Study on the Gonadosomatic Indices of Sea Urchin Echinometra mathaei in Persian Gulf, Iran
Study on the Gonadosomatic Indices of Sea Urchin Echinometra mathaei in Persian Gulf, Iran
Mousa Keshavarz1*, Ehsan Kamrani1, Narges Amrollahi Biuki1 and Hossein Zamani2
1Department of Marine Biology, Faculty of Marine Science and Technology, University of Hormozgan, Post Box: 3995 Bandar Abbas, Iran
2Department of Statistics, Faculty of Basic Sciences, University of Hormozgan, Post Box:3995 Bandar Abbas, Iran
ABSTRACT
The gonad developments of 217 sea urchins (Echinometra mathaei) were monitored monthly from March to September 2014 in the intertidal zones of Bandar Lengeh in the Persian Gulf. The numbers of males, females, unsexual samples were counted for 71, 126 and 20, respectively. Females were totally 1.77 times heavier than males. Wet weights of gonads were nearly 8.25% of the mean total wet body weights. Only females were observed by gonad wet weights more than 12 g. The gonad wet weights of females (3.74±3.26) was heavier than males (3.46±2.63). March to August was the spawning months, as a U-shaped trend by August (mid-summer) as its concave curve and it means that the most stores of gonads is in spring. The operational sex ratio (OSR) of E. mathaei was female-biased and was not significantly different during the study. The monthly adult sex ratio (ASR) was significantly different. It means that the sex ratio has no effects on OSR. Moreover, the gonadosomatic indices GSI 1, GSI 2, and GSI 3 were 8.2±0.6, 4.1±0.3 and 9.4±0.9, respectively. GSIs did not showed differ significantly in females; while in males, they were different. It was also obvious difference for males in spring and summer. All three indices were higher in the spring than summer (for example for GSI 2: 1.6 (August) ≤ 7.3 (March)).
Article Information
Received 16 July 2016
Revised 22 August 2016
Accepted 18 October 2016
Available online 02 May 2017
Authors’ Contributions
MK was the main investigator; he designed the study and laboratory experiment, collected data, statistically analysis and interpreted them and wrote the manuscript. EK and NAB revised the manuscript. HZ statistically checked the results. All of the authors read and approved the final manuscript.
Key words
Gonadosomatic index, Operational sex ratio, Echinometra mathaei, Sea urchin.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.3.923.933
* Corresponding author: [email protected]
0030-9923/2017/0003-0923 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
INTRODUCTION
Sea urchins are found widely in tropical and subtropical marine zones, although they can also be found in other places like Antarctic (for example Sterechinus neumayeri, Klinger et al., 1997). They have often been overfished in several countries in the last few decades (Siikavuopio, 2009). Their gonads, also known as roe or uni (Arafa et al., 2012) are popular luxury seafood in Asian, Mediterranean and some other countries (Unuma et al., 2002). The right color of the gonad is an important marketing factor (Lawrence, 2013) so that color measurements have been standardized. For example, Paracentrotus lividus (Lamarck) is preferred when it has yellow gonads which have a caviar-like appearance and a bittersweet flavor (Robbins et al., 1990).
In the Persian Gulf region the local urchins are not eaten locally. Moreover,they have been neglected and little research has been done, especially on Echinometra mathaei (Keshavarz, 2016).These are the dominant urchin found in patches along the rocky coasts of this area between the average low tide and a maximum depth of 10 m (McClanahan and Muthiga, 2013).
Echinometra mathaei is widely distributed species. It scrapes surfaces in the process of grazing (Coppard and Campbell, 2006). It has been studied because of its significant ecological role in coral reef environments like other species of the genus Echinometra. It was reported to have densities of 0.1 to 100 individuals m-2 in a limited area of New Caledonia in the South Pacific (Dumas et al., 2007). At high densities, their bioerosion role has been considered to be a limiting factor for the growth and survival of coral reef ecosystems (Bronstein and Loya, 2014).
Since sea urchins have separate sexes and fertilization is external, they release their gametes into the sea at spawning time via 5 visible gonopores on their external shells (Keshavarz, 2016). The growth and gonad size of sea urchins in primarily rocky coastal regions are affected by the abundance and species of algal foods (Walker et al., 2006). For example, growth and gonad production of Strongylocentrotus nudus, which is commercially harvested in northern Japan, are greatest in kelp beds, followed by fucoid beds and small perennial algal turf beds such as those formed by the red alga Chondrus ocellatus, and least in Crustose coralline dominated ‘‘barren’’ beds (Agatsuma, 1997; Sano et al., 2001; Agatsuma et al., 2005a; Nakabayashi et al., 2006).
Various gonadosomatic indices have been used to describe the nature of the annual cycles of a variety of marine invertebrates. These measurements, particularly when used in conjunction with histological techniques, have generally been used in characterizing the annual reproductive cycles of a number of species including urchins (Moore, 1934).
Operational sex ratio (OSR) defined by Emlen and Oring (1977) as the proportion of sexually mature males divided by the total number of sexually mature adults (Emlen and Oring, 1977; Kvarnemo and Ahnesjo, 1996) is a major factor influencing the intensity of sexual selection (Clutton-Brock and Parker, 1992) may help elucidate sex differences during the life-history of a population (Kokko et al., 2012). For example, in a population where 50% of males remain unmated, 50% mate once (Kokko et al., 2012). Any changes in OSR usually leads to increased competition for mates among all members of the more abundant sex, while members of the other sex may have a greater opportunity to exercise mate choice (Emlen and Oring, 1977).
The objective of the present study was to study the OSR and gonadosomatic indices of E. mathaei in the Bandar Lengeh area of the Persian Gulf.
MATERIALS AND METHODS
Study area
The study area was located in Bandar Lengeh, Iran, a small inlet along a rocky shore in the northern part of the Persian Gulf (26˚32ʹ28̋ N, 54˚52ʹ28̋ E; water depth: 0.5–1 m), covered with a high density of dominant macroalgae species of Sargassum wightii and Padina antillarum, from March to September, 2014. Due to the location of the studied area on subtropical zone, the first three months (March, April, May) were considered as spring while the next four months (June, July, August, September) were considered as summer season.
Laboratory measurements
Thirty one random size (height= [2.60 to 40.35 mm]) samples of urchins were collected individually each month using a handy stainless steel forceps in the intertidal zone during low tides of spring tides. They were transported in fiber tanks of sea water with continuous aeration by a portable aquarium air pump (Boyu D-200) of a 1.5V-waterproof battery as fresh E. mathaei to a marine biology laboratory in Hormozgan University. It is useful to mention that the temperature of sea water during the total sampling months were 22.46 to 34.05 ˚C.
Before their dissections, every urchin was placed on a paper towel for about 1–2 min to eliminate any surface water, and the attached algae and crushed seashells of the urchins were discarded. The total wet weight of the animal was measured using a portable electric stainless steel balance [model: the professional digital table top scale (weighing scale 0.01 - 500 g, 0.01 g precision)]. After measuring the weight, the dissection started. The spines of the urchins were removed by a slim pointed tip Lodestar stainless steel tweezers, L605014; the peristome was cut using a scalpel; the Aristotle’s lantern was removed using forceps, and finally the coelomic fluid was removed using a one-milliliter sterile Q Ject Ultra insulin syringe. Two small cuts were made on the sample shell by using a scissors to divide the shell into two parts. The contents of the digestive system were removed and the urchin rinsed several times using sterile natural sea water. Then, the gonads were separated from the shell using small forceps, and kept inside pre-weighed 10 cc glass sterile vials. The wet weights of the gonads were obtained. And finally, sex was determined by observing a small piece of gonad using the easily observed color differences between males and females
Important indices
As mentioned previously, OSR is one of the indicators on the study of sexual behaviors of animals that can be defined as follows (Emlen and Oring, 1977):
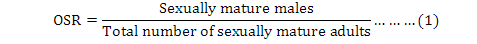
The above formula was used to determine the monthly trend of male adults in compared with the general adult population and specify monthly differences. In this study, the sex ratio (males/females) was also calculated monthly.
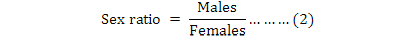
Moreover, the Gonadosomatic Indices (GSIs) were calculated for each individual (Martínez-Pita et al., 2008; Lozano et al., 1995) as equation 3 to 5, and then monthly and seasonally means and standard deviations were also investigated:
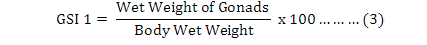

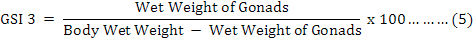
It is useful to mention that for achieving dry weights of each individual on equation 4, samples were put inside a 60 ˚C oven for 24 hours, and then they were weighted as “primary dry weights”. This step has repeated continuously until a fixed unchanged weight named “Dry Weight” was found for each individual sample in compare with previous step. It was found useful to measure and analyze the weight of the gonads (Feng et al., 2014) by equation 6, where “GMC” is gonad moisture content:
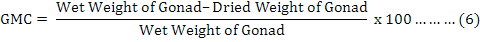
Statistical analysis
Statistical analysis was done using different software programs suitable for windows as the operating system: IBM SPSS statistics 32-bit version 22.0.0.0, MINITAB Inc. 814-238-328 version 11.12 32-bit, Microsoft office Excel (2007) and MATLAB R2014a (8.3.0.432) 64-bit, here after SPSS, MINITAB, Excel, MATLAB, respectively.
The gonad weights and total wet weights distributions were calculated by using SPSS (Mean ± Standard Deviation). The data outputs were also checked by Skewness to see whether the data was symmetric (-2≤Standard Error of Skewness≤2) or asymmetric (-2> Standard Error of Skewness>2).
The data checked for the homogeneity of the variance and normal distribution in SPSS. OSR, sex ratio and gonad indices were compared by one-way ANOVA. Chi-squared test and Tukey’s HSD were used to determine significant differences.
Finally, MINITAB was used to calculate the gonad moisture content index (GMC) for the two genders, Quartiles (Q1(xi) and Q3(xi)), Interquartile Range (IQR = Q3(xi) – Q1(xi)), and Range (R= Maximum (xi) – Minimum (xi)), where xi refer to males, females, or total data regards to equation 6.
RESULTS
Out of 217 E. mathaei, 71 were males, 126 were females and the genders of 20 of samples were not obvious, because they were small size without any gonad. The largest sample was 103.77 g while the smallest one was only 0.07 g.
Table I represents the most frequent data of each parameter and if they are symmetric by calculating Skewness. Multi-high frequencies were seen for wet weights of gonads. Figure 1 presents the distribution of the measured weights for all samples by considering their genders.
Figure 2 shows the monthly OSR of E. mathaei in October (0.46± 0.106), and April (0.11± 0.106). The adult sex ratios are significantly different between September and other months (P<0.05). There was significant deviation at overall sex ratio of E. mathaei from 1:1 (Table II) without any hermaphrodites samples.
Table I.- The weight of sea urchin and their gonads of E. mathaei, derived by SPSS analysis.
|
Parameter |
Weight of Wet Gonads (g) |
Total Weight (g) |
|
Mean ± SD |
3.3 ± 2 |
40 ± 21 |
|
Skewness |
1.4 |
0.083 |
|
Symmetric/Asymmetric |
Asymmetric |
Symmetric |
|
Most frequent data |
0.00–0.83, 2.51–3.34 |
35.0–45.0 |
Table III shows gonadosomatic indices separated by season and gender without including unsexual samples. The numbers of female, male and unsexual samples were 59, 31 and 7 in spring and 67, 40 and 13 in summer, respectively. Tukey’s test showed that in spring (March-May) there were no significant differences between GSI 1 and GSI 3 while in summer (June-September) it was found no significant differences among three gonadosomatic indices (GSI 1, GSI 2, GSI 3) (p>0.05).
Figure 2 also represents monthly changes in the gonadosomatic indices of E.mathaei of Bandar Lengeh. It is clear that maximum and minimum GSI 1 occurred in March and August, respectively. GSI 2 declined March to August. Maximum of GSI 3 is March, and GSI 3 gradually decreases until August. Broadly speaking, all 3 gonadosomatic indices decreases from April to August, and there is a slight increase in September.
A 3D scatter gonadosomatic indices of females and males are shown in Figure 3. In spring, gonadosomatic indices of females scattered more than summer (Fig. 3A). This feature can also be observed in males (Fig. 3B). Although in summer, gonadosomatic indices of females more scattered in compare with males.
Table IV shows that the mean of GMC has no significant differences between populations. In females, IQR for GMC were lower than the males. Since GMC is the ratio of the difference between wet and dry weight to wet weight, R as a difference between maximum and minimum of GMC shows lower values in males in compare with females which shows that less moisture of gonads of males in compare with females.
Table II.- Binomial test analysis for the sex ratio of on E. mathaei.
| No. | Observed prop. | Test prop. | Asymp. Sig. (2-sided) | |
| Females | 126 | 64.14 | 0.5 | 0.157 |
| Males | 71 | 30.86 | ||
| Total | 197 | 100 |
Table III.- The calculated GSI from March to September, 2014.
| GSI 1 | GSI 2 | GSI 3 | ||
| Season |
Spring (March-May) |
11 ± 6a |
5.5 ± 3b |
13 ± 7a |
|
Summer (June-September) |
3.6 ± 3b |
2.0 ± 2b |
3.8 ± 3b |
|
| Gender |
Female (126 samples) |
7.7 ± 5a |
4.0 ± 3a |
10 ± 8b |
|
Male (71 samples) |
8.6 ± 6a |
4.3 ± 4b |
8.7 ± 6a |
|
|
Total |
8.2±0.6a |
4.1 ± 0.3b |
9.4 ± 0.9a |
|
The different superscript alphabets above numbers in each rows show significant differences between rows (p<0.05).
Table IV.- The gonad moisture contents index (GMC) for the two genders of E. mathaei.
| Parameters | No. | Mean ± SD | Q1 | Q3 | IQR | R |
| Male | 71 | 74 ± 10 | 70 | 81 | 11 | 57 |
| Female | 126 | 75 ± 11 | 72 | 80 | 7.6 | 91 |
| Total | 217 | 68 ± 24 | 70 | 80 | 10 |
99 |
DISCUSSION
Sea urchins’ gonads have a positive potential for presentation of a valuable sea food and therefore the culture of sea urchin can be very important and lead to job creation and income for local people. In the discussion of sea urchin growing, gonad weights compared to the total weight of the body is of great importance. In Bandar Lengeh, according to Figure 1A, in the total weights less than 12 g, the gender was mostly unknown. Higher than 12 g, the gender was known. The highest frequency of the total weights referred to 40-44 g (both female and males). It was seen that the frequency of females was more than males in the total weights more than 70 g (70-92 g). It means that the females were heavier than males in studied samples. Figure 1B also shows the gonad weight was less than 16 g. Mostly, the frequency of the gonad wet weight is less than 10 g. Table I shows obviously that the mean wet weights of gonads was nearly 8.25% of the mean total body weight. The most frequent wet weights of gonads (female frequencies: 80, male frequencies: 60, unknown sex frequencies: 20) were observed in weights less than 4 g. A sharp drop of frequency was observed in gonad wet weights of 5-12 g. Only females were observed in gonad wet weights of 12-16 g. By looking carefully at Figure 1, it can be understand that total wet weight distribution is wider than gonad weight distribution. Briefly, the gonad wet weight distribution is not normal.
The GSIs as indicators are particularly helpful in identifying days and seasons of spawning, if some reasonable numbers of adults’ gonads collected each month over a year. In our study, the spawning period was determined March to August (Fig. 3). In Kenyan coral reef lagoons, effects of seasons on the reproduction of the Indo-Pacific echinoid Echinometra mathaei were studied by Muthiga and Jaccarini (2005) in 3 stations. In Kanamai station as an example, which is equivalent to GSI1 (equation 3) of the current study, a roughly U-shaped continuous trend with a minimum value of GSI can easily be seen in mid-summer (July-August) during different years (1986-1987, 1992-1994). The same rhythm is also exists in our studied months (Fig. 3, the dash line) by August as its concave curve. Although our studied months (March-September) do not cover the whole year but a slight increase can be seen in September (Fig. 3). This carefully proves that our studied months corresponded with the study of Muthiga and Jaccarini (2005). Moreover, on study of Paracentrotus lividus, the gradual maturation of gonads’ peak was seen in April. In P. lividus (Tomšić et al., 2010), the range of GSI1 was 1.33 (September) ≤ GSI1 ≤ 4.83 (April), while we have found it between 2.05 (August) and 14.09 (March). In another study of P. lividus (Murillo-Navarro and Jimenez-Guirado, 2012), the monthly variation of GSI1 peak value was produced in February and the lowest values were observed from October to January. In the study of Fabbrocini and D’Adamo (2010a), it was found more than 90% of GSI1 in P. lividus occurred on April without any decrease
Table V.- Echinometra mathaei spawning season.
| Location | Spawning months | References |
| Bandar Lengeh, Iran | March to August | Current study |
| Zanzibar Island, Tanzania | June to September | Bronstein and Loya (2014) |
| S.E. Honshu, Japan | January to September | Kobayashi (1969) |
| Minatogawa, Japan | May to December | Fujisawa and Shigei (1990) |
| Sakurajima (Kagoshima Bay), Japan | June to September | Fujisawa and Shigei (1990) |
| Shirahama (Kii Pen), Japan | July to August | Fujisawa and Shigei (1990) |
| Seto, Japan | July to August | Onoda (1936) |
| Sesoko Island, Japan | September to October | Arakaki and Uehara (1991) |
| Hawaii | January to December | Kelso (1971) |
| Gulf of Suez | July to September | Pearse (1969) |
| NW Red Sea | January to December | Pearse (1969) |
| Diani, Kanamai and Vipingo, Kenya | January to May & November to December | Muthiga (1996), Muthiga and Jaccarini (2005) |
| Eastern coast South Africa | January to March | Drummond (1995) |
| Rottnest Island | January to December | Pearse and Phillips (1968) |
until summer-time (50%). In the study of Bronstein and Loya (2014) on the coral reefs of Zanzibar, of E. mathaei reached an annual peak during August–September and was followed by a drop in the GSI value in October, indicating a single annual spawning event. GSI values reached their annual minimum from March through May, after which GSI build up was observed (Bronstein and Loya, 2014). Table V represents a very good comparison of spawning months of our study with other studies of E. mathaei in different locations. Again, it is valuable to remind that different factors effect spawning months, and in different locations through a year, their months’ variations are reasonable.
The factors that influence the timing of the reproductive cycle of Echinometra, however, are not well understood (Muthiga, 1996; Muthiga and Jaccarini, 2005). Pearse (1974) suggested that E. mathaei might have a restricted spawning period in the higher latitudes and continuous spawning throughout the year closer to the equator where the environmental factors, especially temperature, are presumed more stable. It was found that different complicated factors effect spawning time of Echinometra, which were mainly environmental parameters as temperature, salinity, light, availability of food, the population density, wave-swept areas or even pH in different studies. The seasonal reproductive pattern of E. mathaei on the Kenyan coast is closely correlated to seawater temperatures, which are influenced by the monsoons (McClanahan, 1988). Temperature variations may be the key factor in E. mathaei rather than an absolute temperature minimum or threshold for spawning (See Byrne, 1990; Siikavuopio et al., 2006, 2008) for further information). Carballeira et al. (2011) tested embryo-larval development (ELD) of sea urchin and its fertilization by a bioassays attempt to find out an optimum range of salinity (15-40.5 psu) with two species of Atlantic sea urchin: Arbacia lixula and Paracentrotus lividus. It was discovered wider salinity range for A. lixula (29-35.5 psu) than for P. lividus (29-33psu). Lessios (1981) postulated that salinity could act as a proximate cue for controlling the timing of spawning as it coincided with increased salinity for E. lucunter and E. viridis at Fort Randolph, Caribbean. Light is another factor which effects spawning time (e.g. Iliffe and Pearse, 1982). Temporal and spatial variability of reproductive conditions of sea urchins is closely related to trophic conditions (Scheibling and Hatcher, 2007). Fabbrocini and D’Adamo (2010b) have tested the effects of food on gametogenesis of Paracentrotus lividus and found that starvation significantly affected gametogenesis, whereas developing gametes were always observed in fed animals, whose GSI had doubled by the end of the four-week trial. For example, growth and gonad production were lower in beds dominated by D. divaricata and Laurencia spp. than in beds of small algae without defense chemicals (Agatsuma et al., 2005b). Moreover, the fertilization ability of gametes from starved urchins was significantly lower (Fabbrocini and D’Adamo, 2010b). Even more, availability of food for the larvae may be also an important factor in controlling spawning. Spawning occurs just prior to the peak of phytoplankton concentrations (Lessios, 1981) (See also Azad et al., 2011; Bayed et al., 2005). Lessios (1981) showed that spawning synchrony is tighter in sparsely populated populations of E. viridis and less important in densely populated populations of E. lucunter (See also Muthiga and Jaccarini, 2005). Spawning also occurs in E. lucunter during a discrete period from July to October in wave-swept areas, and occurred during several periods in populations living in calm waters (Lewis and Storey, 1984). pH, (e.g. Moulin et al., 2011), the farm effluents, organic pollution (Cook and Kelly, 2007) and contamination of heavy metals (e.g. Bayed et al., 2005) can also effect spawning time.
As it is evident, the cause of spawning in any region is faced with extremely complex parameters. Therefore, the comparison of results with other previous observations (the same as Table V) is not completely right and the details of how spawning occurs need to be checked. We have separated GSI values due to gender and seasons. Table III represents our calculated data of GSIs. The GSIs showed the higher values in spring, when Acinal wall of gonads were filled of both nutritive phagocytes and mature gametes. In summer, there is no significant difference between the indices spatially GSI1 (equation 3) and GSI2 (equation 4), then the most stores of gonads is in spring. Figure 3 is also confirmed this claim that after April, the amount of storage gonads is reduced sharply so that in August (mid-summer) it reaches its minimum value. This may refer to the environmental condition of this area, that spawning season starts at the begging of spring and ends in middle of summer. The indices GSI1 and GSI2 did not differ significantly in females by gender separation, while in males, they were different (Table III) and GSIs scatter graphs of samples (Fig. 3) also showed the obvious difference of males in spring and summer. We have followed the sexual relationships of GSI by OSR. Many species encounter spatial variation of OSR (Rohr et al., 2005). In a male-biased situation, it is often correct to follow the traditional approach of this assumption that finding mates for females are easy (Kokko et al., 2012) and females are selective (Balshine-Earn, 1996). A male-biased OSR can be affected by three major parameters: a) the adult sex ratio (Kvarnemo et al., 1995); b) the sex-specific time that individuals spend outside the mating pool and c) environmental factors (Kokko et al., 2012).
Sex ratio is important to the notion of echobiological and genetic balance for species in land and marine ecosystems. It provides information about the representations of sea urchin males and females (as the proportion of male to female, equation 2), and indicates the gender dominance species in a given population. The adult sex ratio is such as sex-biased mortality (Moore and Wilson, 2002), the primary sex ratio, and differential reproductive investment between the two sexes (Bateman, 1984). In this study, the sex ratio of E. mathaei, showed no significant differences in individual males and females by the Chi-square test (p=0.157 (P>0.05)). It means that there is no preference between males and females as the sex ratio and then no effects on OSR. The same result was found in other studies. No significant differences were found by Bronstein and Loya (2014), from a ratio of 1:1 for Echinometra sp. in Zanzibar. Zhao et al. (2010) also found no significant differences by the Chi-square test for Strongylocentrotus intermedius in China.
In the study of E. mathaei, we have found that the adult sex ratios, ASR, had significant differences between months (by ANOVA-test, P<0.05) but OSR had no significant differences between months (by ANOVA-test, P>0.05). Gianguzza et al. (2009) showed that the OSR had no significant differences (P>0.05 and P>0.001) for Paracentrotus lividus, the same as our study.
Therefore, OSR can be affected by two other parameters. The sex-specific time that individuals spend outside the mating pool, are like providing parental care (Kokko and Jennions, 2008) which is not included for a sea urchin, replenishing gamete supplies, regaining body condition (Clutton-Brock and Parker, 1992) or different mating behavior between sexes (Xiao and Kumar, 2004). Environmental factors are such as food supplies (Gwynne and Simmons, 1990) and hunger (Rowe et al., 1994), nesting sites, predators (Gianguzza et al., 2009), density of population (DeRivera, 2003; Gianguzza et al., 2007) or even temperature (which effects on breeding cycle; see Kvarnemo (1994) as described previously. As an example, the females of sea urchin Paracentrotus lividus were more vulnerable to the starfish Marthasteria glacialispredation at high densities of this starfish during the summer spawning period and therefore OSR is female-biased (Gianguzza et al., 2009). Or another example, if males are required to provide a nutrient high gift before mating (most likely food) then when the available nutrients are high, the OSR will be male biased because there are plenty of nutrients available to provide gifts. However, if nutrients is low, less males will be ready to reproduce, causing the population to have a female biased OSR. (See Gwynne and Simmons, 1990; Kvarnemo and Simmons, 1999). In this study of E. mathaei, since the standard definition of OSR is adult males to total adults (equation 1), it is assumed that OSR should be male-biased, but surprisingly it was found that OSR in this species is female-biased (male: 71; female: 126). It means that adult females are roughly 1.77 times in compare with adult males. In another study on Paracentrotus lividus, it was suggested that OSR depends on density, so that, at higher densities, there was a more equal sex ratio whereas at low density sites males of P.lividus were significantly more abundant (Gianguzza et al., 2007) and therefore OSR is male-biased. Another male-biased OSR for Strongylocentrotus franciscanus was also found at intermediate densities by the study of Levitan (2004). Levitan (2004) mentioned that at low and high densities, OSR is roughly 50% of total for both sexes. It means that the results of our study for E. mathaei, in Bandar Lengeh is completely opposite with those two studies of P.lividus and S. franciscanus. But on the other hand, by regarding to ANOVA-test, we had the same results as Gianguzza et al. (2009). Overall, due to no effects of sex ratio on OSR, more studies are required to check the effects of time spending outside mating pool and spatially environmental factors on OSR to understand why E. mathaei is female-biased in Bandar Lengeh, Iran.
CONCLUSIONS
In this study, we have collected random size of E. mathaei once per month (March – September, 2014), Bandar Lengeh, Iran. Their gonads were tested by two methods of operational sex ratio (OSR) and gonadosomatic indices. We have discovered out E. mathaei is female-biased and consequently finding mates for males is convenient. It was also found out March to August, 2014, as spawning months of this species. Since sex ratio has no effects on OSR, therefore, the other factors can affect OSR: 1) the time that individuals spend outside the mating pool; 2) regaining body condition; 3) different mating behavior between sexes; 4) environmental factors, which all are needed continuous studies during years on E. mathaei natural conditions of Bandar Lengrh; and finally, 5) different replenishing gamete supplies of females and males that can be more highlighted because of a female-biased OSR in E. mathaei.
ACKNOWLEDGEMENTS
A special acknowledgement is due to Dr. Maryam Soyuf Jahromi, Associate Professor of Physical Oceanography, Department of Marine Science and Technology, Hormozgan University, for her creativity and overwhelming enthusiasm in helping us with the MINITAB plots. We also thank her for her reading of the entire paper and her valuable suggestions and comments. An additional acknowledgement is needed for Professor Emeritus Joe M. Regenstein at Cornell University for major efforts to improve this manuscript’s English and scientific presentation.
Conflict of interest statement
We declare that we have no conflict of interest.
REFERENCES
Agatsuma, Y., 1997. Ecological studies on the population dynamics of the sea urchin Strongylocentrotus nudus. Scientific Reports of the Hokkaido Fisheries Experimental Station, Japan, pp. 443-457.
Agatsuma, Y., Sato, M. and Taniguchi, K., 2005a. Factors causing brown-colored gonads of the sea urchin Strongylocentrotus nudus in northern Honshu, Japan. Aquaculture, 249: 449-458. https://doi.org/10.1016/j.aquaculture.2005.04.054
Agatsuma, Y., Nakabayashi, N., Miura, N. and Taniguchi, K., 2005b. Growth and gonad production of the sea urchin Hemicentrotus pulcherrimus in the fucoid bed and algal turf in northern Japan. Mar. Ecol., 26: 100-109. https://doi.org/10.1111/j.1439-0485.2005.00046.x
Arafa, S., Chouaibi, M., Sadok, S. and Abed, A. El., 2012. The influence of season on the gonad index and biochemical composition of the sea urchin Paracentrotus lividus from the Golf of Tunis. Sci. World J., 2012: 1-8. https://doi.org/10.1100/2012/815935
Arakaki, Y. and Uehara, T., 1991. Physiological adaptations and reproduction of the four types of Echinometra
mathaei (Blainville). In: Biology of echinodermata (eds. Yanagisawa, T, Yasumasu, I., Oguro, C., Suzuki, N., Motokawa, T., ), A.A. Balkema, Rotterdam, pp. 105–112.
Azad, A. K., Pearce, C. M. and McKinley, R. S., 2011. Influence of microalgal species and dietary rations on larval development and survival of the purple sea urchin, Strongylocentrotus purpuratus (Stimpson, 1857). Aquaculture, 322: 210-217. https://doi.org/10.1016/j.aquaculture.2011.09.029
Balshine-Earn, S.,1996. Reproductive rates, operational sex ratios and mate choice in St Peter’s fish. Behav. Ecol. Sociobiol., 39: 107-116. https://doi.org/10.1007/s002650050272
Bateman, A. J., 1984. Intra-sexual selection in Drosophila. Heredity, 2: 349-368. https://doi.org/10.1038/hdy.1948.21
Bayed, A., Quiniou, F., Benrha, A. and Guillou, M., 2005. The Paracentrotus lividus populations from the northern Moroccan Atlantic coast: growth, reproduction and health condition. J. mar. Biol. Assoc. U.K., 85: 999-1007. https://doi.org/10.1017/S0025315405012026
Bronstein, O. and Loya, Y., 2014. Echinoid community structure and rates of herbivory and bioerosion on exposed and sheltered reefs. J. exp. mar. Biol. Ecol., 456: 8-17. https://doi.org/10.1016/j.jembe.2014.03.003
Byrne, M., 1990. Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol., 104: 275-289. https://doi.org/10.1007/BF01313269
Carballeira, C., Martín-Díaz, L. and DelValls, T., 2011. Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar. environ. Res., 72: 196-203. https://doi.org/10.1016/j.marenvres.2011.08.008
Clutton-Brock, T. H. and Parker, G. A., 1992. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol., 67: 437-456. https://doi.org/10.1086/417793
Cook, E. and Kelly, M., 2007. Enhanced production of the sea urchin Paracentrotus lividus in integrated open-water cultivation with Atlantic salmon Salmo salar. Aquaculture, 273: 573-585. https://doi.org/10.1016/j.aquaculture.2007.10.038
Coppard, S. E. and Campbell, A. C., 2006. Taxonomic significance of test morphology in the echinoid genera Diadema Gray, 1825 and Echinothrix Peters, 1853 (Echinodermata). Zoosystema, 28: 93-112.
DeRivera, C. E., 2003. Causes of a male-biased operational sex ratio in the fiddler crab Uca crenulata. J. Ethol., 21: 137-144.
Drummond, A.E., 1995. Reproduction of the sea urchins Echinometra mathaei and Diadema savignyi on the
South African coast. Mar. Freshwater Res., 46: 751–757. https:// doi.org/10.1071/mf9950751
Dumas, P., Kulbicki, M., Chifflet, S., Fichez, R. and Ferraris, J., 2007. Environmental factors influencing urchin spatial distributions on disturbed coral reefs (New Caledonia, South Pacific). J. exp. mar. Biol. Ecol., 344: 88-100. https://doi.org/10.1016/j.jembe.2006.12.015
Emlen, S. T. and Oring, L. W., 1977. Ecology, sexual selection, and the evolution of mating systems. Science, 197: 215-223. https://doi.org/10.1126/science.327542
Fabbrocini, A. and D’Adamo, R., 2010a. Histological examination of the gonads of Paracentrotus lividus (Lamarck, 1816) from the Southern Adriatic coast. Biol. Mar. Mediterr., 17: 272-273.
Fabbrocini, A. and D’Adamo, R., 2010b. Gamete maturation and gonad growth in fed and starved sea urchin Paracentrotus lividus (Lamarck, 1816). J. Shellf. Res., 29: 1051-1059. https://doi.org/10.2983/035.029.0407
Feng, W., Chang, Y., Zhao, C., Sun, P. and Wei, J., 2014. Effects of inbreeding on growth, gametogenesis, gonad production, quality and MYP expression in the sea urchin Strongylocentrotus intermedius. Aquacult. Int., 23: 903-912. https://doi.org/10.1007/s10499-014-9849-4
Fujisawa, H. and Shigei, M., 1990. Correlation of embryonic temperatures sensitivity of sea urchins with spawning
season. J. Exp. Mar. Biol. Ecol., 136: 123–139. https://doi.org/10.1016/0022-0981(90)90191-e
Gianguzza, P., Badalamenti, F. and Riggio, S., 2007. Operational sex ratio in the edible sea urchin Paracentrotus lividus at Ustica Island MPA(western Mediterranean, Italy). Biol. Mar. Mediterr., 14: 108-109.
Gianguzza, P., Badalamenti, F., Gianguzza, F., Bonaviri, C. and Riggio, S., 2009. The operational sex ratio of the sea urchin Paracentrotus lividus populations: the case of the Mediterranean marine protected area of Ustica Island (Tyrrhenian Sea, Italy). Mar. Ecol., 30: 125-132. https://doi.org/10.1111/j.1439-0485.2008.00267.x
Gwynne, D. T. and Simmons, L. W., 1990. Experimental reversal of courtship roles in an insect. Nature, 346: 172-174. https://doi.org/10.1038/346172a0
Iliffe, T. M. and Pearse, J. S., 1982. Annual and lunar reproductive rhythms of the sea urchin, Diadema antillarum (Philippi) in Bermuda. Int. J. Inverteb. Reprod., 5: 139-148. https://doi.org/10.1080/01651269.1982.10553463
Keshavarz, M. 2016. Reproductive cycle and biometerical parameters and feeding behavior in sea urchin Echinometra mathaei. Ph.D thesis, University of Hormozgan, Iran. Bandar Abbas.
Kelso, D.P., 1971. Morphological variation, reproductive periodicity, gamete compatibility and habitat specialization in two species of the sea urchin Echinometra in Hawaii. Ph.D Thesis, University of Hawaii.
Klinger, T., Lawrence, J. and Lawrence, A., 1997. Gonad and somatic production of Strongylocentrotus droebachiensis fed manufactured feeds. Bull. aquacul. Assoc., 1: 35-37.
Kobayashi, N., 1969. Spawning periodicity of sea urchins at Setto III. Tripneustes gratilla, Echinometra mathaei,
Anthocidaris crassipina and Echinostrephus aciculatus. Sci. Eng. Rev., 9: 254–269.
Kokko, H. and Jennions, M. D., 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol., 21: 919-948. https://doi.org/10.1111/j.1420-9101.2008.01540.x
Kokko, H., Klug, H. and Jennions, M. D., 2012. Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient and the scope for competitive investment. Ecol. Lett., 15: 1340-1351. https://doi.org/10.1111/j.1461-0248.2012.01859.x
Kvarnemo, C., Forsgren, E. and Magnhagen, C. 1995. Effects of sex ratio on intra- and inter-sexual behaviour in sand gobies. Anim. Behav., 50: 1455–1461. https://doi.org/10.1016/0003-3472(95)80002-6
Kvarnemo, C. and Ahnesjo, I., 1996. The dynamics of operational sex ratios and competition for mates. Trends Ecol. Evol., 11: 404-408. https://doi.org/10.1016/0169-5347(96)10056-2
Kvarnemo, C. and Simmons, L. W., 1999. Variance in female quality, operational sex ratio and male mate choice in a bushcricket. Behav. Ecol. Sociobiol., 45: 245-252. https://doi.org/10.1007/s002650050559
Kvarnemo, C., 1994. Temperature differentially affects male and female reproductive rates in the sand goby: consequences for operational sex ratio. Proc. R. Soc. Lond. B. Biol. Sci. 256: 151-156. https://doi.org/10.1098/rspb.1994.0063
Lawrence, J. M., 2013. Sea urchins: biology and ecology. Academic Press, San Diego, CA. pp. 135-154. https://doi.org/10.1016/B978-0-12-396491-5.00009-5
Lessios, H., 1981. Reproductive periodicity of the echinoids Diadema and Echinometra on the two coasts of Panama. J. exp. mar. Biol. Ecol., 50: 47-61. https://doi.org/10.1016/0022-0981(81)90062-9
Levitan, D. R., 2004. Density dependent sexual selection in external fertilizers: variances in male and female fertilization success along the continuum from sperm limitation to sexual conflict in the sea urchin Strongylocentrotus franciscanus. Am. Nat., 164: 298-309. https://doi.org/10.1086/423150
Lewis, J. and Storey, G. S., 1984. Differences in morphology and life history traits of the echinoid Echinometra lucunter from different habitats. Mar. Ecol., Prog. ser., 15: 207-211. https://doi.org/10.3354/meps015207
Lozano, J., Galera, J., Lopezl, S., Turon, X. and Morera, G., 1995. Biological cycles and recruitment of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats. Mar. Ecol. Prog. Ser., 122: 179–191. https://doi.org/10.3354/meps122179
Martínez-Pita, I., Sánchez-España, A.I. and García, F.J., 2008. Gonadal growth and reproduction in the sea urchin Sphaerechinus granularis (Lamarck 1816) (Echinodermata: Echinoidea) in southern Spain. Sci. Mar., 72:603–611.
McClanahan, T. R.,1988. Coexistence in a sea urchin guild and its implications to coral reef diversity and degradation. Oecologia, 77: 210-218. https://doi.org/10.1007/BF00379188
McClanahan, T.R. and Muthiga, N.A. 2013. Echinometra. In: Sea Urchin: Biology and ecology (ed. J.M. Lawrence) Academic Press, Florida, MA, pp. 337–353. https://doi.org/10.1016/B978-0-12-396491-5.00023-X
Moore, H. B., 1934. A comparison of the biology of Echinus esculentus in different habitats. J. mar. Biol. Assoc. U.K., 19: 869-885. https://doi.org/10.1017/S002531540004683X
Moore, S. L. and Wilson, K., 2002. Parasites as a viability cost of sexual selection in natural populations of mammals. Science, 297: 2015-2018. https://doi.org/10.1126/science.1074196
Moulin, L., Catarino, A. I., Claessens, T. and Dubois, P., 2011. Effects of seawater acidification on early development of the intertidal sea urchin Paracentrotus lividus (Lamarck 1816). Mar. Pollut. Bull., 62: 48-54. https://doi.org/10.1016/j.marpolbul.2010.09.012
Murillo-Navarro, R. and Jimenez-Guirado, D., 2012. Relationships between algal food and gut and gonad conditions in the Mediterranean sea urchin Paracentrotus lividus. Mediterr. Mar. Sci., 13: 227-238. https://doi.org/10.12681/mms.302
Muthiga, N. A., 1996. The role of early life history strategies on the population dynamics of the sea urchin Echinometra mathaei (de Blainville) on reefs’ in Kenya, University of Nairobi, Mombasa.
Muthiga, N. and Jaccarini, V., 2005. Effects of seasonality and population density on the reproduction of the Indo-Pacific echinoid Echinometra mathaei in Kenyan coral reef lagoons. Mar. Biol., 146: 445-453. https://doi.org/10.1007/s00227-004-1449-9
Nakabayashi, N., Miura, N., Agatsuma, Y. and Tanigushi, K., 2006. Growth and gonad production of the sea urchin Strongylocentrotus nudus in relation to rarine algal communities along the Japan sea coast of Akita Prefecture, Northwestern Japan. Aquacult. Sci., 54: 365-374.
Onada, K., 1936. Notes on the development of some Japanese echinoids with special reference to the structure of the larval body. Jap. J. Zool., 6: 635–654.
Pearse, J.S. and Phillips, B.F., 1968. Continuous reproduction in the Indo-Pacific sea urchin Echinometra mathaei at
Rottnest Island, Western Australia. Aust. J. Mar. Freshwater Res., 19: 161–172. https:// doi.org/10.1071/mf9680161
Pearse, J.S., 1969. Reproductive periodicities of Indo-Pacific invertebrates in the Gulf of Suez II. The echinoid
Echinometra mathaei (de Blainville). Bull. Mar. Sci., 19: 580–613.
Pearse, J.S., 1974. Reproductive patterns of tropical reef animals: three species of sea urchins. Proceeding of 2nd International Coral Reef Symposium, Brisbane, Australia, pp. 235-240.
Robbins, N.J., McKeever, T.J. and Trenholm, R., 1990. Developing a Newfoundland sea urchin fishery. Trinity-Placentia Development Association. Newfoundland, Canada. Newfoundland Inshore Fisheries Development Agreement.
Rohr, J. R., Park, D., Sullivan, A. M., McKenna, M., Propper, C. R. and Madison, D. M., 2005. Operational sex ratio in newts: field responses and characterization of a constituent chemical cue. Behav. Ecol., 16: 286-293. https://doi.org/10.1093/beheco/arh164
Rowe, L., Arnqvist, G., Sih, A. and Krupa, J.J., 1994. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol., 9: 289-293. https://doi.org/10.1016/0169-5347(94)90032-9
Sano, M., Omori, M., Taniguchi, K. and Seki, T., 2001. Age distribution of the sea urchin Strongylocentrotus nudus (A. Agassiz) in relation to algal zonation in a rocky coastal area on Oshika Peninsula, northern Japan. Fish. Sci., 67: 628-639. https://doi.org/10.1046/j.1444-2906.2001.00299.x
Scheibling, R. E. and Hatcher, B. G., 2007. Ecology of Strongylocentrotus droebachiensis. In: Edible sea urchin: Biology and ecology (ed. J.M. Lawrence) Tampa, Florida, The Netherlands: Elsevier Science, 37: 353-392.
Siikavuopio, S. I., 2009. Green sea urchin (Strongylocentrotus droebachiensis), Müller in aquaculture: the effects of environmental factors on gonad growth. Ph.D thesis, University of Tromsø, Tromsø, Norway.
Siikavuopio, S. I., Mortensen, A. and Christiansen, J. S., 2008. Effects of body weight and temperature on feed intake, gonad growth and oxygen consumption in green sea urchin, Strongylocentrotus droebachiensis. Aquaculture, 281: 77–82. https://doi.org/10.1016/j.aquaculture.2008.05.033
Siikavuopio, S. I., Christiansen, J. S. and Dale, T., 2006. Effects of temperature and season on gonad growth and feed intake in the green sea urchin (Strongylocentrotus droebachiensis). Aquaculture, 255: 389-394. https://doi.org/10.1016/j.aquaculture.2005.12.021
Tomšić, S., Conides, A., Dupčić Radić, I. and Glamuzina, B., 2010. Growth, size class frequency and reproduction of purple sea urchin, Paracentrotus lividus (Lamarck, 1816) in Bistrina Bay (Adriatic Sea, Croatia). Acta Adriat., 51: 67–77.
Unuma, T., Exton, P. and Balkema, A., 2002. Gonadal growth and its relationship to aquaculture in sea urchins. The sea urchin: from basic biology to aquaculture. Swets & Zeitlinger, Liss, The Netherlands. pp. 115-127.
Walker, C., Unuma, T. and Lesser, M., 2006. Gametogenesis and reproduction of sea urchins. In: Edible sea urchin: Biology and ecology (ed. J.M. Lawrence), Elsevier Science, The Netherlands, MA, pp. 11-33.
Xiao, Y. and Kumar, M., 2004. Sex ratio, and probability of sexual maturity of females at size, of the blue swimmer crab, Portunus pelagicus Linneaus, off southern Australia. Fish. Res., 68: 271-282. https://doi.org/10.1016/j.fishres.2003.11.012
Zhao, C., Zhang, W., Chang, Y. and Liu, P., 2010. Test and gonad characteristics in different genders of cultivated sea urchins (Strongylocentrotus intermedius, Agassiz): First insight into sexual identification. Afr. J. Biotechnol., 9: 7560-7563. https://doi.org/10.5897/AJB10.1332
To share on other social networks, click on any share button. What are these?










