Seven Local Commercial Wheat Cultivars Tested for Resistance against Rhopalosiphum padi L. in Pakistan
Seven Local Commercial Wheat Cultivars Tested for Resistance against Rhopalosiphum padi L. in Pakistan
Zafrullah Khan1, Shah Alam Khan1*, Hamayoon Khan2, Naeem Khan3, Khwaja Junaid1 and Inamullah Khan1
1Department of Plant protection, University of Agriculture, Peshawar, Pakistan
2Department of Agronomy, University of Agriculture, Peshawar, Pakistan
3Department of Weed Science, University of Agriculture, Peshawar, Pakistan
ABSTRACT
Bird cherry oat aphid (Rhopalosiphum padi L.) is a serious pest of wheat (Triticum aestivum L.) crop and causes significant production losses to the wheat crop in Pakistan. So far eight genes from the conventional wheat cultivars that are resistant to the attack of R. padi have been incorporated in the commercial wheat cultivars to help manage R. padi. During this study seven wheat genotypes were evaluated as antixenosis, antibiosis and tolerance. These tested genotypes were (Pir Sabaq-2004, Atta-Habib, Saleem-2000, Serin-2010, Khyber-87, Sarhad-82, and Khushal-69) in three separate experiments. Antibiosis resistance was identified to R. padi in wheat cultivar (Serin-2010), where a minimum number of R. padi progeny were produced in 11.4 days. Wheat cultivar (Atta Habib) was found significantly susceptible to R. padi as it produced higher number of progeny in only 7 days duration. The other wheat cultivars (Pirsabaq-2004, Saleem-2000, Atta-Habib, Khushal-69, Sarhad-82 and Khyber–87) also did not show any antixenosis or tolerance category of resistance to the R. padi. This study concludes, Serin-2010’ as standard resistant cultivar for future breeding program.
Article Information
Received 31 July 2015
Revised 09 may 2016
Accepted 28 November 2016
Available online 25 April 2017
Authors’ Contribution
SAK designed the study. ZK conducted the experimental work and wrote the article. HK statistically analyzed the data. NK helped in technical writing and analysis. SAK and IK supervised the study. KJ maintained plant and insect culture.
Key words
Rhopalosiphum padi, Antixenosis, Antibiosis, Tolerance, Progeny, Wheat crop.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.3.793.799
* Corresponding author: [email protected]
0030-9923/2017/0003-0793 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
INTRODUCTION
Bird cherry-oat aphid (Rhopalosiphum padi L.) is a major pest of several important cereal crops such as wheat (Triticum aestivum L.), maize (Zea mays L.), barley (Hordeum Vulgare L.), oats (Avena sativa L.) and rye (Secale cereale L.) and is one of the first aphids that appears on small grain cereal crop in the spring season, and often persists on winter cereal crops. This species spreads in North America, Europe, Asia, New Zealand and Canada (Wiktelius et al., 1990; Halbert and Voegtlin, 1995; Hesler et al., 1999).
R. padi is a pest that transmits barley yellow dwarf virus (BYDV) disease in early sown winter crops (Stern, 1967; Irwin and Thresh, 1990). R. padi is the sole aphid of spring and is common in fall. R. padi moves in early September to the native grasses, newly planted wheat and other winter cereals crops from the stubbles of cereals. When the number of aphids per tiller is more than 20 and lady beetles is fewer than 10% of tiller the treatment may be justified for a yield potential (Aheer et al., 1993).
Among several control methods including cultural, physical, mechanical, chemical and biological, the host plant resistance have been used to prevent losses to the wheat crop by aphids worldwide (Junaid et al. 2016). R. padi has been reported from Punjab and Khyber Pakhtunkhwa provinces where late sown wheat crop suffered more due to high population of this aphid attack (8.9 aphid/tiller) as compared to early sown wheat crop, where the numbers of this aphid remained less (2.33-3.28 per tiller) (Aheer et al., 1993). Limited work has been done on the population dynamics of cereal aphids (Aheer et al., 1997). Most of the research work has been done collectively on the cereal aphids without any information about the species composition. Hashmi et al. (1983) found two aphid species viz. Sitobion avenae and R. padi which are harmful to wheat crop. A preliminary survey conducted in the Punjab and Khyber Pakhtunkhwa provinces of Pakistan showed a medium to heavy infestation of cereal aphids at Muree and Multan districts of Punjab and Charsada district of Khyber Pakhtunkhwa (Ihsanulhaq, 1985). Further work on species identification and distribution has already been done by Khan et al. (2007) but no work regarding categories of resistance has ever been done on this species in Khyber Pakhtunkhwa province.
Keeping in view, the importance of this pest R. padi, the present study was undertaken to determine categories of resistance components to R. padi with a specific aim to screen the commercial wheat cultivars for potential resistance to R. padi
MATERIALS AND METHODS
Plant materials and seedling preparation
Twenty commercial wheat cultivars (viz. Khaniwal, NARC, Tatara-98, Nowshera-96, K2L, Takbeer, PirSabaq-85, Ghaznavi-98, Bakhtawar-92, Dera-98, KRL14, Suliman-96 Pirsabaq-2004, Serin-2010, Saleem-2000, Atta-Habib, Khyber-87, Sarhad-82, and Khushal-69) were selected on the basis of their local adaptation to the local agro-climatic conditions for screening under the laboratory conditions and were tested for their resistance capability to R. padi. The seed of all the wheat cultivars were obtained from certified companies (AUP). Four experimental trays (each 61 × 40 cm diameter) were used to undertake the proposed experiment. Ten seeds of each cultivar were sown per row. The row to row and seedling to seedling distances were kept 7 and 5 cm, respectively. After seed germination, the seedlings were grown for 14 d and then infested with R. padi. After 14 d of R. padi infestation, only seven local commercial wheat cultivars out of the 20 were selected on the basis of their resistance capabilities, for detailed assay. The nymphs of R. padi species were collected from a typical wheat field at New Developmental Research Farm (NDF) Malakand area (71° E and 34° N) during the winter season in 2011. Previously grown seedlings of a local commercial wheat cultivars (PirSabaq-85) that is susceptible to R. padi in pots were used for rearing the R. padi nymphs, under the laboratory conditions, where the wheat cultivars were grown in pots containing potting material.
For all of three tests (i.e. antixenosis, antibiosis and tolerance) plastic pots (each 16.5 cm diameter) were used and maintained at 24/20oC day and night and a photoperiod of 14:10 (L:D) hours inside the Plant Protection Laboratory growth chamber, The University of Agriculture Peshawar Khyber Pakhtunkhwa, Pakistan for 80 d.
The three different tests of resistance (i.e., antixenosis, antibiosis and tolerance) were determined for the seven selected wheat cultivars (PirSabaq-2004, Atta-Habib, Saleem-2000, Serin-2010, Khyber-87, Sarhad-82 and Khushal-69) using the modified method of Flinn et al. (2001).
Antixenosis
Ten seeds of each cultivar was pre-germinated in Petri dish for 2 d, and the newly germinated seedlings were transplanted equidistantly of every cultivar in individual pots (each 16.5 x 7.5 cm diameter) till two leaf growth stage. Each of the pot was 10 times replicated containing a seedling of each the Pirsabaq-2004, Saleem-2000, Atta-Habib, and Serin-2010, Khyber-87, Sarhad-82 and Khushal-69 wheat cultivars. The experiment was run using a completely randomized design. After 2 d, 20 late instars wingless adults of R. padi were released to the pot center containing the seedling on a piece of paper to achieve the seedling infestation. Then the seedlings were caged with the nylon mesh cloth or caged inside the pots. The number of aphids were recorded on each seedling of each cultivar 12, 24 and 48 h of R. padi infestation to determine the degree of antixenosis.
Antibiosis
Antibiosis to R. padi was also determined by caging an individual aphid on each cultivar at two leaf stage. New germinated seedling of the selected cultivars were transplanted from Petri-dish to a separate pots after two days. The pots were replicated 10 times in completely randomized design. When the seedling of each cultivar reached two leaf stage the mid-section of older leaf were caged by plastic drinking straw 3-4 cm long. By piercing with insect pin the straw cages were ventilated with 35-40 holes. One end of straw cage was placed at the plant leaf tip and moved towards the middle portion for caging. The leaf of each plant was infested with wingless adult R. padi nymph p1 (Md) and both ends of cage were closed by cotton bolls. To record the first nymphal production, the caged aphids were observed twice a day. When the caged female produces its first nymph (F1), the time and date were recorded. The caged mother which produce its first offspring were shifted from one leaf to another on the same plant and caged as described earlier. The time in days were recorded when the first offspring (F1) produced by the first caged last instar wingless adult produced its first offspring and the number of nymphs were counted and recorded which were produced by p1 (Md). For all the selected cultivars, p1 Md and d were calculated for each of the aphid. To calculate the mean intrinsic rate of increase (rm) of female aphids on each plant cultivar were calculated for analysis using the equation;
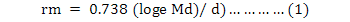
In equation (1), d represents the time required for a newly emerged F1 to produce its first offspring and Md represents the total number of progeny produced by the mother of F1 [p1] , and 0.738 is the mean regression slope of Md over d for four aphid species (Wyatt and White, 1977).
Tolerance
Proportional plant dry weight change (DWT) and tolerance index (TI) of seven specific wheat cultivars plants were used to calculate the Tolerant component. Tolerant component can be measured by using the following equation;
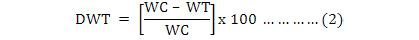
In equation (2) WC represent the dry weight of the non-infested control plant, WT represents the dry weight of the infested plant and TI is the DWT/ number of aphids produced on the infested plant (Dixon et al., 1990; Reese et al., 1994). To determine and compensate the TI for confounding effect of differing numbers of R. padi on infested plants. Cultivars with TI values significantly lower than those of the susceptible control were considered tolerant.
The newly germinated seeds in petri dishes were transplanted to the pots, until they reach the two leaf stage. Twelve plants were monitored for growth and height within each cultivar. Treatment plants were infested with R. padi in each pair of plants and considered the paired plant (second additional plant/ control plant) as a non-infested control plant.
In both parts of experiment the plants are enclosed in the nylon mesh cloth/cage and the aphids are allowed to feed on plants for 15 days and the plants were arranged in complete randomized design. After 15 days the cages were removed and the aphids of infested plants were collected on a waxy paper sheet, fixed in 70% alcohol and then counted. The shoots of infested and control or non-infested plants were cut at the base and kept in pre-weighed aluminum foil pouches. Roots were washed well in clean water and then kept in pouches as mentioned above for shoots. The roots and shoots were dried in an oven for three days 75°C. The weight of tissues were calculated.
Table I.- R. padi infestation (Mean±SEM) on different wheat cultivars noted 12, 24 and 48 h after infestation.
| Wheat cultivars |
12 h |
24 h |
48 h |
| Pir-Sabaq-2004 |
4.7±0.73a |
4.6±0.64a |
4.5±0.34a |
| Salim-2000 |
4.3±0.49a |
4.5±0.45a |
4.4±0.40a |
| Serin-2010 |
4.1±0.44a |
4.3±0.38a |
4.2±0.38a |
| Atta-Habib |
4.8±0.74a |
4.60±0.47a |
4.7±0.44a |
| Sarhad-82 |
4.4±0.68a |
4.30±0.89a |
4.5±0.50a |
| Khushal-69 |
5.3±0.61a |
5.50±0.34a |
5.9±0.54a |
| Khyber-87 |
5.5±0.40a |
5.10±0.27a |
5.8±0.44a |
| Means ± SE |
33.1±0.22a |
32.90±0.20a |
34±0.17a |
The means within the columns followed by different small letters are significantly different at P < 5.
RESULTS AND DISCUSSION
The results of antixenosis experiment are shown in Table I. Wheat cultivar Khyber-87 sustained the highest infestation (5.5 aphids per plant) at 12 h post infestation period and was followed by Khushal-69 (5.3 aphids per plant), Atta-habib (4.8 aphids per plant), Pirsabaq-2004 (4.7 aphids per plant), Sarhad-82 (4.4 aphids per plant) and Salim-2000 (4.3 aphids per plant). The cultivar Serin-2010 has attracted the minimum (4.1 aphids per plant) compared to the tested wheat cultivars. Almost similar response of antixenosis resistance was recorded after 24 h post infestation and the trend did not changed rather continued for two days (48 h post infestation). However, no statistical difference were recorded among the tested wheat cultivars at all the three time points 12, 24 and 48 h post infestation (f = 1.77, df = 6, P = 0.11).
Table II shows the results of antibiosis resistance where R. padi (P1 female aphid) when caged on a single leaf of wheat cultivar produced significantly higher number of progeny on cultivar Atta-habib (26.10) aphids per female compared to the tested cultivars, Serin-2010 (14.30), Pir-Sabaq-2004 (19.90) Salim-2000 (20.20), Sarhad-82 (19.10), Khushal-69 (19.40) and Khyber-87 (19.10), respectively; however, within the genotypes they were non significance differences except cultivar Serin-2010. Thus wheat cultivar, Serin-2010, exhibit strong antibiosis resistance compared to the tested genotypes, against aphid R. padi (Column 1, Table II).
Table II.- Number of progeny produced by P1 pre-reproductive period (d) of late instars R. padi and rm value on different wheat varieties.
|
Wheat cultivars |
Number of progeny by P1 | Nymph pre- reproductive period (d) |
Mean ± SE* rm values |
| PirSabaq-2004 | 19.90±1.22b | 7.33±0.15b | 0.08±0.005b |
| Salim-2000 | 20.20±1.16b | 7.28±0.17 b | 0.08±0.006b |
| Serin-2010 | 14.30±0.59c | 11.40±0.37a | 0.04±0.002c |
| Sarhad-82 | 19.10±1.10b | 7.43±0.25b | 0.07±0.004b |
| Khushal-69 | 19.40±0.74b | 7.55±0.29b | 0.08±0.004b |
| Khyber-87 | 19.10±0.90b | 7.71±0.11b | 0.07±0.004b |
| Atta-Habib | 26.10±1.29a | 7.36±0.24b |
0.11±0.007a |
The means within the column followed by different letters are significantly different at 5% level of significance.
The trend in pre-reproductive period (total time taken to complete one generation on the leaf of wheat cultivar only ‘Serin-2010’ took significantly longer time during the nymphs pre-reproductive period compared to all the tested cultivars (f = 11.13, df = 6, p = 0.0001). However, within the tested cultivars no statistical significance was recorded except ‘Serin-2010’ regarding nymphs pre-reproductive period (Column- 2, Table II).
This pattern was almost the same in time period taken to complete one generation of progeny by a female aphid. There was no significant difference between PirSabaq-2004, Salim-2000, Sarhad-82, Khushal-69 and Khyber-87. However, R. padi had produced maximum numbers of nymph or progeny on the cultivar Atta-Habib compared to cultivars Pir-Sabaq-2004, Salim-2000, Sarhad-82, Khushal-69 and Khyber-87 (f = 37.78, df = 6, P = 0.0001). The number of pre-reproduction period time in days, where the aphid has taken longer time on wheat cultivar Serin2010 compared to all other wheat cultivars. The aphid nymph (F1) took 11.40 days and produced 14.30 nymphs (f = 15.55, df = 6, p = 0.0001).
The response regarding the rate of natural intrinsic increase (rm) value was recorded significantly minimum in wheat genotype ‘Serin-2010’ and maximum value was noted in ‘Atta-Habib. However, within the genotypes: Pir-Sabaq-2004, Salim-2000, Sarhad-82, Khushal-69 and Khyber-87 no statistical significance were found (f = 15.55, df = 6, p = 0.0001). Thus (Column-3, Table II), depict the same picture as shown in case of number of progeny production column.
Table III.- Percentage of (Mean ± SE) DWT and TI values for shoots of different wheat varieties infested with R. padi.
| Variety |
DWT |
TI |
| PirSabaq-2004 |
3.97 ± 0.48 a |
0.02 ± 0.0020a |
| Salim-2000 |
3.47 ± 0.30 a |
0.02 ± 0.002 a |
| Serin-2010 |
4.48 ± 0.34 a |
0.02 ±0.002 a |
| Sarhad-82 |
3.60 ± 0.40 a |
0.02 ±0.003 a |
| Khushal-69 |
4.32 ± 0.30 a |
0.02 ± 0.002 a |
| Khyber-87 |
3.29 ± 0.29 a |
0.01 ± 0.002 a |
| Atta-Habib |
3.93 ± 0.43 a |
0.05 ± 0.03 a |
The means within the column followed by different letters are significantly different at 5% level of significance.
The results of proportional plant dry weight change and TI values for shoots are presented in Table III, when the leaf of wheat genotypes were infested with aphid to the R. padi at 14 days post infestation period. The proportion plant dry weight change (DWT) data shows no significant differences in all the tested cultivars to R. padi in-terms of shoot tissue. All the cultivars showed mixed response of resistance to the aphid R. padi with no statistical difference. However, some of the cultivars values as in case of Serin-2010 (4.48) and Khushal-69 were greater compared to Khyber-87 (3.29) and Saleem-2000 (3.47) but no statistical difference were recorded (f = 2.23, df = 6, p = 0.051).
The tolerance index (TI) experiment for shoot of the tested wheat cultivars showed similar response to the aphid in all the tested cultivars. However, Khyber-87 have minimum weight (0.01) and was followed by Saleem-2000 (0.01) > PirSabaq (0.02) > Sarhad-82 (0.02) > Khushal-69 (0.024) > Serin-2010 (0.02) and Atta-habib (0.05) respectively but these differences were statistically non-significant (f = 0.92, df = 6, p = 0.4897). This indicated that no comparatively tolerance resistance category was shown by any wheat cultivar to the aphid during this experiment.
Table IV shows the ratio of dry weight of roots of the tested cultivars after 14 days of R. padi infestation period. ‘Serin-2010’ has shown significantly maximum proportion plant dry weight change compared to ‘PirSabaq-2004’ where as all other tested cultivars have shown no significant difference in response to the aphid feeding (f = 2.23, df = 6, p = 0.051) though all these cultivars were found statistically non-significant to each other.
Table IV.- Percentage (Mean ± SE) of DWT and TI values for roots of wheat varieties, infested with R. padi.
| Variety |
DWT |
TI |
| PirSabaq-2004 |
3.44 ± 0.34 b |
0.02 ± 0.002 b |
| Salim-2000 |
4.18 ± 0.39 ab |
0.02 ± 0.003 ab |
| Serin-2010 |
5.66 ± 0.60 a |
0.03 ± 0.004a |
| Sarhad-82 |
3.92 ± 0.58 ab |
0.02 ± 0.004 ab |
| Khushal-69 |
3.98 ± 0.67 ab |
0.02 ± 0.004 ab |
| Khyber-87 |
3.58 ± 0.53 ab |
0.02 ± 0.003 b |
| Atta-Habib |
4.71 ± 0.30 ab |
0.02 ± 0.002 ab |
The means within the column followed by different letters are significantly different at 5% level of significance.
Among the tested genotypes Serin-2010 has shown significantly less tolerance to the feeding of R.padi compared to Pirsabaq-2004 and Khyber-87 regarding the (TI) ratio of root tissues. However, the cultivars (Salim-2000, Sarhad-82, Khushal-69) had no significance value between each other (f = 2.71, df = 6, p = 0.020).
The inhibitory compounds naturally present in crops can affect feeding magnitude of the insect pests (Smith et al., 1991; Dutoit, 1987; Khan et al., 2009). Such compounds and other identified gene expression studied by Boyko et al. (2006) may explain the antibiosis in R. padi.
Furthermore, tolerance components to R. padi was recorded in our tested genotypes in ‘Khyber-87’ and pirsabaq-2004 and in any of the tested wheat cultivars. In case of antixenosis experiment Atta-Habib sustained the maximum number of aphids (Tables I and III), while the Serin-2010 had fewer R. padi compared to all other tested cultivars. Our results are in conformity with Khan et al. (2009), however, their experiments were conducted with Russian wheat aphid which may have different feeding behavior to the tested entries. The findings of Akhtar et al. (2009) who studied R. maidis, (Fitch) Schizaphis graminum (Rondani) and R. padi of cereal crops showed different resistance compared to our results. The cultivars like Saleem-2001 and Iqbal-2000 were susceptible but the cultivars Inqilab-91, Margalla-99 and Wafaq-2001 were more susceptible to the aphids. Another report mentioned by Hesler et al. (1999), found no differences in antixenosis among ‘MV4’ Vista’ and five other wheat accession in antixenosis test. Though, it is really important to find cultivars with antixenosis resistance however, very few cultivars carry this type of resistance to arthropods. Thus, in our study none of the tested genotypes showed Antixenosis resistance to R. padi.
The antibiosis resistance category seem to be the predominant category of resistance in many cultivars. In our study the wheat cultivar ‘Serin-2010’ resistant to R. padi manifested lower progeny production, prolonged nymphal pre-reproductive period and numerically lesser (rm-0.04) value, compared to PirSabaq-2004, Salim-2000, Sarhad-82, Khushal-69, Khyber-87 and Atta-Habib. Our results are in line with the results of Hesler and Dashiell (2011) and Khan et al. (2009) who have worked with D. noxia and reported H871 as antibiosis resistance based on number of progeny produced and pre-reproductive period interval and minimum number of aphids were produced in maximum time on antibiosis resistant leaves of resistant. Since antibiosis resistance may inhibit progeny production and can stop further build up population to avoid economic injury level and will keep the pest below the threshold level. Such type of resistant category will help in developing integrated pest management strategy to avoid pesticides load on environment. Thus, in our study among the tested cultivars only ‘Serin-2010’ exhibit antibiosis category of resistance.
As far as the tolerance category are concerned where the proportional plant dry weight change of shoot ratio have indicated no resistance factor and similar picture was depicted in case of shoots tolerance index (TI) which is very common in many cases and is also in line with Diaz-Montano et al. (2007), concluded research on R. padi and a total of 4,056 wheat accessions were used against aphids. During their studies 97 % of entries were rated worse and only 92 accessions considered as a infested by the R. padi, which can affect the rate of growth of root and shoot in an equal non-infested control treatment. Tolerance which is a form of host plant resistance may differ its applicability to fully manage R. padi to limit losses and diseases caused by R. padi (Porter et al., 2009). Such resistant component is durable and can be integrated with other method of pest control like an IPM strategy where partially resistant cultivar combined with biological agents may work for long time and with less cost.
In case of tolerance index (TI) and proportional plant dry weight change (DWT) of roots the cultivar, ‘Serin-2010’ had maximum proportional plant dry weight change compared to the tested cultivars ‘Pirsabaq-2004’ and ‘Khyber-87’ however among the other tested cultivars no statistical differences were noticed. Our results are not in conformity with Aheer et al. (1997), who have reported no tolerance in 28 genotypes out of 30 accessions, however, they did not categorize the component of resistance which was only based on the number of aphid/tiller but in our study the experiment was conducted on different component of resistance to R.padi under laboratory conditions in controlled environment. Thus in our study among the tested genotypes ‘Khyber-87’ and Pirsabaq-2004 showed strong vigor against R. padi.
It is evident from our results that none of the tested genotypes exhibit Antixenosis category of resistance while strong antibiosis was shown by the cultivar ‘Serin-2010’ out of the tested entries. As far as the tolerance categories of resistance is concerned among the tested genotypes ‘PirSabaq-2004’ and ‘Khyber-87’carried tolerance factor only in roots tolerance index (TI). Thus it is concluded that out of seven cultivars only Serin-2010 exhibit antibiosis resistance, while tolerance category was also present in two genotypes (PirSabaq-2004 and Khyber-87) in root tolerance index.
Thus, it is concluded that out of seven cultivars only ‘Serin-2010’ have proved strong antibiosis resistance to R. padi while cultivars Khyber-87, Pirsabaq-2004, showed root tolerance index as resistant factor but the cultivar Atta-Habib, proved susceptible cultivar to R. padi infestation under laboratory conditions.
It is important to mention that cultivars with partially/fully tolerance type of resistant may play a sustainable role in crop protection program to have minimum selection pressure on varieties and avoid resistant breaking biotypes. Furthermore, such variety can be integrated with other IPM tools like biological control agents and partially resistant variety may play viable performance to arthropods and other pests. Thus in our current study wheat variety ‘Serin-2010’ proved antibiosis resistant to R. padi while ‘Khyber-87,and ‘Pirsabaq-2004’ partially tolerance resistant to R. padi and recommended for future screening program as standard and also for the wheat growers in Pakistan, particular in this part of the country.
Statement of conflict of interest
Authors have declared no conflict of interest.
REFERENCES
Aheer, G.M., Haq, I., Ahmad, K.J. and Ali, A., 1993. Varietal resistance and sowing date effect on Aphid density and wheat yield. J. agric. Res., 31: 313-317.
Aheer, G.M., Rashid, A., Afzal, M. and Ali, A., 1997. Varietal resistance susceptibility of wheat to aphids, Sitobion avenae F. a Rhopalosiphum rufiabdominalis Sasaki. J. agric. Res., 31: 307-311.
Akhtar, I.H., Naeem, M., Javed, H. and Aziz, M.A., 2009. Could host plant resistance affect on cereal aphids (Aphididae; Homoptera) population on wheat crop. Int. J. Agric. Vet. med. Sci., 3: 31-42.
Boyko, E.V., Smith, C.M., Vankatappa, T., Bruno, J., Deng, Y., Starkey, S.R. and Klaahsen, D., 2006. The molecular basis of plant gene expression during aphid invasion; wheat Pto- and Pite-like sequences modulate aphid-wheat interaction. J. Ent., 99: 1430-1445.
Diaz-Montano, J., Reese, J.C., Louis, J., Campbell, L.R. and Schapaugh, W.T., 2007a. Feeding behavior by the soybean aphid (Hemiptera: Aphididae) on resistant and susceptible soybean genotypes. J. econ. Ent., 100: 984-989. https://doi.org/10.1093/jee/100.3.984
Dixon, A.G.O., Brammel-Cox, P. J., Reese, J. C. and Harvey, T.L., 1990. Mechanism of resistance and their interaction in twelve sources of resistance to biotype ‘E’ green bug (Homoptera;Aphididae) in sorghum. J, econ. Ent., 83: 234-240.
Dutoit, F., 1987. Resistance in wheat (Triticum aestivum) to Diuraphis noxia (Homoptera: Aphididae). Cereal Res. Comm., 15: 175–179.
Flinn, M., Smith, C.M., Reese, J.C. and Gill, B.S., 2001. Categories of resistance to green bug (Homoptera;Aphididae) biotype in Aegilops tauschii germplasm, J. econ. Ent., 85: 558-563.
Halbert, S., and Voegtlin. D., 1995. Biology and taxonomy of vectors of barley yellow dwarf viruses. In: Barley yellow dwarf 40 years of progress (eds. CJ D’Arcy and P.A. Burnett). APS Press, St. Paul, MN, USA, pp. 217–258.
Hashmi, A.A., Hussain, M. and Ulfat, M., 1983. Insect pest complex of wheat crop. Pakistan J. Zool., 15: 169-176.
Hesler, L.S., Riedell, W.E., Kieckhefer, R.W., Haley, S.D., and Collins, R.D., 1999. Resistance to Rhopalosiphum padi (Homoptera: Aphididae) in wheat germplasm accessions. J. econ Ent., 92: 1234-1238.
Hesler, L.S. and Dashiell, K.E., 2011. Antixenosis to Aphis glycines (Hemiptera: Aphididae) among soybean lines. Open Ent. J., 5: 39–44.
Ihsan Ul Haq, 1985. incendance of green bug S Graminum (Rondani) (Homopetra aphididae) in Pakistan and resistance in wheat against it. Insect. Sci. Applicat., 14: 247-254.
Irwin, M.E. and Thresh, J.M., 1990. Epidemiology of barley yellow dwarf: a study in ecological complexity. Annu. Rev. Phytopathol., 28: 393–424. https://doi.org/10.1146/annurev.py.28.090190.002141
Junaid, K., Khan, S.A., Khan, I., Shah, S.R.A. and Khan, Z., 2016. Study on different components of resistance in wheat genotypes to green bug (Schizaphis graminum) (Rondani). Pakistan J. Zool., 48: 981-987.
Khan, S.A., Hussain, N., Farmanullah and Hayat, Y., 2007. Screening of wheat genotypes for resistance against cereal aphids. Sarhad J. Agric., 23: 427-434.
Khan, S.A., Murugan, M., Starkey, S., Manley, A. and Smith, C.M., 2009. Inheritance and categories of resistance in wheat to Russian wheat aphid (Hemiptera: Aphididae) biotype 1 and biotype 2. J. econ. Ent. 102: 1654-1662. https://doi.org/10.1603/029.102.0433
Kieckhefer, R.W. and Thysell, J.R., 1981. Host preferences and reproduction of four cereal aphids on 20 triticale cultivars. Crop Sci., 21: 322-324. https://doi.org/10.2135/cropsci1981.0011183X002100020029x
Neil, K.A., Gaul, S.O. and McRae, K.B., 1997. Control of the English grain aphid [Sitobion avenae (F.)] (Homoptera: Aphididae) and the oat-birdcherry aphid [Rhopalosiphum padi (L.)] (Homoptera: Aphididae) on winter cereals. Can. Entomol., 129: 1079-1091. https://doi.org/10.4039/Ent1291079-6
Porter, D., Rassenti, R., Shobe, W., Smith, V. and Winn, A., 2009. The design, testing and implementation of Virginia’s NOx allowance auction. J. econ. Behav. Organ., 69: 190–200. https://doi.org/10.1016/j.jebo.2007.09.007
Reese, J.C., Schwenke, J.R., Lamont, P.S. and Zehr, D.D., 1994. Importance and quantification of plant tolerance in crop pest management programs for aphids: greenbug resistance in sorghum. J. agric. Ent., 11: 255-270.
Riazuddin, Anayatullah, M. and Khattak, M.K., 2004. Screening resistant wheat lines against aphids. Pak. Entomol., 26: 144-147.
Smith, C. M., Schotzko, D., Zemetra, R. S., Souza, E. J. and Schroeder-Teeter, S., 1991. Identification of Russian wheat aphid (Homoptera: Aphididae) resistance in wheat. J. econ. Ent., 84: 328–332. https://doi.org/10.1093/jee/84.1.328
Souza, E.J., 1988. Host plant resistance to the Russian wheat aphid (Homptera: Aphididae). In: wheat and barley (eds. S. S. Quisenberry and F.B. Peairs Thomas) Say Publications response model for Russian wheat aphid. Entomological Society of America, Lanham, MD. pp. 122-147.
Stern, V.M., 1967. Control of the aphids attacking barley and analysis of yield increases in the Imperial Valley, California. J. econ. Ent., 60: 485–490.
Wiktelius, S., Weibull, J. and Pettersson, J., 1990. Aphid host plant ecology: the bird cherry oat aphid as a model. In: Aphid plant genotype interactions (eds. R.K. Campbell and R.D. Eikenbary), Elsevier, Amsterdam, The Netherlands, pp. 21–36.
Wyatt, I.J. and White, P.F., 1977. Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J. appl. Ecol., 14: 757–766. https://doi.org/10.2307/2402807
To share on other social networks, click on any share button. What are these?










