease (Balakrishnan and Rekha 2018). Maintaining herd immunity is crucial for controlling FMD disease
outbreak, as such breaking of this immunity might provide a window of opportunity for the silently circulating virus to spread to the entire population (Singh et al., 2019).
The break of herd immunity could happen when there is a varying level of antibody titers (protective and non-protective) within a population. An earlier study by Smitsaart et al. (1998) showed a positive correlation between detected FMD antibody titers with the protection against infection challenge. The threshold of mean titer log10 value for sufficient population immunity against FMD using virus neutralization test (VNT) and VNT-calibrated liquid phase blocking ELISA (LBPE) is between 1.6 to 1.8 (Cloete et al., 2008; Sharma et al., 2017). While this study was limited by the use of competitive ELISA of which resulting titers cannot be directly extrapolated as above, the variation of antibody titers found in the single-dose vaccination group should be considered as a risk for the integrity of herd immunity that should be mitigated.
Effective biosecurity measures, such as sanitation, isolation, and controlled traffic, can significantly reduce disease risks. This is crucial because the risk of disease introduction is predominantly linked to indirect transmission through inadequately monitored routes (Sansamur et al., 2020). The FMD virus can be spread through fomites carried by people, animals, equipment, and vehicles, which highlights the importance of stringent biosecurity (Auty et al., 2019; Aslam and Alkheraije, 2023). Alternative strategies, such as administering multiple doses in one session, may also be viable to reduce handling stress and improve productivity (Ulziibat et al., 2023; Endris and Feki, 2021; Vaz et al., 2023).
CONCLUSIONS AND RECOMMENDATIONS
The study found no significant difference in immune response between the two-dose and single-dose groups following FMD vaccination. However, the single-dose group showed greater variability in antibody titers. While a single-dose FMD vaccination program can be viable in feedlots, it should be implemented alongside stringent biosecurity measures to reduce the risk of FMD virus introduction.
We recommend that the relevant authorities review and revise the regulation for FMD vaccination in the field, namely by classifying livestock groups based on origin (free/affected by FMD country) and duration of rearing before harvest, as well as determining the prerequisites for implementing FMD vaccination procedures in each group. This revision will allow for efficient allocation of the PMK vaccine while maintaining the effectiveness of the implemented vaccination program.
ACKNOWLEDGEMENTS
We would like to thank the management of PT Rumpinary Agro Industry for allowing this research to be conducted at their facility. Special thanks to Dr. Satya Prawira for his invaluable help with sample collection, daily observations, and for promoting this research to PT Rumpinary Agro Industry. We are also grateful to Dr. Titin Supriyatin of PT Diagnostik Indonesia for supplying the necessary tools and materials for the study.
NOVELTY STATEMENT
This article reports the first haematological and immunological evaluation of the two-dose FMD vaccination regimen in commercial feedlot in Indonesia.
AUTHORS’ CONTRIBUTIONS
ECS was responsible for research conceptualization, sample collection, laboratory analysis, data analysis, and preparation of the original draft. SM handled funding acquisition and laboratory analysis. IWT and ONP contributed to the review and editing of the manuscript and supervised the overall research activities.
Ethical Clearance
This research complies with Article 80 of the Indonesian Law on Livestock and Animal Health (UU 18/2009). The animal experiments were conducted in accordance with institutional regulations and were approved by the IPB University Institutional Animal Ethics Commission under certificate number 200/KEH/SKE/IV/2024.
Conflict of Interest
The authors declare no conflict of interest with any financial, personal, or other relationships with people or organizations related to the material discussed in the manuscript.
REFERENCES
Antari LD, Kusumastuti T, Juwari A, Widiati R (2024). Policy response on handling of foot and mouth disease outbreaks in Indonesia. In: IOP Conference Series: Earth Environ. Sci., 1341: 012089. https://doi.org/10.1088/1755-1315/1341/1/012089
Arifin WN, Zahiruddin WM (2017). Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci., 24(5): 101-105. https://doi.org/10.21315/mjms2017.24.5.11
Aslam M, Alkheraije KA (2023). The prevalence of foot-and-mouth disease in Asia. Front. Vet. Sci., 10. https://doi.org/10.3389/fvets.2023.1201578
Auty H, Mellor D, Gunn G, Boden LA (2019). The risk of foot and mouth disease transmission posed by public access to the countryside during an outbreak. Front. Vet. Sci., 6: 381. https://doi.org/10.3389/fvets.2019.00381
Ayele G, Getachew B, Bari FD (2023). Combined adjuvant formulations enhanced an immune response of trivalent foot and mouth disease vaccine in cattle. Veterinary Medicine: Research and Reports. Published online:47-62. https://doi.org/10.2147/VMRR.S397590
Balakrishnan S, Rekha VB (2018). Herd immunity: an epidemiological concept to eradicate infectious diseases. J. Entomol. Zool. Stud., 6(9): 2731-2738.
Barteling SJ (2002). Development and performance of inactivated vaccines against foot and mouth disease: -EN- -FR- -ES-. Rev. Sci. Tech. OIE, 21(3): 577-588. https://doi.org/10.20506/rst.21.3.1361
Bazid AH, Amer HM, Nayel M (2023). Assessment of the potency and effectiveness of a heptavalent oil-adjuvanted (ISA 206) foot-and-mouth disease vaccine in Egypt. Arch. Virol., 168(2): 62. https://doi.org/10.1007/s00705-022-05624-2
Biogénesis B (2022). Biogénesis Bagó Foot and Mouth Disease Vaccine. Biogénesis Bagó; 2022. Accessed December 18.
Cloete M, Dungu B, Van Staden LI, Ismail-Cassim N, Vosloo W (2008). Evaluation of different adjuvants for foot-and-mouth disease vaccine containing all the SAT serotypes. Onderstepoort J. Vet. Res., 75(1): 17-31. https://doi.org/10.4102/ojvr.v75i1.84
Cirelli KM, Carnathan DG, Nogal B, (2019). Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell. 2019;177(5):1153-1171.e28. doi:10.1016/j.cell.2019.04.012
Diaz-San Segundo F, Medina GN, Ramirez-Medina E (2016). Synonymous Deoptimization of Foot-and-Mouth Disease Virus Causes Attenuation In Vivo while Inducing a Strong Neutralizing Antibody Response. Perlman S, ed. J. Virol., 90(3): 1298-1310. https://doi.org/10.1128/JVI.02167-15
Endris M, Feki E. 2021. Review on effect of stress on animal productivity and response of animal to stressors. J Anim Vet Adv., 20(1):1-14.
Ekanayaka P, Weerawardhana A, Chathuranga K, Park JH, Lee JS (2022). Foot-and-Mouth Disease Virus 3Cpro Cleaves BP180 to Induce Blister Formation. Viruses, 14(9): 2060. https://doi.org/10.3390/v14092060
Golde WT, Pacheco JM, Duque H (2005). Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: use in emergency outbreak response. Vaccine, 23(50): 5775-5782. https://doi.org/10.1016/j.vaccine.2005.07.043
Gunasekera U, Biswal JK, Machado G (2022). Impact of mass vaccination on the spatiotemporal dynamics of FMD outbreaks in India, 2008–2016. Transboundary Emerg. Dis., 69(5): e1936-e1950. https://doi.org/10.1111/tbed.14528
Habel J, Sundrum A (2023). Dairy Cows Are Limited in Their Ability to Increase Glucose Availability for Immune Function during Disease. Animals, 13(6): 1034. https://doi.org/10.3390/ani13061034
Ismail I, Indarjulianto S, Yusuf S, Purba FY (2023). Clinical Examination of Foot and Mouth Disease of Dairy Cows in Sukamurni, Cilawu, Garut, West Java, Indonesia. In: IOP Conference Series: Earth Environ. Sci., 1174: 012005. https://doi.org/10.1088/1755-1315/1174/1/012005
Kementerian Pertanian Republik Indonesia (2022). Keputusan Menteri Pertanian Republik Indonesia No. 510/KPTS/PK.300/M/6/2022 tentang Vaksinasi dalam Rangka Penanggulangan Penyakit Mulut dan Kuku (Foot-And-Mouth Disease), https://ditjenpkh.pertanian.go.id/uploads/download/surat-keputusan-menteri-pertanian-republik-indonesia-nomor-5001-tahun-2022-tentang-vaksinasi-dalam-rangka-penaggulangan-penyakit-mulut-dan-kuku-foot-and-mouth-disease-1658804951.pdf
Li K, Wang C, Yang F, Cao W, Zhu Z, Zheng H (2021). Virus–host interactions in foot-and-mouth disease virus infection. Front. Immunol., 12: 571509. https://doi.org/10.3389/fimmu.2021.571509
Ozturk N, Kocak O, Vosough Ahmadi B (2020). Economic Analysis of Increasing Foot-and-Mouth Disease Vaccination Frequency: The Case of the Biannual Mass Vaccination Strategy. Front. Vet. Sci., 7: 557190. https://doi.org/10.3389/fvets.2020.557190
Pedersen GK, Wørzner K, Andersen P, Christensen D. 2020. Vaccine Adjuvants Differentially Affect Kinetics of Antibody and Germinal Center Responses. Front Immunol. 11. doi:10.3389/fimmu.2020.579761.
Quishpe X, Recalde MM, Chuquimarca AC, Aranda JP, Guevara E, Robalino E (2020). Respuesta Inmunitaria de Autovacuna de Papiloma Virus en Bovino. In: Tendencias Actuales En Medicina Veterinaria y Zootecnia. Centro de Investigación y Desarrollo Ecuador.
Raina AA, Patel M, Somagond A (2023). Effect of foot-and-mouth disease vaccination on acute phase response and milk production in the Holstein-Friesian crossbred cow. J. Appl. Anim. Res., 51(1): 495-500. https://doi.org/10.1080/09712119.2023.2229409
Rivera-Villegas A, Carrillo-Muro O, Rodríguez-Cordero D (2024). Effects of Supplemental Calcium Propionate and Concentrate Level: Growth Performance, Body Fat Reserves, and Health of High-Risk Beef Calves. Vet. Sci., 11(8): 336. https://doi.org/10.3390/vetsci11080336
Rodríguez-Habibe I, Celis-Giraldo C, Patarroyo ME, Avendaño C, Patarroyo MA (2020). A comprehensive review of the immunological response against foot-and-mouth disease virus infection and its evasion mechanisms. Vaccines, 8(4): 764. https://doi.org/10.3390/vaccines8040764
Sansamur C, Arjkumpa O, Charoenpanyanet A, Punyapornwithaya V (2020). Determination of Risk Factors Associated with Foot and Mouth Disease Outbreaks in Dairy Farms in Chiang Mai Province, Northern Thailand. Animals, 10(3): 512. https://doi.org/10.3390/ani10030512
Sharma GK, Mahajan S, Matura R (2017). Herd Immunity Against Foot-and-Mouth Disease Under Different Vaccination Practices in India. Transbound Emerg Dis, 64(4): 1133-1147. https://doi.org/10.1111/tbed.12478
Singh RK, Sharma GK, Mahajan S (2019). Foot-and-Mouth Disease Virus: Immunobiology, Advances in Vaccines and Vaccination Strategies Addressing Vaccine Failures—An Indian Perspective. Vaccines (Basel), 7(3): 90. https://doi.org/10.3390/vaccines7030090
Smitsaart EN, Zanelli M, Rivera I (1998). Assessment using ELISA of the herd immunity levels induced in cattle by foot-and-mouth disease oil vaccines. Prev. Vet. Med., 33(1): 283-296. https://doi.org/10.1016/S0167-5877(97)00014-7
Sordillo LM (2016). Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci., 99(6):4967-4982. https://doi.org/10.3168/jds.2015-10354
Terry SA, Basarab JA, Guan LL, McAllister TA (2021). Strategies to improve the efficiency of beef cattle production. Can. J. Anim. Sci., 101(1): 1-19. https://doi.org/10.1139/cjas-2020-0022
Tizard IR (2021). Adjuvants and adjuvanticity. Vaccines for Veterinarians. Published online:75. https://doi.org/10.1016/B978-0-323-68299-2.00016-2
Ulziibat G, Raizman E, Lkhagvasuren A (2023). Comparison of vaccination schedules for foot-and-mouth disease among cattle and sheep in Mongolia. Front. Vet. Science, 10. https://doi.org/10.3389/fvets.2023.990043
Urrutia N, Bomberger R, Matamoros C, Harvatine KJ (2019). Effect of dietary supplementation of sodium acetate and calcium butyrate on milk fat synthesis in lactating dairy cows. J. Dairy Sci., 102(6): 5172-5181. https://doi.org/10.3168/jds.2018-16024
Vaz RZ, Dutra MMM, Pacheco RF, 2024. Factors intrinsic to handling and transport in pre-slaughter behavioural changes in beef cattle. Veterinary Research Communications. Published online. 1-6.
Xiao C, Rajput ZI, Hu S (2007). Improvement of a commercial foot-and-mouth disease vaccine by supplement of Quil A. Vaccine, 25(25): 4795-4800. https://doi.org/10.1016/j.vaccine.2007.04.027
Yang L, He K, Li J (2019). Correlation of Antibody Levels with Peripheral Lymphocyte Subsets and Routine Hematological Parameters after Vaccination with FMD Vaccine in Young Sires. PVJ, 39(02): 251-255. https://doi.org/10.29261/pakvetj/2019.045
To share on other social networks, click on any share button. What are these?






