Studies on Antimicrobial Properties of Neem (Azadrichta indica) Seed Oil
Research Article
Studies on Antimicrobial Properties of Neem (Azadrichta indica) Seed Oil
Hailemariam Assefa1*, Tesfahun Dagnaw2
1Department of Chemistry, Mekdela Amba University, Ethiopia; 2Department of Chemistry, Wolaita Sodo University, Ethiopia.
Abstract | This study aimed to determine the in vitro antimicrobial activities of Neem seed oil by r disk diffusion method. Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus and Streptococcus pyogenes were used as tested bacterial species whereas Yeast, Mold and Dandruff were selected as tested fungi. The effect was seen high on Staphylococcus aureus, while the Klebsiella pneumoniae was less affected by any of the used extracts. Methanolic extract was more active than petroleum ether and ethyl acetate extract on all three tested fungal species. The oil was obtained by soxhlet extraction method. The maximum oil yield using petroleum ether was found 18.4% at a temperature of 70oC and a minimum yield of 13.5% at 30oC while at the same operating condition ethyl acetate and methanol resulted a maximum yield of 17.0% and 16.5% and a minimum of 12.0% and 13.0% respectively. So petroleum ether is a good solvent to afford high yield in this work. The oil was analyzed by GC-MS and result in the identification of the compounds, (Palmitoleic acid methyl ester, Palmitic acid methyl ester, Linoleic acid methyl ester, Oleic acid methyl ester, Stearic acid methyl ester and etc) was obtained. In the present study various physical and chemical characteristics have been also studied. The results also indicated that the oil extracted from Neem seed is the most acidic and non edible.
Keywords | In vitro antimicrobial, Paper disk diffusion, Neem seed, Soxhlet extraction, GC-MS, non-edible, Identification of the compounds
Received | April 08, 2021; Accepted | June 21, 2021; Published | January 04, 2022
*Correspondence | Hailemariam Assefa, Department of Chemistry, Mekdela Amba University, Ethiopia; Email: haileassefa100@gmail.com
Citation | Assefa H, Dagnaw T (2022). Studies on antimicrobial properties of neem (Azadrichta indica) seed oil. Adv. Anim. Vet. Sci. 10(2): 244-252.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.2.244.252
ISSN (Online) | 2307-8316
INTRODUCTION
Historically, plants have provided a source of inspiration for novel drug compounds, as plant derived medicines which have made large contributions to human health and well-being. Although many drugs that come from trees generally have been replaced by more potent synthetic ones, trees remain a source for some drug ingredients. Medicinal plants have become important for the treatment of different disease conditions, such as diabetes, malaria, anemia for a long time now, but the potential of higher plants as source for new drugs is still largely unexplored. Systematic screening of them may result in the discovery of novel effective compounds (Uwimbabazi et al., 2015).
Infectious disease caused by bacteria, fungi, viruses and parasites are still a major threat to human and animal health. The impact is particularly large in developing countries due to lack of availabilities of modern medicines and the emergence of widespread drug resistance (Alberto et al., 2009). The development of drug resistance as well as lack of availabilities of certain antibiotics has led to the search of new antimicrobial agent mainly among plant extracts with the goal to discover new chemical structures, which overcome the above disadvantages (Benharref et al., 2006). Current research on natural molecules and products primarily focuses on natural plants since they are locally available, less costly and can be selected based on their ethno-medicinal uses (Arora and Kaur, 2007). Natural products perform various functions, and have interesting and useful biological activities (Adam et al., 1991). There are more than 35,000 plant species being used in various human cultures around the world for medicinal purpose (Jantan, 1998).
Among many well-known medicinal plants, Neem (Azadirachta indica) is one of the plants that have been identified in curing various infections. The plant Neem belongs to the big mahogany family of Meliaceae and the genus Azadirachta of A. indica. This plant was originally from India but now it has wide distribution in huge numbers in tropical, subtropical, semi-arid and wet-tropical regions of the world. The roots, stems, barks, leaves, seeds, flowers and fruits of A. indica have chemically bioactive substances such as peptides, alkaloids, tannins, phenols, sterols, flavonoids and glycosides that contribute in fighting against the bacteria. Among all the parts of A. indica, the seeds are listed as one of the most popular source of medicaments in antibacterial activity as the oil contains extensive spectrum against antibacterial infections (Jessinta et al., 2013).
Medicinal plants are part and parcel of human society to combat diseases, from the dawn of civilization. Azadirachta indica A. juss (syn. Melia azadirachta) is well known in India and its neighboring countries as one of the most versatile medicinal plants (Susmithaet al., 2013). Neem oil and the bark and leaf extracts have been therapeutically used as folk medicine to control leprosy, intestinal helminthiasis, respiratory disorders, and constipation and also as a general health promoter. Its use for the treatment of rheumatism, chronic syphilitic sores and indolent ulcer has also been evident. Neem oil finds use to control various skin infections. Bark, leaf, root, flower and fruit together cure blood morbidity, biliary afflictions, itching, skin ulcers, burning sensations and pthysis (Kausik et al., 2002).
Various parts of Neem have been extensively used to treat human ailments since ancient time. For example, the Neem twigs have been used by millions of people for cleaning teeth. Neem has been used as a deterrent for smallpox and other infectious diseases and is known to possess potent anti-plasmodial activity. The plant has also been used for urinary tract problems, leprosy, gastrointestinal problems, hair problems, ulcers, diabetes, and blood pressure. Traditionally, the plant is known to possess potential to ward off evil spirits (Martin and Macleod, 1934). The Neem leaves are known to possess 0.13% essential oil that provides smell to the leaves (Light et al., 1977). Two major classes of phytochemicals (i) isoprenoids and (ii) non-isoprenoids have been isolated from different parts of Neem. The most common isoprenoids include diterpenoids, triterpenoids, vilasinins, limonoids, and C-secomeliacins. The nonisoprenoids include proteins, polysaccharides, sulphur compounds, polyphenolics, dihydrochalcone, coumarin, tannins and aliphatic compounds (Beith, 1999).
Plant oils are oils derived from plant sources, as opposed to animal fats or petroleum. Oils derived from plants have been used for thousands of years. Plant oils have been a healthy alternative to animal derived oils since their discovery. Plant oils are composed of compounds called triglycerides; which may exist in a highly unsaturated form or less (Ahmedet et al., 1989).
Vegetable oils are substances derived from oil plants, they are composed of triglycerides which contain primarily polyunsaturated and monounsaturated fatty acids. Oil is extracted primarily from seeds. However, crude oil obtained needs to be refined in order to transform it into a range of useful products for industry and consumers. Oils improve flavor, lubricity, texture, and satiety to foods. They have also been found to have a major role in human nutrition. Oils and fats contain fatty acids essential for health that are not manufactured by the human body (O’Brien, 2009).
There are broadly two classes of plant oils: Essential oils and fixed oils. Essential oils are volatile, and are usually derived from the non-seed parts of the plants. Most fixed oils are the so-called fatty oils, and a majority of the fatty oils are derived from the seeds hence the term oilseeds, meaning oil-bearing seeds. Some of the fixed oils are derived from vegetables and nuts (Boby, 2000). Factors that determine the composition and yield of the essential oil obtained are numerous. In some instances it is difficult to segregate these factors from each other, since many are interdependent and influence one another (Terblanche, 2000). These variables may include seasonal and maturity variation, geographical origin, genetic variation, growth stages, part of plant utilized and postharvest drying and storage (Marotti et al., 1994). Sesonal and maturity variations. These two factors are interlinked with each other, because the specific on to genic growth stage will differ as the season progresses. There are variations in the chemical profile of essential oils from various plants collected during different seasons. The essential oils yields varied considerably from season to season and was also influenced by the micro-environment (sun or shade) in which the plant was growing. Results obtained by Badi (Sanguanpong, 2010) also indicated that timing of harvest is critical to both yield and oil composition. There are many reports in the literature showing the variation in the yield and chemical composition of the essential oil with respect to geographical regions (Uribe-Hernandez et al., 1992). Variations in the yield and chemical profile of essential oils, collected from different geographical locations, respectively. Such differences could be linked to the varied soil textures and possible adaption response of different populations, resulting in different chemical products being formed, without morphological differences being observed in the plants (Hussain et al., 2008). Genotype is typically defined as the genetic make-up of an organism, as characterized by its physical appearance or phenotype, while chemotype is generally defined as a group of organisms that produce the same chemical profile for a particular class of secondary metabolites. Genetic makeup of the plant is one of the most important contributors to their essential oil composition (Abdullah, 2009). Other factors which affect the growing plants thus leading to variations in oil yield and composition, include part of plant used; post-harvest drying; length of exposure to sunlight, availability of water, height above sea level, plant density, time of sowing and the presence of fungal diseases and insects. The oil composition and yield may also change as a result of the harvesting methods used, the isolation techniques employed, the moisture content of the plants at the time of harvest and the prevailing extraction conditions (Abdullah, 2009). This study aimed to determine the in vitro antimicrobial activities of Neem seed oil by paper disk diffusion method and also to find the compounds that exist in Neem seed oil by GC-MS.
MATERIALS AND METHODS
Materials
Soxhlet extractor, dry oven (DAIHAN Scientific natural flow type oven, Japan),analytical balance (RADWAG; PS 360/C/1, China), heating mantle, rotary evaporator (RE-2S-VD, German), petri-dishes, laminar flow, autoclave, Soxhlet extractor, dry oven (DAIHAN Scientific natural flow type oven, Japan),analytical balance (RADWAG; PS 360/C/1, China), sieve, heating mantle, thermometer, rotary evaporator (RE-2S-VD, German), pH meter (HI 99161, China), refractometer (HI 96841 Beer Refractometer, India), GC-MS (Agilent Technologies 7890B-5977A, Chin).
Chemicals (Reagent)
Petroleum ether, methanol, ethyl acetate, starch, DMSO, gentamicine, n-hexane, ethanol, chloroform, petroleum ether, methanol, ethyl acetate, phenolphthalein, bromophenol, starch, DMSO.
Material preparation
Neem seed used in this study was obtained from Bahir Dar. Prior to use the Neem seeds was repeatedly washed to remove dirty and other impurities. Then, Neem seeds were ground to different particle sizes.
Growth media
Muller Hinton agar media (CM00337, India) is the best growth media for bacteria. The media was composed of Beef extract 2.0 g, Agar 17.0 g, acid hydrolysate of casein 17.5 g, starch 1.50 g, and distilled water 1000 mL. One liter media was prepared by dissolving 38 g of nutrient agar in 700 mL of distilled water. After complete dissolution, the final volume of the media was raised to 1000 mL by adding more distilled water. The media was boiled using a hot plate. The pH was adjusted to 7.0 at 25 ºC, using 0.1M NaOH and 0.1M HCl. The needed media and all glassware were sterilized through autoclaving at 15psi at 121 ºC for 20 minutes.
Potato dextrose agar (PDA) medium (CM0139, India) was used for growth and maintenance of the fungal species. The medium composed of 0.4% potato starch, 2.0% dextrose, 1.5% agar and the PH was adjusted to 5.8. Ten (10 mL) of the medium was then taken into 15mL autclavable screw caped Falcon tubes and autoclaved at 121ºC for 15 minutes under pressure.
Antibacterial and antifungal activity
The 20mL of nutrient agar was plated in Petri dishes and allowed to solidify for 30 minutes. Wells of 6mm in diameter and about 2cm apart was punctured in the culture media using sterile cork borers to make three to five uniform wells in each petri dish. A drop of molten muller hinton agar was used to seal the base of each petri dish well (Adam et al., 1991). The antibacterial activities were determined after 24 hours at 32ºC incubation in the incubator. The diameter of zone of inhibition produced by the extract on two gram negative bacteria: Escherichia coli, Klebsiella pneumoniae and two gram positive bacteria: Sterptococcus pyogenes, Staphylococcus aureus and there fungi species: Yeast Mold and Dandruff was measured and compared with standard. Each sample was used in triplicate for the determination of both antibacterial and antifungal activity. The work was carried out according to methods described by (Adam et al., 1991).
Sample analysis
Determination of moisture content of the seeds
The cleaned sample was weighed and dried under shaded area until a constant weight was obtained. The percentage moisture in the kernel was calculated in accordance to methods described by (Adam et al., 1991).
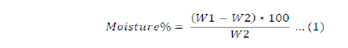
Where; W1 = Original weight of the sample before drying; W2= Weight of the sample after drying.
Size reduction and sieve analysis of the seeds
The dried Neem seed was crushed by mechanical grinder (Retsch GmbH). The sample was sieved using set of sieves sizes arranged in descending order 1mm, 500µm and 200µm to obtain particular sizes of 500-1000 µm, 200-500 µm and ≤ 200 µm. This is because to investigate the effect of particle size on yield and quantity of oil.
Extraction procedure
Neem seeds powder were weighed and put into the thimble of the soxhlet extractor. 1:10 mL of the solvent (petroleum ether, ethyl acetate and methanol) was measured with a measuring cylinder and poured into the still pot of the soxhlet extractor. The heat source was a heating mantle operating at a different temperature level. The solvent evaporated through the distillation path, thimble and the expansion adapter after which it condensed at the condenser unit of the soxhlet extractor. At this point the condensed vapour returned to the thimble as liquid droplets and got in contact with the sample therein. When the solvent in the thimble rose to the level of the siphon top, the entire content of the thimble and siphon was emptied back into the still pot of the soxhlet extractor. The process was repeated several refluxes till the extraction process were completed. Temperature was regulated using a thermometer. It was done according to methods described by (Adam et al., 1991).
Determination of the yield of neem oil extracted
The percentage of oil extracted was determined as:
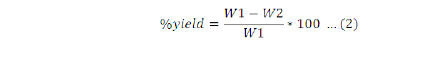
Where, W1= the sample powder placed in the thimble; W2= cake weight.
Determination of the chemical properties of neem oil
Determination of saponification value
This parameter is defined as the weight of potassium hydroxide, in milligrams, needed to saponify one gram of oil. The method for SV determination is based on the oil sample saponification by refluxing with a known excess of alcoholic potassium hydroxide solution. The alkali required for saponification is determined by titration of the excess potassium hydroxide with standard hydrochloric acid; in the presence of phenolphthalein as indicator. A blank is also simultaneously prepared 2 g of crude oil was added to 250 mL conical flask and 0.6 g of KOH was added to 30 mL of rectified spirit (absolute alcohol). Similarly, blank solution (all chemicals except oil) was prepared in another conical flask. Reflux condenser was attached to both sample and refluxed for 25 min while stirring. After completion of refluxing, 5 drops of phenolphthalein indicator wereadded, while it was cool, titrated against 0.5 M HCl to the end point. It was done according to method described by (Gohari et al., 2011). S.V was calculated using the following formula:
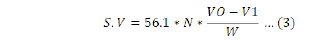
Where: N= concentration of Hydrochloric acid; V0 = volume of HCl before titration; V1 = volume of HCl used after titration; W= Weight in gram of sample taken.
Determination of acid value
Acid values are used as an indicator for edibility or otherwise of oils and suitability for use in industries. Oil (10 g) was added to 250 mL and 50 mL of neutral alcohol was added. Then the mixture was heated to 70 oC for 3 minutes. Phenolphthalein indicator (5 drops) was added to the prepared solution and titrated against 0.1 N NaOH to the end point, which is appearance of pink color. Similarly, blank solution was prepared and titrated against 0.1 N NaOH to the end point in accordance to standard methods described by (Sadoudi and Ali, 2017). Acid value was calculated using the following formula:
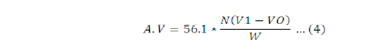
Where; N= concentration of NaOH; V0= volume NaOH used for the blank; V1= volume of NaOH used for sample; W= Weight of sample taken.
Determination of iodine value
The iodine value (IV) indicates the degree of unsaturation of the oil. It is defined as the number of grams of iodine absorbed by 100 grams of oil.A Neem oil (0.3 g) with 10 mL carbon tetrachloride was dissolved in 250 mL. 20 mL of 0.2 N Wijs reagents, which is prepared by dissolving 8 g iodine trichloride in a mixture of 150 mL glacial acetic acid and 10 mL of carbon tetrachloride, were added and mixed after it was plugged with stopper. Then, the mixture was placed in dark at room temperature for 30 min and 15 mL of 10% potassium iodine solution and 100 ml distilled water was added. 4 drops of 1% starch indicator was added and titrated against 0.1 M sodium thiosulfate solution till the disappearance of blue color. The color of samples with starch was dark-blue color and titrated till straw color appears.This method was carried as stated in (Sadoudi and Ali, 2017).
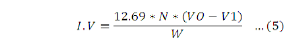
Where; N= concentration of sodium thiosulfate; Vo= volume of sodium thiosufate used for blank; V1= volume of sodium thiosufate used for sample; W= Mass of the sample.
Determination of peroxide value
The PV of an oil or fat is used as a measurement of the extent to which rancidity reactions have occurred during storage. A. indica oil weighing 5 g was added to 250 mL conical flask and 30 mL of solvent (acetic acid chloroform) was added. Then 0.5 mL of KI, 30 mL of distilled water was added to the mixtures. A few drops of starch were added to insure the presence of free iodine by the appearance of blue color. The mixture was titrated by 0.1N sodium-thiosulfate until the blue color just vanishes. Similarly, blank solution (all chemicals except oil) was prepared in another conical flask. It was carried out according to methods described by (Sadoudi and Ali, 2017). P.V was calculated using the following formula:
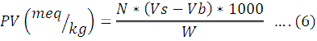
Where: N= concentration of sodium thiosulfate; Vo= volume of sodium thiosufate used for blank; V1= volume of sodium thiosufate used for sample; W= Mass of the sample
Gas chromatography-mass spectroscopy
Analysis of the Neem oil was done using Gas Chromatography with Mass spectrometer to know the composition of oil. Gas Chromatography Mass Spectrometry (GC-MS) is a method that combines the features of liquid chromatography and mass spectrometry to identify different substances within a test sample.
RESULTs AND DISCUSSION
Antibacterial and antifungal activity
Azadirachta indica extracts used in this study had shown an antibacterial effect as presented in different tables used in results interpretation below, but the effect was seen on Staphylococcus aureus highly, while the Klebsiella pneumoniae was less affected by any of the used extracts, as shown in Table 1. This might be due to the fact that K. pneumoniae can alter their genetic makeup with astonishing rapidity. In general, gram negative bacteria show resistance to antibiotics because of their cell wall. Resistant bacteria change their cell walls slightly, so the antibiotics cannot attach or they produce enzymes to disable the antibiotics, so the K. pneumoniae might have done the same and consequently, Azadirachta indica (Neem) extracts show less effect on it. Methanol extract were more effective against S.aureus compared to petroleum ether and ethyl acetate extracts. In all cases, methanol extracts were the best in terms of effectiveness against S. aureus.
Azadirachta indica extracts used in this study had shown also an antifungal effect as presented Table 2 below, but the effect was seen on Yeast highly by methanol and ethyl acetate extract, while the Yeast was less affected by petroleum ether as shown Table 2.
For all extracts used during this study, the bactericidal and fungicidal activity increased with the increase of the extract concentration, this means that the inhibition zone was higher on plates that contain extract with low dilution factor, as shown in Tables 1 and 2. This show that extract of plants inhibit the growth of various microorganisms at different concentrations. This is similar to the results recorded in this study where the increase in the concentration of extracts corresponded to the increase of diameter of inhibition zones.
Table 1: Mean zones of inhibition of different extracts of Neem oil at different concentrations against both gram negative and positive bacteria.
| Test bacteria | Conc (µg/ml) | Mean zone of inhibition ± S.D (mm) | P values | ||
| PE | EtOAc | ME | |||
| Escherchia coli |
500 | 7±2.0 | 7.3±2.1 | 14.3±2.5 | 0.016 |
| 400 | 4.3±1.5 | 5.0±1.7 | 10.7±2.1 | 0.000 | |
| 300 | 2.3±2.1 | 3.0±2.6 | 7.0±1.7 | 0.000 | |
| 200 | Growth | 1.0±1.7 | 3.0±2.6 | 0.000 | |
| 100 | Growth | Growth | Growth | 0.002 | |
| Klebsiella pneumoniae |
500 | 5.3±1.5 | 5.7±1.5 | 7.3±1.5 | 0.012 |
| 400 | 2.3±2.1 | 2.7±2.3 | 4.7±0.6 | 0.000 | |
| 300 | Growth | 1.0±1.7 | 1.7±2.8 | 0.000 | |
| 200 | Growth | Growth | Growth | 0.000 | |
| 100 | Growth | Growth | Growth | 0.000 | |
| Staphylococcus aureus |
500 | 12.3±4.2 | 11.3±4.0 | 17.3±6.1 | 0.002 |
| 400 | 7.3±2.3 | 6.7±2.5 | 9.7±1.15 | 0.000 | |
| 300 | 4.0±1.0 | 3.3±2.9 | 6.3±0.6 | 0.003 | |
| 200 | Growth | 1.3±2.3 | 4.7±0.5 | 0.000 | |
| 100 | Growth | Growth | Growth | 0.000 | |
|
Streptococcus pyogenes |
500 | 8.7±1.5 | 8.3±2.1 | 11.3±2.5 | 0.013 |
| 400 | 5.0±1.0 | 5.7±1.2 | 6.3±1.5 | 0.003 | |
| 300 | 2.3±2.1 | 1.3±2.3 | 3.0±2.6 | 0.002 | |
| 200 | Growth | Growth | Growth | 0.000 | |
| 100 | Growth | Growth | Growth | 0.000 | |
In this work the drug reference for anti-bacterial test was Gentamicin at 0.3%w/vas a positive control and DMSO as a negative control. Gentamicin has 27, 17, 29 and 19mm zone of inhibition on E. coli, K. pneumoniae, S. aureus and S. pyogenes respectively. While DMSO has no activity on the above four bacterial species, i.e. it has zero zone of inhibition (as a negative control).
In this study the negative results of antibacterial activity of plants did not mean absence of bioactive constituents nor that is the plant inactive. Active compounds may be present in insufficient quantities in the extracts to show activity with the concentration employed. Neem extracts with low concentrations didn’t show effect on S. aureus but by increasing the concentration of extract, effect was obtained as the dilution level decreases.
Table 2: Mean zones of inhibition of different extracts of Neem oil at different concentrations against different fungus.
| Test fungi | Conc (µg/ml) | Mean zone of inhibition ± S.D (mm) | P values | ||
| PE | EtOAc | ME | |||
|
Yeast |
500 | 4.7±1.1 | 7.0±2.6 | 11.7±2.1 | 0.000 |
| 400 | 2.3±2.1 | 5.0±2.0 | 8.0±2.0 | 0.000 | |
| 300 | 1.0±1.7 | 2.3±2.0 | 5.0±1.0 | 0.001 | |
| 200 | Growth | Growth | 1.3±2.3 | 0.000 | |
| 100 | Growth | Growth | Growth | 0.000 | |
|
Mold |
500 | 8.7±1.1 | 7.7±2.5 | 9.0±1.7 | 0.003 |
| 400 | 5.3±1.5 | 4.7±1.5 | 4.7±1.1 | 0.000 | |
| 300 | 2.3±2.1 | 2.0±1.7 | 2.7±2.3 | 0.013 | |
| 200 | 1.0±1.7 | 1.0±1.7 | Growth | 0.000 | |
| 100 | Growth | Growth | Growth | 0.003 | |
|
Dandruff |
500 | 7.5±0.5 | 6.0±1.0 | 10.5±0.5 | 0.000 |
| 400 | 6.5±0.5 | 5.5±0.5 | 8.7±0.6 | 0.000 | |
| 300 | 4.6±0.6 | 1.0±1.0 | 5.5±0.5 | 0.000 | |
| 200 | Growth | Growth | 2.5±1.5 | 0.000 | |
| 100 | Growth | Growth | Growth | 0.000 | |
In this work the drug reference for anti-fungal test was Gentamicine at 0.3% w/vas a positive control and DMSO as a negative control. Gentamicine has 23, 18, 20 mm zone of inhibition on yeast, mold and dandruff respectively. While DMSO has no activity on the above three fungal species, i.e.it has zero zone of inhibition. MICs, MBCs and of different extracts from A. indica seed against different bacteria of both gram-positive and gram-negative strains and fungi were determined and results are shown in (Tables 3 and 4).
Table 3: Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) of different extracts of A. indica seed oil at different dilutions against different bacteria.
| Test bacteria | Extracts | Concentrations (µg/ml) | ||||||
| 500 | 400 | 300 | 200 | 100 | MIC (µg/ml) | MBC (µg/ml) | ||
| E. coli |
PE | + | + | + | - | - | 200 | 100 |
| EtOAc | + | + | + | + | - | 100 | 50 | |
| ME | + | + | + | + | - | 100 | 50 | |
|
K. pneumonia
|
PE | + | + | - | - | - | 300 | 200 |
| EtOAc | + | + | + | - | - | 200 | 100 | |
| ME | + | + | + | - | - | 200 | 100 | |
|
S. aureus
|
PE | + | + | + | - | - | 200 | 100 |
| EtOAc | + | + | + | + | - | 100 | 50 | |
| ME | + | + | + | + | - | 100 | 50 | |
| S. pyogenes |
PE | + | + | + | - | - | 200 | 100 |
| EtOAc | + | + | + | - | - | 200 | 100 | |
| ME | + | + | + | - | - | 200 | 100 | |
Table 4: Minimum Inhibitory Concentrations (MICs) and Minimum Fungicidal Concentrations (MBCs) of different extracts of A. indica seed oil at different dilutions against different fungi.
| Test fungi | Extracts | Concentrations (µg/ml) | ||||||
| 500 | 400 | 300 | 200 | 100 | MIC (µg/ml) | MFC (µg/ml) | ||
| Yeast | PE | + | + | + | - | - | 200 | 100 |
| EtOAc | + | + | + | - | - | 200 | 100 | |
| ME | + | + | + | + | - | 100 | 50 | |
| Mold | PE | + | + | + | + | - | 100 | 50 |
| EtOAc | + | + | + | + | - | 100 | 100 | |
| ME | + | + | + | - | - | 200 | 200 | |
| Dandruff | PE | + | + | + | - | - | 200 | 100 |
| EtOAc | + | + | + | - | - | 200 | 100 | |
| ME | + | + | + | + | - | 100 | 50 | |
Percent Yield of Soxhlet Extracts
Percent yield of Neem Oil can affected by extraction time, temperature, solvent type, Particle Size, and other components in the seed as Shown Table 5-7 and Figure 1. And from those results we can realized that the oil components are more extracted with non polar solvents under long extraction time and fine particle size.
Table 5: Total %yield for soxhlet extractor for different particle size and time using petroleum ether as a solvent.
| Particle size (µm) | Cake wt, and % yield | Extraction time (hours) | ||
| 2 | 4 | 6 | ||
| ≤200 | cake wt (gm) | 6.9 | 8.0 | 8.6 |
| % yield | 17.5 | 20.0 | 21.5 | |
| 200-500 | cake wt (gm) | 6.3 | 7.3 | 8.4 |
| % yield | 15.75 | 18.25 | 21.0 | |
| 500-1000 | cake wt (gm) | 5.0 | 5.9 | 7.2 |
| % yield | 12.5 | 14.75 | 18.0 | |
Table 6: Total %yield for soxhlet extractor for different particle size and time using ethyl acetate as a solvent.
| Particle size (mm) | Cake wt, and % yield | Extraction time (hours) | ||
| 2 | 4 | 6 | ||
| ≤200 | cake wt (gm) | 4.9 | 5.7 | 6.4 |
| % yield | 12.25 | 14.25 | 16.00 | |
| 200-500 | cake wt (gm) | 4.3 | 5.3 | 5.9 |
| % yield | 10.75 | 13.25 | 14.75 | |
| 500-1000 | cake wt (gm) | 3.0 | 3.9 | 4.2 |
| % yield | 7.50 | 9.75 | 10.5 | |
Table 7: Total %yield for soxhlet extractor for different particle size and time using methanol as a solvent
| Particle size (µm) | Cake wt, and % yield | Extraction time (hours) | ||
| 2 | 4 | 6 | ||
| ≤200 | cake wt (gm) | 4.2 | 4.6 | 5.0 |
| % yield | 10.5 | 11.5 | 12.5 | |
| 200-500 | cake wt (gm) | 3.7 | 4.2 | 4.9 |
| % yield | 9.25 | 10.5 | 12.25 | |
| 500-1000 | cake wt (gm) | 2.0 | 2.7 | 3.2 |
| % yield | 5.0 | 6.7 | 8 | |
Determination of physical properties of the Oil
The properties of Neem Oil extracted with different solvent system were summarized in Table 8 & 9
Chemical Characteristic of Neem Oil
The quality of the product could be determine based on the values of the Saponification, Acid, Iodine, and Peroxide. The effect of solvent type in general on Oil qualities were presented in this finding as depicted Figure 2. Analysis of Neem Oil was done Using Gas Chromatography with Mass Spectrometer to know the composition of Oil as shown Table 10 and 11.
Table 8: Physical property determination, experimental result of the characterization of extracted oil by petroleum ether.
| Physical property | Unit | Experimental value | Standard value |
| Refractive index at 20°C | - | 1.47381 | 1.45-1.4705 |
| Density | g/ml | 0.9506 | 0.900-0.9850 |
| Odour | - | Garlic | Garlic-peanut |
| Colour | - | Yellow | Yellow |
| Specific gravity | - | 0.9538 | 0.948 - 0.964 |
| pH | - | 3.4 | 3.4-5.5 |
| Brix at 20°C | % | 73.385 | ND |
Table 9: Physical property determination, experimental result of the characterization of extracted oil by ethyl acetate.
| Physical property | Unit | Experimental value | Standard value |
| Refractive index at 20°C | - | 1.43746 | 1.45-1.4705 |
| Density | g/ml | 0.9862 | 0.900-0.985 |
| Odour | - | Garlic | Garlic-peanut |
| Colour | - | Yellow | Yellow |
| Specific gravity | - | 0.9479 | 0.948 - 0.964 |
| pH | - | 3.3 | 3.4-5.5 |
| Brix at 20°C | % | 58.014 | ND |
ND: not determined before.
Table 10: Chemical composition of A. indica seed oil extracted by petroleum ether
| Peak | RT | Area % | Height % | Chemical name | Molecular formula |
| 8 | 36.858 | 100 | 100 |
9, 12-Octadecadienoic acid (Z,Z)-, methyl ester |
C19H34O2 |
| 9 | 36.934 | 28.22 | 70.46 |
9-Octadecenoic acid, methyl ester, (E) |
C19H36O2 |
| 5 | 33.561 | 17.64 | 40.36 | Hexadecanoic acid, methyl ester |
C17H34O2 |
| 11 | 37.316 | 6.32 | 16.97 | Methyl stearate |
C19H38O2 |
| 10 | 36.978 | 1.41 | 5.25 | 11-Octadecenoic acid, methyl ester |
C19H38O2 |
| 23 | 53.871 | 1.16 | 0.28 |
β-Sitosterol |
C29H50O |
| 15 | 40.741 | 0.62 | 1.57 | Eicosanoic acid, methyl ester |
C21H42O2 |
| 14 | 40.299 | 0.49 | 1.31 | cis-11-Eicosenoic acid, methyl ester |
C21H40O2 |
| 16 | 42.375 | 0.44 | 0.98 |
Phenol, 2,2'-methylene bis [6-(1, 1-dimethylethyl)-4-methyl |
C23H32O2 |
| 12 | 39.187 | 0.32 | 0.6 | 7,10-Octadecadienoic acid, methyl ester |
C19H34O2 |
| 18 | 44.905 | 0.29 | 0.43 | Docosanoic acid, methyl ester |
C23H46O2 |
| 19 | 45.37 | 0.28 | 0.38 | Diisooctyl phthalate |
C24H38O4 |
| 7 | 35.448 | 0.27 | 0.72 | Heptadecanoic acid, methyl ester |
C18H36O2 |
| 3 | 29.405 | 0.26 | 0.77 | Methyl tetradecanoate |
C15H30O2 |
Table 11: Chemical composition of A. indica seed oil extracted by ethyl acetate.
| Peak | RT | Area | Height | Chemical Name | Molecular formula |
| 1 | 15.705 | 407351.41 | 129215.95 | Benzene acetic acid, methyl ester |
C9H10O2 |
| 2 | 24.86 | 277276.55 | 94242.71 | Undecanoic acid, 10-methyl-, methyl ester |
C13H26O2 |
| 3 | 26.442 | 279148.04 | 104321.61 |
7-Hexadecene, (Z)- |
C16H32 |
| 4 | 29.401 | 447024.83 | 159424.44 | Methyl tetradecanoate |
C15H30O2 |
| 5 | 33.095 | 258057.05 | 75362.09 |
(Z)-Methyl hexadec-11-enoate |
C17H32O2 |
| 6 | 33.553 | 30952280.97 | 9498069.61 | Hexadecanoic acid, methyl ester |
C17H34O2 |
| 7 | 34.842 | 1278854.68 | 430551.61 | Hexadecanoic acid, ethyl ester |
C18H36O2 |
| 8 | 34.978 | 275859.62 | 67589.95 | Cyclopropaneoctanoic acid, 2-hexyl-, methyl ester |
C18H34O2 |
| 9 | 35.448 | 460130.74 | 157519.26 | Heptadecanoic acid, methyl ester |
C18H36O2 |
| 10 | 36.83 | 176380248.8 | 26284257.53 |
9,12-Octadecadienoic acid (Z,Z)-, methyl ester |
C19H34O2 |
| 11 | 36.906 | 52247248.12 | 17346907.35 | 9-Octadecenoic acid (Z)-,methyl ester |
C19H36O2 |
| 12 | 36.958 | 2257340.67 | 995940.84 |
9-Octadecenoic acid, methyl ester, (E) |
C19H36O2 |
| 13 | 37.303 | 11963927.04 | 4078428.14 | Methyl stearate |
C19H38O2 |
| 14 | 37.91 | 9793271.76 | 3463530.45 | Linoleic acid ethyl ester |
C20H36O2 |
| 15 | 38.01 | 3021336.5 | 936795.04 | Ethyl Oleate |
C20H38O2 |
CONCLUSIONs and Recommendations
The effect of Azadirachta indica crude extracts was tested on the four bacteria species (E. coli, K. pneumoniae, S. aureus, S. pyogenes), and three fungi species (Yeast, mold and dandruff). Neem effect on these bacteria was determined; K. pneumoniae showed high resistance on all extracts used than other bacteria. Lastly, based on the information got from this study the antimicrobial effect of A. indica (Neem) can change depending solvent system used, moreover the extract concentration matters a lot because each extract has its minimum inhibition concentration (MIC) which is the highest dilution of a plant extract that still retain an inhibitory effect against the growth of a microorganism. The extraction process is solvent selective as certain solvents failed to extract the required solute while other solvents had greater affinity for some samples than others, hence the choice of petroleum ether as the optimal solvent in this work. The quality and properties of Neem seed oils were evaluated through this study using different parameters. Results obtained indicated that saponification value, peroxide value, acid value, free fatty acid and iodine values that fell within the range of those acceptable for soap production although with low storage property. The measurement of the mentioned physico-chemical characteristics indicate that Neem seed oil has high value of rancidity among all vegetable oil samples and this is may be attributed to the presence of higher free fatty acids, degree of unsaturation, hydrolysis of triglycerides, storage and date of expire. This work also hints that the Neem seed oil is non edible oil. GC-MS analysis revealed that many different chemical components were identified in the fixed oil of Neem, including (Palmitoleic acid methyl ester, Palmitic acid methyl ester, Linoleic acid methyl ester, Oleic acid methyl ester, Stearic acid methyl ester and etc.) as listed in the result.
ACKNOWLEDGEMENTS
We would like to thank the Almighty God and his mother St. Merry. Our gratitude extends to our family and those who contributed directly and indirectly for the accomplishment of this study.
Novelty Statement
In this research article we have done Antimicrobial properties in addition to different physicochemical properties assessment and GC-MS characterization of phytochemical constituent from the seed parts of Azadrichta indica. Despite the existence of excess information regarding the prolonged and uneventful local use of this plant, scientific evidences regarding their efficacy on different biological activity are less abundant.
Author’s Contribution
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdullah R (2009). Characterization and biological, activities of essential oils of some Speciesof lamiaceae. Dep. Chem. Biochem. Fac. Sci. Univ. Agric. Faisalabad, Pak., 34(2): 56-67.
Adam D, Galal M, Bashir M, Salih D (1991). Activity of water extracts of Albizia anthelmintica and Alebbek Backs against experimental Hymenolepisdiminuta infection in rats. J. Ethnol. Pharmacol., 31(4): 333-337. https://doi.org/10.1016/0378-8741(91)90019-A
Ahmed S, Bamofleh M, Munshi A (1989). Cultivation of Neem (Azadirachta indica) in Saudi Arabia. J. Econ. Bot., 43(6): 35-38. https://doi.org/10.1007/BF02859323
Alberto R, Zampini C, Cuello S, Ordonez M, Almeida D, Solorzano E, Isla I (2009). Antimicrobial activity of selected plant species from the Argentine puna against sensitive and multi resistant bacteria. J. Ethnol. Pharmacol., 124(9): 499-505. https://doi.org/10.1016/j.jep.2009.05.011
Arora S, Kaur J (2007). Antibacterial activity of some Indian medicinal plants. J. Nat. Med., 61(6): 313-317. https://doi.org/10.1007/s11418-007-0137-8
Benharref A, Bouamama H, Noel T, Villard J, Jana M (2006). Antimicrobial activities of the leaf extract of two Moroccan Cistus L species. J. Ethnol. Pharmacol., 104(2): 104-107. https://doi.org/10.1016/j.jep.2005.08.062
Gohari A, Farhoosh A, Haddad K (2011). Chemical composition and physicochemical properties of pumpkin seeds (Cucurbitapepo Var. Styriaka) grown in Iran. J. Agric. Sci. Technol., 13(4): 1053-1063.
Hussain A, Anwar S, Sherazi T, Przybylski R (2008). Antioxidant and antimicrobial activities of basil (Ocimumbasilicum) essential oils depend on seasonal variations. J. Food Chem., 108(10): 986-995. https://doi.org/10.1016/j.foodchem.2007.12.010
Jessinta D, Sandanasamy A, Saiful N, Abdurahman H (2013). Fatty acid composition and antibacterial activity of neem (Azadirachta indica) seed oil. J. Open Conf. Proc., 4(10): 43-48. https://doi.org/10.2174/2210289201304020043
Light J, Flora S, Benjamin W (1977). Extraction of essential oil from Eucalyptus leaves. J. Essential Oil Extract., 4(7): 56-60.
Marotti M, Piccaglia R, Giovanelli E (1994). Effects of variety and ontogenicstage on the essential oil composition and biological activity of fennel. J. Essential Oil Res., 6(4): 57-62. https://doi.org/10.1080/10412905.1994.9698325
O’Brien D (2009). Fats and Oils. CRC Press, Taylor Francis Group, 3(2): 25-27.
Sadoudi R, Ali A (2017). Studies on physico-chemical characteristics and fatty acid composition of commercially available Algerian frying edible oils. J. Int. Food Res., 24(9): 60-67.
Sanguanpong U (2010). Productivity of rit-pilot plant for small-scale industrial production of the Neem-based extract. J. Agric. Eng. Technol., 12(5): 78-89.
Susmitha K, Vidyamol P, Ranganayaki K and Vijayaragavan (2013). Phytochemical extraction and antimicrobial properties of Azadirachta indica (Neem). Glob. J. Pharmacol., 7(2): 316-320.
Terblanche F (2000). The characterization, utilization and manufacture of products recovered from Lippiascaberrima Sond. PhD. thesis, Pretoria, University of Pretoria. 23(10): 45-67.
Uribe-Hernandez C, Hurtado-Ramos, E, Olmedo-Arcega E, Martinez-Sosa M (1992). The essential oil of Lippiagraveolens H.B.K. from Jalisco, Mexico. J. Essent. Oil Res., 4(4): 647-649. https://doi.org/10.1080/10412905.1992.9698152






