Root Rot Disease Complex of Cotton: A Menace to Crop in Southern Punjab and its Mitigation through Antagonistic Fungi
Root Rot Disease Complex of Cotton: A Menace to Crop in Southern Punjab and its Mitigation through Antagonistic Fungi
Muhammad Arslan Khan1,*, Sajid Aleem Khan1, Imran-ul-haq1 and Rashad Waseem Khan2
1Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan
2Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
ABSTRACT
Root rot is very drastic disease prevalent in major cotton producing areas of Pakistan. Complex nature of disease due to root rot fungi and root-knot nematode cause huge losses to cotton. Experiments were conducted to assess the role of fungi and root knot nematodes in root rot disease complex of cotton and to optimize their control. For this purpose fifteen cotton growing areas in Southern Punjab were surveyed in year 2013-14. Data were recorded on disease prevalence, incidence, frequency percent of root rot fungi and nematodes population. Maximum disease prevalence was recorded in Tibba Sultanpur (100%) and Dunyapur (88%) whereas disease incidence calculated was 5% and 4.4%, respectively, in these areas. Rhizoctonia bataticola (Taub.) was frequently isolated (78%) from roots as well as in soil samples (81.7%) collected from infected fields. Among nematodes, Meloidogyne incognita (Kofoid and White) chitwood showed the maximum frequency percentage (72.3%). Results confirmed the presence of fungi and nematodes interacting each other to enhance the disease. Paecilomyces lilacinus (Thom) and Trichoderma harzianum (Rifai) (alone and in combination) were used as bio-control agents against isolated pathogens at three different concentration viz., R, R/2 and R/4. Combination of bio agents (T+P) gave maximum results at R concentration against R. bataticola and M. incognita.
Article Information
Received 11 December 2016
Revised 24 April 2017
Accepted 08 June 2017
Available online 14 September 2017
Authors’ Contribution
MAK carried out the lab work. SAK and IH provided technical support and helped in writing the manuscript. RWK helped in statistical analysis.
Key words
Root rot disease complex, Gossypium hirsutum L., R. bataticola, M. incognita, Paecilomyces lilacinus and Trichoderma harzianum.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1817.1828
* Corresponding author: [email protected]
0030-9923/2017/0005-1817 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Cotton (Gossypium hirsutum L.), a perennial shrub cultivated as an annual crop, is a major cash crop and has a greater share in the world’s economy. About two third of cotton is cultivated in US, India, China and Pakistan. Pakistan ranks at fourth in cotton producing countries of the world while at 10th in term of yield. Cotton and its products contribute 10 percent to GDP and 55 percent to foreign exchange earning of Pakistan (Azam et al., 2013). Nearly 60% of the Pakistan’s total population lives in Punjab. Rice and cotton are the major crops of Punjab (Nia, 2000). It is a warm season crop and grows well in areas having 50 mm annual rainfall with heavy showers at the time of boll formation (Nazir, 2007). Now a days cotton production is on decline due to fungi, bacteria, nematodes and viruses that reduce the yield drastically. Among these, nematodes are considered major yield reducing pests (Agrios, 2005). Root rot is very severe and devastating disease of cotton prevailing in Punjab and Sindh (Iqbal et al., 2012). Climatic conditions of Pakistan favor root knot nematodes and root rot fungi and their further interaction results in crop failure (Anwar and McKerny, 2007). Disease outbreak causes severe losses to cotton crop (Summy, 1992). Plant disease complexes cause 16% of total crop reduction per anum in the world. In view of root rot disease complex, local and systemic modifications induced by root knot nematodes make plants vulnerable to attack by other soil borne fungi (Siddique et al., 2004).
Chemicals are used to control nematodes but due to their high cost and hazardous effects, nematicides are not always attractive to farmers (Khan et al., 2016a). Environment friendly and economical affordable products are now being used due to their advantage of less resistance against the targeted pathogens. As well as people becoming more conscious about environmental and health hazard that is why pesticides are removing from markets (Gerhardson, 2002; Khan et al., 2016b). In the past few years use of chemical pesticides was strictly prohibited due to contamination of ground water, death of avian, toxemia of mammals and accumulation of hazardous chemicals in the food web that is why it is a need of the time to develop some biological management strategies (Bird and Kaloshian, 2003). A great number of efforts have been made for identification of the microorganisms capable of reducing the activity of soil borne pathogens. The majority of such strategies for biological control of such pathogens depend on a single microbial biological control agent for pathogen reduction (Larkin et al., 1998). In diverse rhizophere and soil conditions application of an individual biological control agent may not always perform against all the pathogens of the similar crop. A very little work has been done on the management of root rot disease complex of cotton in Pakistan. Present study was designed to check the prevalence and incidence of disease in different cotton growing regions of Southern Punjab and their control through bio-control agents.
Materials and Methods
Survey of cotton growing areas for root rot of cotton
During 2013-14, an extensive survey of cotton fields was carried out in Southern Punjab, Pakistan to assess the root rot incidence. Different areas selected in these locations were Kabirwala, Tibba Sultanpur, Dunyapur, Dakota, Basti dharik, Fatehpur, Khanewal, Lodhran, D G Khan, Muzaffargarh, Grhamor, Bahawalpur, Multan, Vehari and Rajanpur of Punjab Province, Pakistan. Hundred samples of healthy and infected plants were collected from each locality. The diseased samples were first packed in paper bags and then in 15×20-cm polyethylene bags, labelled, brought to the lab and stored at 4°C until processed for identification. The disease prevalence was measured by following formula:
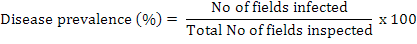
The disease incidence was measured by the following formula (Rao et al., 2016):
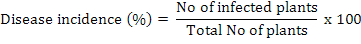
Relative density of nematodes was calculated by using following formula (Anwar and McKerny, 2012):
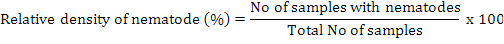
Isolation of plant parasitic fungi from infected samples
The infected roots were cut in to 5-6 cm pieces, washed with tap water and surface disinfected by 2 percent sodium hypochlorite for two minutes. The pieces were given two washings in sterilized water and were blotted on sterilized filter paper sheet for drying. The segments were then plated on potato dextrose agar (potato starch, 20 g; agar agar, 20 g; dextrose, 20 g; distilled water, 1 L) for the isolation of suspected fungus in petri plates for each isolate collected from different locations. All plates were placed at 28±2°C in an incubator, exposed to 12 h photoperiod under white light of wavelength between 350 and 750 nm for 5-7 days for recovery of pathogens (Sharma et al., 2012). The plates were examined under the microscope and identified on the basis of their morphological characters and the growth of fungal hyphae from the segments of plant roots was assessed regularly (Ellis, 1971). It was determined whether more than one fungal species are growing. Purification was done at approximately 5 mm of hyphal growth. A small block of medium from the margin of each colony was transferred to another Petri plate containing PDA and incubated for 5 days at 28±2°C. The frequency of each isolated fungus from each part in the cultured pieces was calculated by using the formula (Saxena and Singh, 1987):
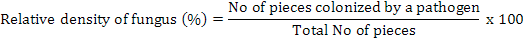
Isolation and identification of fungi from soil
The soil sample were air-dried and grinded it lightly with a mortar and pestle (a marble kitchen mortar and pestle is appropriate) and mixed thoroughly. Then transferred a 10 g sub sample to 100 mL of sterile 0.01% WA (water agar) in a bottle, to give a dilution of 1:10. After that 10 mL was transferred to a second bottle containing 90 mL of 0.01% WA to give a dilution of 1:100 and mixed well to ensure an even spread of the soil in the solution. Repeated this step to give a 1:1000 dilution, which is usually satisfactory for the isolation of Fungus from vegetable or field crop soils. One mL of soil suspension was Dispersed (spread) across the medium in a 9 cm diameter Petri dish. Plates were prepared and let them dry for a few days to eliminate water from the surface of the plate. One mL of soil suspension was carefully pipetted onto the edge of the medium to one side of the plate. Plate was held on a slight slope away from the suspension and gently shaked at right angles to the slope, spreading the suspension in a uniform wetting front across the plate. Isolated plates were incubated under lights for 5-7 days. The colonies were sub-cultured and purified using the single spore technique on PDA. The number of these colony-forming units (CFU) per gram of soil were then be calculated for each species using the following formula (Warcup, 1955):
Dilution × Mean No. colonies of fungal species on isolation plates = CFU/g soil
Pure cultures were identified by observing the following features: micro-morphological features (e.g. asexual and sexual fruiting bodies), spore morphology or morphology of sclerotia, mode of formation of spores (e.g. nature of the conidiogenous cell and the presence or absence of chains of condia). Some important plant pathogens were distinguished on the presence and morphology of sclerotia (e.g. Rhizoctonia).
Collection of diseased samples for nematode isolation and identification
For nematode isolations soil, and plant roots were taken at 4-6 inches depth. Samples were collected and mixed thoroughly and a composite sample of soil was taken in paper bags and then in 15×20-cm polyethylene bags. Polythene bags having samples were tightly sealed to maintain humidity and brought immediately to Laboratory of Nematology, Department of Plant Pathology, University of Agriculture, Faisalabad. The roots were separated from the soil, washed and weighed. The entire root system was chopped and incubated in a mist chamber for 5 days to hatch the eggs (McKerny and Roberts, 1985). Soil samples were thoroughly mixed and processed by Baermann funnel technique for 3 days to collect nematodes (Thistlethwayte, 1970). The isolation of nematodes from soil and root samples was carried out by Whitehead and Hemming tray method (Whitehead and Hemming, 1965) and Baermann funnel method (McKenry and Roberts, 1985), respectively. Relative density of nematodes was calculated by using following formula (Anwar and McKerny, 2012):
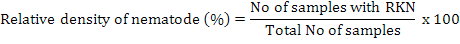
Staining methods
Two staining methods were used. Acid fuchsin was used for staining nematodes within the root systems whereas phloxine B was used to stain Meloidogyne spp. egg masses. Identification of root knot nematodes was done on Perineal patterns of mature females (Eisenback et al., 1981).
Preparation of concentrations
Cultures of antagonistic fungi obtained from Department of Plant pathology, University of Agriculture Faisalabad at different concentrations of culture filtrates of P. lilacinus and T. harzianum R (106 spores/ml), R/2 (104 spores/ml) and R/4 (102 spores/ml) were prepared by adding requisite amount of distilled water.
For mortality test, freshly hatched second stage J2s within 48 h of M. incognita were used. Juveniles of M. incognita were extracted from the eggs and 50 µl of the suspension containing 50 J2s was placed in each petri dish. Petriplate without extract of microbial antagonist was served as control. Four treatments including control of each concentration (P. lilacinus, T. harzianum, P. lilacinus+T. harzianum) with five replications were tested. Data were recorded after 24, 48, 72 h of incubation.
Juvenile mortality was calculated and corrected by Abbot’s formula (Abbott, 1925).
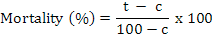
Where t is percent mortality in the chemical (bio/synthetic) and c is percent mortality in the control. Juveniles were considered dead if they did not move when probed with a fine needle (Abbasi et al., 2008) and were considered alive if they moved or appeared as a winding shape (El-Rokiek and El-Nagdi, 2011).
For hatching test, population of M. incognita maintained on the roots of eggplant from single egg mass culture was used, eggs of M. incognita were isolated by the method of Hussey and Barker (1973). A drop of distilled water containing approximately 50 eggs was placed in each petri dish. Three treatments of antagonistic fungi were added in the petri dishes and a control containing only eggs was used. Each treatment was replicated five times and incubated at 28°C ±2 in a completely randomized design. Data were recorded after at 24, 48 and 72 h intervals.
Percent egg hatching was calculated and corrected by Abbot’s formula (Abbott, 1925).
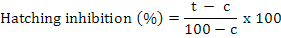
Where t is percent hatching inhibition in the chemical (bio/synthetic) and c is percent hatching inhibition in the control.
Effect of antagonistic potential of T. harzianum and P. lilacinus against R. bataticola
Culture of R. bataticola was maintained on PDA (potato starch, 20 g; agar agar, 20 g; dextrose, 20 g; distilled water, 1 L) in a 9 cm petri plate. Culture T. harzianum and P. lilacinus was maintained on PDA. Dual culture plate technique was used to check antagonistic potential of T. harzianum and P. lilacinus in inhibiting growth of the R. bataticola as described by Morton and Strouble (1955). A mycelial A mycelial disc of 0.3 cm from margin of the 10 days old culture of T. harzianum and P. lilacinus and the pathogen were taken with the help of sterilized needle and placed on the opposite edges of the plate at equal distance from the periphery. Five replications for each treatment alone and in combination of antagonistic fungi were maintained under completely randomized design. Petri plates containing antagonistic fungi alone and in combination and a petri plate containing pathogen were used as control for the comparison of the mycelial growth. All the inoculated plates were incubated at 28 ±2°C until the end of inoculation period (10 days after inoculation). Data was recorded 2, 4, 6, 8 and 10 days interval after inoculation by measuring radial growth of the pathogen and percent inhibition of average radial growth was calculated in relation to growth of controls as follows:
L= [(C-T)/C] x100
Where L represents the inhibition of radial mycelial growth, C is radial growth measurement of the pathogen in control and T is radial growth of the pathogen in the presence of antagonistic isolates (Singh et al., 2002).
Data analysis
Data were analyzed statistically using Statistics 8.1 software and means were compared by LSD test at 5 % significant level (Steel et al., 1997).
Results
Disease prevalence of root rot of cotton in different cotton growing areas of Southern Punjab
Data on disease prevalence was 100% in Tibba Sultanpur which revealed that all the fields visited were infected with root rot. Dunyapur showed 88% while Kabirwala showed 80% prevalence that means 4 out of 5 fields were infected, whereas fifth field was healthy. Rajanpur showed 76% and both Dakota and Khanewal showed 60% prevalence. Bahawalpur represented 56% disease prevalence followed by Multan 48%, Lodhran 36% and Grhamor 36%. Minimum disease prevalence was observed in D G Khan, Fatehpur and Muzaffargarh that was 26%. Results showed that root rot of cotton was most prevalent disease in cotton fields. The results were significantly different from each other at P=0.05 (Fig. 1).
Disease incidence of root rot of cotton in different cotton growing areas of Southern Punjab
During visit the total number of plants from each field were counted and recorded. The total number of plants showing root rot symptoms was recorded. Disease incidence was maximum at Tibba Sultanpur (5%) and Dunyapur (4.4%) while there was less incidence in Kabirwala (4%) followed by Rajanpur (3.8%), Basti dharik (3.4%) and Vehari (3.4%). Khanewal showed 3% while Bahawalpur and Dakota showed 2.8%. Incidence observed in Garhamor and Lodhran was 1.8%. Minimum incidence occurred in D. G. Khan, Fatehpur and Muzaffargarh (1%) (Fig. 1). The results were significantly different from each other at P=0.05.
Frequency % of fungi isolated (root samples) from different locations during survey
Root samples taken from different locations expressed frequency percentage of different fungi. R. bataticola was most frequently isolated fungi. Frequency % of Rhizoctonia bataticola was found to be highest (78%) in root samples collected from Tibba Sultanpur followed by B. theobrome (33.4%), A. alternate (22.8%) and F. oxysporum (17.8%). Frequency % of Rhizoctonia bataticola in Rajanpur was 71.2 % followed by Vehari (71%), Dunyapur (70%), Bahawalpur (66%), Dakota (56.2%) and Lodhran (56%). Basti Dharik and D G khan showed 55% frequency of R. bataticola. Frequency percentage of R. bataticola calculated in Kabirwala was (52.2%) while Grhamor showed (49.6%), Fatehpur showed (49%) and Khanewal (46%). Multan and Muzaffargarh showed 36.2% and 30.6% R. bataticola frequency, respectively. Botryodiplodia theobrome was second frequent fungus (67%) isolated from cotton root samples in Dunyapur followed by Rajanpur (55.2%), Vehari (48%), Bahawalpur (46%), Dakota (43.8%), Fatehpur (39.6%), Tibba Sultanpur (33.4%), D G khan (30.4%), Lodhran (28.8%), and Kabirwala (28.4%). Grhamor and Kanewal showed 25.4% and 25%. Basti dharik (23.4%) showed more frequency percentage of B. theobrome than Multan (22.8%) while minimum percentage was observed in Muzaffargarh (19.2%). Maximum frequency percentage of Alternaria alternata (51.4%) was observed in root samples collected from Rajanpur followed by Vehari (32.6%), Fatehpur (32.4%), Dakota (27.6%), Bahawalpur (26%), Kabirwala (25%) and Basti dharik (24%). Lodhran, D G Khan and Muzaffargarh showed 23.8%, 23.6% and 23.2%, respectively. Grhamor (18.6%) showed more percentage than Dunyapur (17.8%) while Multan (17.4%) showed more percentage than Khanewal (13.2%) and Tibba Sultanpur (12.8%). Fusarium oxysporum was less frequently fungi isolated from all samples collected at different locations. Maximum frequency percentage was observed in Vehari (26.4%) while minimum percentage was observed in Khanewal (10.4%). The results were significantly different from each other at P=0.05 as shown in Figure 2A.
Frequency % of fungi isolated (soil samples) from different locations during survey
By using a soil dilution technique, the mycological analysis of soil samples was studied. Different fungi including R. bataticola, B. theobrome, F. oxysporum, A. alternata, Aspergillus, Penicillium and Mucor were isolated from samples collected at different locations. Maximum frequency percentage (81.7%) of R. bataticola was calculated in Tibba Sultanpur followed by Dunyapur (66.2%), Basti dharik (66.2%), Kabirwala (61.7%), Vehari (52.5%), Bahawalpur (51.2%) and Khanewal (43.7%). R. bataticola isolated from Grhamor (41.7%) soil samples showed higher frequency percentage than Lodhran (40%) while minimum frequency percentage was observed in Multan (25.2%). Maximum frequent fungus was R. bataticola at Tibba Sultanpur followed by A. alternata (34.2%), B. theobrome (33%), F. oxysporum (31%), Aspergillus (25.2%), Penicillium (17.2%) and Mucor (7%). Frequency percentage of fungi isolated from Kabirwala was R. bataticola (61.7%), A. alternata (29.2%), B. theobrome (40%), F. oxysporum (20.7%), Aspergillus (17.2%) and Mucor (4.5%). Frequency percentage of fungi isolated from Multan was R. bataticola (25.2%), A. alternata (22.2%), B. theobrome (29.7%), F. oxysporum (16.2%), Aspergillus (14.5%), Penicillium (5.5%) and Mucor (4%). Frequency percentage of fungi isolated from Dakota was R. bataticola (27.5%), by A. alternata (42.5%), B. theobrome (50%), F. oxysporum (22%), Aspergillus (2.5%), Penicillium (14.5%) and Mucor (11.7%). Frequency percentage of fungi isolated from Khanewal was R. bataticola (43.7%), A. alternata (27.5%), B. theobrome (44.2%), F. oxysporum (23.7%), Aspergillus (8.5%), Penicillium (0.0%) and Mucor (5.5%). Frequency percentage of fungi isolated from Basti Dharik was R. bataticola (66.2%), A. alternata (42.5%), B. theobrome (54.7%), F. oxysporum (29.2%), Aspergillus (33%), Penicillium (0.0%) and Mucor (3.5%). Frequency percentage of fungi isolated from Lodhran was R. bataticola (40%), A. alternata (51%), B. theobrome (30.5%), F. oxysporum (20.2%), Aspergillus (6.5%), Penicillium (7.2%) and Mucor (5%). Frequency percentage of fungi isolated from Fatehpur was R. bataticola (35%), A. alternata (57.5%), B. theobrome (83.7%), F. oxysporum (57.5%), Aspergillus (15.7%), Penicillium (24%) and Mucor (19.5%). Frequency percentage of fungi isolated from D G khan was R. bataticola (26.5%), A. alternata (24.2%), B. theobrome (32.5%), F. oxysporum (11.2%), Aspergillus (4.7%), Penicillium (10.2%) and Mucor (4.7%). Frequency percentage of fungi isolated from Rajanpur was R. bataticola (38.2%), A. alternata (26.5%), B. theobrome (47%), F. oxysporum (27.5%), Aspergillus (2%), Penicillium (30%) and Mucor (9.5%). Frequency percentage of fungi isolated from Muzaffargarh was R. bataticola (26.5%), A. alternata (29.5%), B. theobrome (29.7%), F. oxysporum (11.2%), Aspergillus (10%), Penicillium (5%) and Mucor (2.2%). Frequency percentage of fungi isolated from Grhamor was R. bataticola (41.7%), A. alternata (13.7%), B. theobrome (24.5%), F. oxysporum (32.5%), Aspergillus (2.2%), Penicillium (0.0%) and Mucor (6.2%). Frequency percentage of fungi isolated from Bahawalpur was R. bataticola (51.2%), A. alternata (24.5%), B. theobrome (34.5%), F. oxysporum (23.5%), Aspergillus (24%), Penicillium (11%) and Mucor (3.7%). Frequency percentage of fungi isolated from Dunyapur was R. bataticola (66.2%), A. alternata (42.5%), B. theobrome (61.2%), F. oxysporum (28.2%), Aspergillus (26.7%), Penicillium (7%) and Mucor (13.5%). Frequency percentage of fungi isolated from Vehari was R. bataticola (52.2%), A. alternata (35.5%), B. theobrome (66.2%), F. oxysporum (31.2%), Aspergillus (23.2%), Penicillium (10.5%) and Mucor (15.2%). The results were significantly different from each other at P=0.05 (Fig. 2B).
Population of nematodes at different locations during survey
The results of survey at different locations indicated that maximum nematode population was recorded in Tibba Sultanpur (76.67%) followed by Kabirwala (63.33%). The nematode infestation recorded in Vehari was 56%, Dakota 51%, Bahawalpur 41% and Multan 36%. Infestation recorded in Dunyapur was 33% whereas in Basti Dharik, Lodhran, Muzaffargarh and D G Khan was 26.6%, 17.6%, 13.3% and 13% respectively. Minimum nematode infestation was observed in Fatehpur 12.3 % followed by Grhamor 11.6% and Khanewal 10%. The results were significantly different from each other (P=0.05) as shown in Figure 3A.
Frequency % of nematodes at different locations during survey
M. incognita showed maximum frequency percentage (72.3%) in Tibba Sultanpur followed by Vehari (65.6%) and Bahawalpur (62.6%). Second most frequent nematode was Pratylenchus (53%) in D G Khan followed by Hoplolaimus (33%) in Dunyapur, Heliocotylenchus (33.6%) in Grhamor, Longidorus (26%) in Vehari and Criconema (23.6%) in Dunyapur. Frequency percent of M. incognita in D G khan was 55.33% and Kabirwala showed 52.6%. Grhamor showed 47% followed by Fatehpur (44.6%), Dunyapur (43.3%), Rajanpur (33.3%), Lodhran (32.3%) and Multan (31%). Khanewal showed 28.4% whereas Muzaffargarh showed 28%. Minimum frequency percent of M. incognita was observed in Dakota (23.5%) and Basti Dharik (18.6%). Frequency percent of Pratylenchus in Tibba Sultanpur was 52%, Vehari was 49.3%, in Dunyapur was 44.3%, in Fatehpur was 40.6% and in Grhamor was 40%. Bahawalpur showed 36.6% frequency followed by Kabirwala (30.6%), Multan (29%), Lodhran (28%), Khanewal (27%), Rajanpur (25%), Muzaffargarh (22%), Dakota (19%) and Basti Dharik (16%). Frequency percent of Hoplolaimus in Vehari was 30.6% followed by Bahawalpur (29.6%), Tibba Sultanpur (27%), Grhamor (27%), D G Khan (21.3%), Muzaffargarh (18%), Fatehpur (16.6%), Rajanpur (14.3%), Lodhran (13%), Multan (12.3%), Dakota (10.6%), Khanewal (7.4%) and Basti Dharik (5.7%). Frequency percent of Heliocotylenchus in Bahawalpur was 36% followed by Muzaffargarh (24.6%), Lodhran (22.7%), D G Khan (21.3%), Khanewal (17.4%), Dakota (16.8%), Vehari (16.7%), Fatehpur (13%), Tibba Sultanpur (11.4%), Basti Dharik (9.6%), Rajanpur (8.6%) and Multan (3.3%). Frequency percent of Longidorus in Tibba Sultanpur was 27% followed by Multan (23.3%), Basti Dharik (19%), Rajanpur (16.3%), Bahawalpur (15%), Lodhran (14.6%) and Dakota (12.4%) (Fig. 3B).
Egg hatching inhibition of M. incognita at R, R/2, R/4 concentration of P. lilacinus and T. harzianum
Hatching percent of M. incognita varied significantly at different concentrations of P. lilacinus and T. harzianum. Antagonistic fungi were applied alone and in combination at R concentration of spores. Concentration of spores was maintained using haemocytometer. At R (1×106 spore/ml), R/2 (1×104 spore/ml) and R/4 (1×102 spore/ml) concentrations P. lilacinus significantly inhibited egg hatching of M. incognita as compared to T. harzianum, whereas combined application showed more significant results after 24, 48 and 72 h. After 24 h, P. lilacinus showed 3.6% egg hatching as compared to T. harzianum (7%) at R concentration, whereas their combined application proved more effective (1.6% egg hatching) against nematode. At R/2 after 24 h, 12.6% eggs were hatched in P. lilacinus treatment and T. harzianum showed 22% hatching inhibition, whereas combine application of both showed significant results. At R/4 after 24 h, 18.6% eggs were hatched in P. lilacinus treatment and T. harzianum showed 22.4% hatching inhibition, whereas combined application of both showed significant results (13.2% egg hatching). After 48 h, P. lilacinus showed maximum inhibition (5.6%) compared to T. harzianum (13.8%) at R concentration, whereas their combine application proved more effective (2.8% hatching inhibition) against nematode. At R/2 after 48 h, 18.4% inhibition was recorded in P. lilacinus treatment and T. harzianum showed 28.6% egg hatching, whereas combined application of both showed significant results (12.4%) hatching inhibition. At R/4 after 48 h, 25% hatching was recorded in P. lilacinus treatment and T. harzianum showed 30.6% hatching, whereas combined application of both showed significant results (20.6%). After 72 h, P. lilacinus showed minimum percentage of hatched egg (9.4%) compared to T. harzianum (18.2%) at R concentration, whereas their combined application proved more effective (4.4% egg hatching inhibition) against nematode. At R/2 after 72 h, 24% eggs were hatched in P. lilacinus treatment and T. harzianum showed 32.4% hatching, whereas combined application of both showed significant results (17%) hatching. At R/4 after 72 h, 29.4% eggs were inhibited in P. lilacinus treatment and T. harzianum showed 32.4% hatching, whereas combine application of both showed significant results (24.6%). The results were significantly different from each other (P=0.05) (Fig. 4).
Juvenile mortality of M. incognita at R, R/2, R/4 concentration of P. lilacinus and T. harzianum
The effect of all concentrations varied significantly on juvenile mortality of M. incognita. Results showed that T. harzianum was found very effective against M. incognita juveniles as compared to P. lilacinus, whereas combined application of both showed significant results at all concentration after 24, 48 and 72 h. At R concentration after 24 h, T. harzianum showed 14.6% J2s mortality compared to P. lilacinus (3.8%), whereas combined application of both showed 17.4% J2s mortality. At R/2 concentration after 24 h, T. harzianum showed 10.8% J2s mortality compared to P. lilacinus (2.4%), whereas combined application of both showed 10.8% J2s mortality. At R/4 concentration after 24 h, T. harzianum showed 4.6% J2s mortality compared to P. lilacinus (1%), whereas combined application of both showed 8.6% J2s mortality. At R concentration after 48 h, T. harzianum showed 23.4% J2s mortality compared to P. lilacinus (9.4%), whereas combined application of both showed 28.4% J2s mortality. At R/2 concentration after 48 h, T. harzianum showed 12.6% J2s mortality compared to P. lilacinus (4.6%), whereas combined application of both showed 16% J2s mortality. At R/4 concentration after 48 h, T. harzianum showed 9.6% J2s mortality compared to P. lilacinus (2.6%), whereas combined application of both showed 12% J2s mortality. At R concentration after 72 h, T. harzianum showed 33.2% J2s mortality compared to P. lilacinus (13.2%), whereas combined application of both showed 38% J2s mortality. At R/2 concentration after 72 h, T. harzianum showed 18.4% J2s mortality compared to P. lilacinus (7.6%), whereas combined application of both showed 21.6% J2s mortality. At R/4 concentration after 48 h, T. harzianum showed 13.4% J2s mortality compared to P. lilacinus (4.6%), whereas combined application of both showed 14.4% J2s mortality. The results were significantly different from each other (P=0.05) (Fig. 5).
Evaluation of antagonistic fungi (T. harzianum and P. lilacinus) against R. bataticola (root rot fungus)
T. harzianum and P. lilacinus were tested against R. bataticola in dual culture plate technique. R. bataticola, T. harzianum, P. lilacinus and both (T. harzianum and P. lilacinus) were cultured separately to check their radial growth and then combined and alone antagonistic effect of T. harzianum and P. lilacinus was studied against R. bataticola. R. bataticola showed 7.84 cm radial growth in control. Radial growth of P. lilacinus in control was 5.3 cm. T. harzianum showed 7 cm radial growth in control whereas control with both P. lilacinus and T. harzianum showed 8.4 cm. Results showed that in separate treatments T. harzianum showed better results as compared to P. lilacinus. Radial growth of R. bataticola measured in case of T. harzianum was 2.83 cm, whereas in the case of P. lilacinus radial growth of R. bataticola was 3.95 cm. R. bataticola growth was significantly suppressed by T. harzianum than P. lilacinus. Maximum growth of R. bataticola was suppressed in combination treatment. R. bataticola showed 1.24 cm radial growth when both T. harzianum and P. lilacinus were applied (Fig. 6).
Discussion
Root knot nematodes are most devastating and wide spread group of plant parasitic nematodes, prevalent in almost all cultivated areas of the world. Root knot nematodes are most common in areas having sandy soil with comparatively warm climate. Root knot nematodes take all the nutrients from the diseased plant and reduce the market value of the crop. Higher reproduction potential of root knot nematodes make them difficult to control. They parasitize roots by making injuries and predispose plants to the other soil borne fungal pathogens such as Rhizoctonia solani, Rhizoctonia bataticola, Fusarium oxysporum and Botryodiplodia theobrome.
Root rot caused by the Rhizoctonia bataticola (Taub.) and Rhizoctonia solani (Kuhn) is one of the most damaging diseases of cotton and considerable losses caused by them have been reported. Various reports on the variability of the pathogens of root rot of cotton i.e. R. solani and R. bataticola are available in literature on the basis of physiology, morphology and pathogenicity of different isolates (Monga et al., 2004). Among the four provinces of Pakistan, Punjab’s share in cotton production is estimated to be approximately 81% (Nia, 2000). Disease incidence of root rot, potential severity and population of nematodes during survey have been calculated by many scientists (Kiran et al., 2006; Wheeler et al., 2000; Haq et al., 2012). In present study, survey of different cotton growing areas in Southern Punjab were conducted to calculate the disease prevalence, incidence and the frequency of pathogens present in roots and rhizosphere of cotton plants. R. bataticola was the most frequently isolated fungi from the samples taken. . In current study, results revealed that the higher density of the fungus causing root rot of cotton was isolated from the roots having higher population of nematodes.
Environment friendly and economically affordable products are now being used due to their advantage of less resistance against the targeted pathogens. Moreover, people are becoming more conscious about environmental and health hazard, as a result of which pesticides are being removed from markets (Gerhardson, 2002). In the past few years use of chemical pesticides was strictly prohibited due to contamination of ground water, death of avian, toxemia of mammals and accumulation of hazardous chemicals in the food web that is why it is a need of the time to develop some biological management strategies (Bird and Kaloshian, 2003). A great number of efforts have been made for identification of the microorganisms capable of reducing the activity of soil borne pathogens. The majority of such strategies for biological control of such pathogens depend on a single microbial biological control agent for pathogen reduction (Larkin et al., 1998). In diverse rhizophere and soil conditions application of an individual biological control agent may not always perform against all the pathogens of the similar crop.
A combination of biological control agents in a single preparation can overcome this inconsistent behavior of the antagonistic agent. A greater variety of traits responsible for reduction of one or more pathogen can be found in a combination of biological control agents. There is a possibility of expression of these traits over a wide range of environmental conditions (Pierson and Weller, 1994; Crump, 1998). Present study pointed that culture of T. harzianum and P. lilacinus have nematicidal activity. Nematicidal effect of culture filtrates of different fungi on hatching and mortality of root knot nematodes have been studied by many scientists (Zaki and Maqbool, 1991). In our study it was observed that culture filtrates of T. harzianum and P. lilacinus has capability to parasitize eggs and juveniles of M. incognita on different concentrations and this phenomenon increase with increase in time of exposure. Maximum egg parasitism and juvenile mortality was observed at higher spore concentration of T. harzianum and P. lilacinus after 72 h. Both antagonistic fungi possess larvicidal and as well as ovipocidal activities to compel M. incognita. Biocontrol agents used in the management of M. incognita are ecofriendly and do not have any human health hazards (Ashoub et al., 2009).
These findings are in synchronized with the results obtained by Bonants et al. (1995). Present study is also similar with the results obtained by Montealegre et al. (2005). It was concluded that T. harzianum releases enzymes such as chitinase, glycanase and protease in culture filtrates. Similar results were obtained by Getha et al. (2005). Our results were in conformity with Lumsden et al. (1992) and Ashoub et al. (2009).
Combined application of both P. lilacinus and T. harzianum have strong ovipocidal activity. In combine application after 72 h at higher concentration of antagonists spore, very few eggs were hatched as compared to control while in P. lilacinus alone showed maximum egg inhibition as compared to T. harzianum. Similar results were obtained by Jatala (1986), Dos Santos et al. (1992) and Carnerio and Gomes (1993). Our results are synchronized with that of Sun et al. (2006) during their in vitro experiments they reported a significant parasitism rate of Meloidogyne spp. egg masses by P. lilacinus. Al Kader (2008) enlisted 30 strains of P. lilacinus which have ability to parasitize Meloidogyne spp. eggs. In the present study, maximum egg inhibition was observed during first few hours this indicate that immature eggs are more prone to parasitism as compared to older ones. These results are harmony with Eapen et al. (2005), who reported that P. lilacinus provide significant parasitism during first 24 h.
Present study revealed that combine application of both antagonistic fungi were responsible for the maximum mortality of juveniles of M. incognita as compared to alone treatment. In combined application after 72 h at higher concentration of antagonist spore, maximum juvenile mortality was observed compared to control while in T. harzianum alone showed maximum juvenile mortality as compared to P. lilacinus. Similar findings were reported by Dennis and Webster (1971), who reported that T. harzianum has the ability to produce various enzymes like chitinase, glucanase and protease. These enzymes digest cuticle of M. incognita juvenile and thus destroy cell wall integrity and kills juveniles. Similar results were obtained by Huang et al. (2004). To invade juvenile fungus need to parasitize cuticle that is composed of collagen, keratin and fiber. When cuticle is invaded by fungal hyphae nematode become paralyzed and digested by fungus (Tunlid and Jansson, 1991).
Biological control potential of antagonistic fungi against R. bataticola has been indicated in number of reports (Vasudeva and Sikka, 1941; Jakkar et al., 1997). Utilization of antagonistic microbes is an attractive, environment friendly and economical strategy alternative to chemical management (Bramawy and Sarag, 2012). Keeping in view the above mentioned facts the present study was designed to find out a management strategy for Rhizoctonia root rot of cotton. In vitro evaluation of antagonistic potential of T. harzianum and P. lilacinus was carried out against Rhizoctonia bataticola. It was found that T. harzianum had higher inhibitory potential than P. lilacinus against the pathogen. The results were supported by Devi et al. (2012) that inhibition of the growth of pathogen by T. harzianum isolates is due to its fast growing potential and competition for space and nutrition. The combined application of T. harzianum and P. lilacinus showed best result by inhibiting the growth of pathogen than alone. The results were supported by Nagesh et al. (2006), who reported that the combined application of T. harzianum and P. lilacinus significantly inhibited mycelia growth of F. oxysporum f.sp. lycopersici.
Conclusion
The results revealed that root rot disease complex is very prevalent disease in the cotton growing areas of the Southern Punjab. Root rot of cotton is caused by Rhizoctonia bataticola and its interaction with root knot nematode cause severe disease complex and is very difficult to manage. Root rot disease complex is becoming a problem in Punjab as no proper measures are taken to manage the disease. Present research is highlighting the disease prevalent areas. This will help the farmers to make an integrated disease management plan and biological control will also help to minimize environmental pollution, preserve the agro-ecosystem and bio diversity and keep management process more economical.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abbasi, M.W., Ahmed, N. and Shaukat, S.S., 2008. Effect of Barleria acanthoides Vahl. on root-knot nematode infection and growth of infected okra and brinjal plants. Pak. J. Bot., 40:2193-2198.
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. econ. Ent., 18: 265-267.
Agrios, G.N., 2005. Plant pathology, 5th edition. Academic Press, USA, pp. 570.
Al Kader, M.A.A., 2008. In vitro studies on nematodes interaction with their antagonistic fungi in the rhizosphere of various plants. Albert-Ludwigs-Universitat, Germany, pp. 58.
Anwar, S.A. and Mckenry, M.V., 2007. Variability in reproduction of four populations of Meloidogyne incognita on six cultivars of cotton. J. Nematol., 39: 105-110.
Anwar, S.A. and Mckenry, M.V., 2012. Incidence and population density of plant-parasitic nematodes infecting vegetable crops and associated yield losses in Punjab, Pakistan. Pakistan J. Zool., 40: 327-333.
Ashoub, A.H., Monster, S.A., Mourad, M.H. and Gamal, M., 2009. Impact of some fungi species as biocontrol agent against the root knot nematode Meloidogyne incognita. Aust. J. Basic appl. Sci., 3: 3617-3624.
Azam, S., Samiulla, T.R., Yasmeen, A., Din, S., Iqbal, A., Rao, A.Q., Nasir, I.A., Rashid, B., Said, A.A., Ahmad, M. and Husnain, T., 2013. Dissemination of Bt cotton growing belt of Pakistan. Adv. Life Sci., 1: 18-26.
Bird, D.M. and Kaloshian, I., 2003. Are roots special? Nematodes have their say. Physiol. Mol. Pl. Pathol., 62: 115-123. https://doi.org/10.1016/S0885-5765(03)00045-6
Bonants, P.J., Fitters, P.F., Thijs, H., Belder, E., Waalwijk, C. and Henfling, J.W., 1995. A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs. Microbiology, 141: 775-784. https://doi.org/10.1099/13500872-141-4-775
Bramawy, L. and Sarag, A., 2012. Biological control of root rot of cotton with antagonistic fungi. Asian J. Pl. Sci., 5: 775-778.
Carneiro, R.M.D.G. and Gomes, C.B., 1993. Metodologia e tetede patogenicidade de Paecilomyces lilacinus, P. fumosoroseus, Meloidogyne javanica. Nematoda, 17: 66-75.
Crump, D., 1998. Biological control of potato and beet cyst nematodes. Aspects appl. Biol., 53: 383-386.
Dennis, C. and Webster, J., 1971. Antagonistic properties of species groups of Trichoderma. Production of volatile antibiotics. Trans. Br. Mycol. Soc., 57: 41-48. https://doi.org/10.1016/S0007-1536(71)80078-5
Devi, S.S., Sreenivasulu, Y., Saritha, S., Kumar, M.R., Kumar, K.P. and Sudhakar, P., 2012. Molecular diversity of native Trichoderma isolates against Fusarium oxysporum f.sp. lycopersici (Sacc.). A casual agent of Fusarium wilt in tomato (Lycopersicum esculentum Mill.). Arch. Phytopath. Pl. Protec., 45: 686-698. https://doi.org/10.1080/03235408.2011.591195
Dos Santos, M.A., Ferraz, S. and Muchovej, J.J., 1992. Evaluation of twenty species of fungi from Brazil for biocontrol of Meloidogyne incognita race 3. Nematropica, 22: 183-192.
Eapen, S.J., Beena, B. and Ramana, K.V., 2005. Tropical soil microflora of spice-based cropping systems as potential antagonists of root knot nematodes. J. Inverteb. Pathol., 88: 218-225. https://doi.org/10.1016/j.jip.2005.01.011
Eisenback, J.D., Hirschmann, H., Sasser, J.N. and Triantaphyllou, A.C., 1981. A guide to the four most common species of root knot nematodes (Meloidogyne spp.) with a pictorial key. North Carolina State University, Graphics and USAID, Raleigh, pp. 48.
Ellis, M.B., 1971. More dematiaceus hypomycetes. CMI, Kew, Survey, England, pp. 507.
El-Rokiek, K.G. and El-Nagdi, W.M., 2011. Dual effects of leaf extracts of Eucalyptus citriodora on controlling purslane and root knot nematode in sunflower. J. Pl. Prot. Res., 51:121-129. https://doi.org/10.2478/v10045-011-0021-0
Gerhardson, B., 2002. Biological substitutes for pesticides. Trends Biotechnol., 20: 338-343. https://doi.org/10.1016/S0167-7799(02)02021-8
Getha, K., Vikineswary, S., Wong, W.H., Seki, T.A. and Goodfellow, M., 2005. Evaluation of Streptomyces sp. for suppression of Fusarium wilt and rhizophere colonization in pot grown banana plantlets. J. Microbiol. Biotech., 32: 24-32. https://doi.org/10.1007/s10295-004-0199-5
Haq, I.M., Sajid, M.R., Zahid, A. and Tahir, M.I., 2012. Incidence of root rot diseases of soy bean in Multan Pakistan and its management by the use of plant growth promoting rhizobacteria. Pak. J. Bot., 44: 2077-2080.
Huang, X., Zhao, N. and Zhang, K., 2004. Extracellular enzymes serving as virulence factors in nematophagous fungi involved in infection of the host. Res. Microbiol., 155: 811-816. https://doi.org/10.1016/j.resmic.2004.07.003
Hussy, R.S. and Barker, K.R., 1973. Comparison of methods for collecting inocula of Meloidogyne spp. including a new technique. Pl. Dis. Rep., 57:1025-1028.
Iqbal, M., Iqbal, M.Z., Khan, R.S.A. and Hayat, K., 2012. Comparison of obsolete and modern varieties in view to stagnancy in yield of cotton (G. hirsutum L.). Asian J. Pl. Sci., 4:374-378.
Jakkar, S.S., Chauhan, M.S. and Duhan, J.C., 1997. Biological control of root rot of cotton (Gossypium spp.) caused by Rhizoctonia species using Trichoderma viridae and Trichoderma harzianum under screenhouse conditions. J. Cotton Res. Dev., 11: 191-195.
Jatala, P., 1986. Biological control of plant parasitic nematodes. Annu. Rev. Phytopath., 24: 453-489. https://doi.org/10.1146/annurev.py.24.090186.002321
Jepson, S.B., 1987. Identification of root knot nematodes (Meloidogyne species). CAB International, Wallingford, UK.
Khan, S.A., Javed, N., Anwar, S.A., Haq, I., Naveed, K., Zia-ullah and Safdar, A., 2016a. Survival of entomopathogenic nematodes in sterilized vs non sterilized soil. Pakistan J. Zool., 48: 1349-1352
Khan, S.A., Javed, N., Kamran, M., Abbas, H., Safdar, A. and Haq, I., 2016b. Management of Meloidogyne incognita race 1 through the use of entomopathogenic nematodes in tomato. Pakistan J. Zool., 48: 763-768.
Kiran, K.S., Lingaraju, S. and Aviver, S.S., 2006. Effect of plant extract on Sclerotium rolfsii, the incite of stem rot of groundnut. J. Mycol. Pl. Pathol., 36: 77-79.
Larkin, R.P., Roberts, D.P. and Gracia-Garza, J.A., 1998. Biological control of fungal diseases. In: Fungicidal activity- Chemical and biological approaches to plant protection (eds. D. Hutson and J. Miyamoto). Wiley, New York, pp. 141-191.
Lumsden, R.D., Locke, J.C., Adkins, S.T., Walter, J.F. and Ridout, C.J., 1992. Isolation and localization of the antibiotics gliotoxin produced by Gliocladium virens from alginate prill in soil and soilless media. Phytopathology, 82: 230-235. https://doi.org/10.1094/Phyto-82-230
Mckerny, M.V. and Roberts, P.A., 1985. Phyto-nematology study guide. Division of Agriculture and Natural Resources, Cooperative Extension University of California, Publication 4045.
Monga, D., Ratore, S.S., Mayee, C.D. and Sharma, T.R., 2004. Differentiation of isolates of cotton root rot pathogens Rizoctonia solani and R. bataticola using pathogenicity and RAPD markers. J. Pl. Biochem. Biotechnol., 13: 135-139. https://doi.org/10.1007/BF03263209
Montealegre, J.R., Herrera, R., Velasquez, J.C., Silva, P., Besoain, X. and Perez, L.M., 2005. Biocontrol of root and crown rot in tomatoes under greenhouse conditions using Trichoderma harzianum and Paenibacillus lentimorbus. Additional effect of solarization. Electronic Biotech., 8: 249-257. https://doi.org/10.2225/vol8-issue3-fulltext-7
Morton, D.T. and Strouble, N.H., 1955. Antagonistic and stimulatory effects of microorganism upon Sclerotium rolfsii. Phytopathology, 45: 419-420.
Nagesh, M., Hussaini, S.S., Ramanujam, B. and Chidanadaswamy, B.S., 2006. Management of Meloidogyne incognita and Fusarium oxysporum f.sp. lycopersici wilt complex using antagonistic fungi in tomato. Nematol. Medit., 34: 63-68.
Nazir, S.L., 2007. Control of root rot of cotton with compost rice straw fortified with antagonistic fungi. J. Nematol., 35:324.
Nia, S.M.A., 2000. Cotton: An important cash crop. Pakistan and Gulf Economist Magazine, Karachi, Pakistan.
Pierson, E.A. and Weller, D.M., 1994. Use of mixtures of fluorescent Pseudomonads tosuppress take all and improve the growth of wheat. Phytopathology, 84: 940-947. https://doi.org/10.1094/Phyto-84-940
Rao, S., Danish, S., Keflemariam, S., Tesfagergish, H., Tesfamariam, R. and Habtemariam, T., 2016. Pathological survey on disease incidence and severity of major diseases on Tomato and Chilli crops grown in Sub Zoba Hamelmalo, Eritrea. Int. J. Res. agric. Sci., 2: 20-31.
Sexena, M.C. and Singh, K.B., 1987. The Chickpea. CAB International, ICARDA, pp. 250-252.
Sharma, M., Ghosh, R., Krishnan, R.R., Nagamangala, U.N., Chamarthi, S.K., Varshney, R.K., and Pande, S., 2012. Molecular and morphological diversity in Rhizoctonia bataticola isolates causing dry root rot of chickpea (Cicer arietinum L.). India. Afric. J. Biotech., 11: 8949-8959. https://doi.org/10.5897/AJB11.3657
Siddique, I.A., Shaukat, S.S. and Khan, A., 2004. Differential impact of some Aspergillus spp. on Meloidogyne javanica biocontrol by Psedumonas flourescens strain CHA0. J. appl. Microbiol., 39: 74-83. https://doi.org/10.1111/j.1472-765X.2004.01540.x
Singh, A., Singh, M.K. and Sun, Y.H., 2002. Eye suppression, a novel function of tea shirt, requires wingless signaling. Development, 129: 4271-4280.
Steel, R.G., Torrie, J.H. and Deekey, D.A., 1997. Principles and procedures of statistics. A biometrical approach, 3rd edition. McGraw Hill Book Co. Inc., New York, U.S.A.
Sun, M.H., Gao, L., Shi, Y.X., Li, B.J. and Liu, X.Z., 2006. Fungi and actinomycetes associated with (Meloidogyne spp.) eggs and females in China and their biocontrol potential. J. Inverteb. Pathol., 93: 22-28. https://doi.org/10.1016/j.jip.2006.03.006
Summy, K.R., 1992. Cultural control of cotton insect pests in the United State. Crop Prot., 11:307-339.
Thistlethwayte, B., 1970. Reproduction of Pratylenchus penetrans (Nematode: Tylenchida). J. Nematol., 2: 101-105.
Tunlid, A. and Jansson, S., 1991. Proteases and their involvement in the infection and immobilization of nematodes by nematophagous fungus Arthrobotrys oligospora. Appl. environ. Microbiol., 57: 2868-2872.
Vasudeva, R.S. and Sikka, M.R., 1941. Studies on the root rot disease of cotton in the Punjab. Effect of certain fungi on the growth of root fungi. Indian J. agric. Sci., 11: 422-431.
Warcup, J.H., 1955. Isolation of fungi from hyphae present in soil. Nature, 175: 953-954. https://doi.org/10.1038/175953b0
Wheeler, T.A., Hake, K.D., and Dever, J.K., 2000. Survey of Meloidogyne incognita and Thielaviopsis basicola: Their impact on cotton fruiting and producers’ management choices in infested fields. J. Nematol., 32: 576-583.
Whitehead, A.G. and Hemming, A.K., 1965. Comparison of quantitative method of extracting the small vermiform nematode from soil. Annls. appl. Biol., 55: 25-38.
Zaki, M.J. and Maqbool, M.A., 1991. Paecilomyces lilacinus controls Meloidogyne javanica on chickpea. Int. Chickpea Newsl., 25: 22-23.
To share on other social networks, click on any share button. What are these?










