Relative Susceptibility of Chickpea Varieties to Callosobruchus chinensis L. (Coleoptera: Bruchidae) and Appraisal of its Quantitative and Qualitative Losses
Relative Susceptibility of Chickpea Varieties to Callosobruchus chinensis L. (Coleoptera: Bruchidae) and Appraisal of its Quantitative and Qualitative Losses
Zaigham Abbas1*, Abu Bakar Muhammad Raza1, Muhammad Zeeshan Majeed1, Muhammad Anjum Aqeel1, Talha Nazir2
1Department of Entomology, College of Agriculture, University of Sargodha, Sargodha 40100, Pakistan
2Centre for Agriculture and Bioscience International (CABI), Rawalpindi 46000, Punjab, Pakistan
Abstract | Pulse beetle, Callosobruchus chinensis L., is one of the primary pests of stored grain commodities and causes considerable quantitative and qualitative losses. This study was conducted to assess the relative susceptibility of some desi (Punjab-2008 and Thal-2006) and Kabuli (Punjab-2009 and CM-2008) varieties of chickpea, Cicer arietinum L. Quantitative and qualitative losses incurred by the infestation of C. chinensis were determined under laboratory storage conditions. Maximum grain weight loss was recorded in Thal-2006 (9.07%) after 120 days of infestation followed by CM-2008 (8.37%), while relatively lower weight loss (6.95%) was recorded in case of Punjab-2009. After 90 days of infestation, mean weight loss values for Thal-2006, CM-2008, Punjab-2008 and Punjab-2009 were recorded as 8.21, 5.95, 5.36 and 5.13%, respectively. Relatively lower values of grain weight loss were recorded at 60 and 30 days post-infestation. Minimum grain loss value (0.75%) was recorded in case of control (with no pest infestation). Highest and lowest moisture contents were recorded for Thal-2006 (16.32%) and Punjab-2009 (14.25%), respectively. Similarly, C. chinensis infestation caused highest and lowest grain germination reduction values for Thal-2006 (23.67%) and Punjab-2009 (10.32%), respectively recorded at 120-day post-treatment. Results of qualitative losses revealed that protein and ash contents reduced maximally up to 12.46 and 1.11% in CM-2008 variety, respectively. While minimum protein and ash content reductions were noted in Punjab-2008 variety (i.e., 8.58 and 0.75%), respectively. Similarly, highest reduction in crude fat and carbohydrate contents (i.e., 1.18 and 13.21%) were noted for Thal-2006 and CM-2008 varieties, respectively. While, Punjab-2008 and Punjab-2009 exhibited minimum reductions (i.e., 0.91 and 7.8%) in crude fat and carbohydrate contents, respectively. From overall study results, it is concluded that C. chinensis can result in considerable damage to stored chickpea grains. Moreover, rough surface chickpea varieties were comparatively less preferred by C. chinensis than smooth surface varieties which are found more susceptible to pest infestation.
Novelty Statement | This study is novel as it demonstrates that pulse beetle Callosobruchus chinensis cause considerable qualitative and quantitaive damage to smooth surfaced chickpea varieties than rough surface chickpea varieties.
Article History
Received: October 10, 2023
Revised: March 25, 2024
Accepted: April 12, 2024
Published: May 24, 2024
Authors’ Contributions
ZA performed experiments, recorded data and wrote the manuscript. ABMR conceived and designed the experimental protocol, and supervised the study. MZM prepared tables and figures and proofread the manuscript. MAA provided technical assistance in experimentation. TN performed statistical assistance and helped in results preparation.
Keywords
Pulse beetle, Chickpea varieties, Varietal screening, Qualitative loss, Quantitative parameters, Relative resistance
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding author: Zaigham Abbas
To cite this article: Abbas, Z., Raza, A.B.M., Majeed, M.Z., Aqeel, M.A. and Nazir, T., 2024. Relative susceptibility of chickpea varieties to Callosobruchus chinensis L. (Coleoptera: Bruchidae) and appraisal of its quantitative and qualitative losses. Punjab Univ. J. Zool., 39(1): 87-96. https://dx.doi.org/10.17582/journal.pujz/2024/39.1.87.96
Introduction
Chickpea (Cicer arietinum L.) is one of the important pulse crops being grown in Indo-Pak regions. It is grown in different rainfed areas of the world including Pakistan, India, Turkey, Mexico, Europe, Syria, Australia and Iran (Aslam et al., 2006). Chickpea grains are very nutritious having 24% protein content, 40% starch content, 6% crude fiber, 5% fat content, 3.5% ash and other minerals (calcium, phosphorous, iron) and vitamins (Righi-Assia et al., 2010; Hirdyani, 2014). It is helpful in controlling and even reducing blood sugar level. Chickpea consumption is a good remedy for lowering cholesterol level (Kumar et al., 2009). However, during storage, this commodity is liable to both quantitative and qualitative losses (Ahmed et al., 2003). Qualitative losses result in decreasing aesthetic and nutritional value of grains and quantitative damage results in decreasing germination percentage and weight of chickpea grains (Kim et al., 2003; Islam et al., 2013).
Chickpea grains are often infested by certain Bruchid beetles (Coleoptera: Bruchidae) which cause a considerable qualitative as well as quantitative losses to the grains (Eker et al., 2018). The Bruchids have long been recognized as destructive insect pests of stored chickpea grains (Srinivasan et al., 2008). Among these, two Callosobruchus species, specifically Callosobruchus chinensis L. and Callosobruchus maculatus F. have been most frequently observed in stored chickpeas world widely (Erler et al., 2009; Singh et al., 2012). Both species possess identical habitat and lifestyle and are difficult to distinguish from each other (Kyogoku and Nishida, 2013). The C. chinensis is relatively more damaging and frequently observed species in stored chickpea than C. maculatus (Purohit et al., 2013). It is commonly known as gram dhora or pulse beetle and causes substantial quantitative and qualitative losses (Aslam et al., 2006; Upadhyay et al., 2011). Its infestation results in reduced germination capacity, grain weight and seed value (Singh et al., 2016). It is a destructive pest of stored pulses in Africa and Asia (Kiradoo and Srivastava, 2010). Being cosmopolitan, it also damages other crop grains like mung bean, lentil, cowpea, maize and sorghum. Pulse beetle attacks in field by entering inside grains and making holes and feed on grains (Thakur and Pathania, 2013). Grubs and pupae of pulse beetle are internal feeders and both grubs and adult beetles cause damage to grains (Khalequzzaman and Goni, 2009). Damaged grains are not suitable for human consumption and the germination percentage of grains is also severely affected along with reduced market value (Herald et al., 2022).
Management of the stored grain insect pests is primarily being done by the application of synthetic insecticides including pirmiphos-methyl, chlorpyriphos-methyl and deltamethrin (Daglish et al., 2018). At present, indiscriminate use of fumigants and synthetic insecticides to control insect pest has led to many problems such as pest resistance, resurgence and food poisoning (Stejskal et al., 2021). Fumigation with phosphine gas is the foremost tactic for protection of stored grain against insect pests (Collins, 2006). Currently, phosphine is the most widely used fumigant, but its use is limited due to its adverse effects on the environment and development of pest resistance against phosphine (Collins, 2006; Hossain et al., 2014; Jaiswal et al, 2019).
Therefore, in order to reduce over-reliance on the extensive use of synthetic chemicals against C. chinensis infestations, exploration for host plant resistance in leguminous crops has become a compelling alternative in the last few years (Shaheen et al., 2006). Improvement and utilization of resistant chickpea varieties provide an inexpensive and simple method for the management of pulse beetle infestation, and also increases the efficiency of other pest control methods such as biological and cultural control strategies (Ashok et al., 2020). Therefore, several studies were done from time to time to assess the relative susceptibility of different available legume varieties for resistance to different Bruchid beetles (Shaheen et al., 2006; Sarwar, 2012; Singh et al., 2012; Raghuwanshi et al., 2016).
Keeping in view the above-mentioned information, an in-vitro effort was taken to determine the quantitative and qualitative losses incurred by the pulse beetle C. chinensis to grains of four major chickpea varieties during storage conditions.
Materials and Methods
Experimentation was performed in the Laboratory of Entomology, College of Agriculture, University of Sargodha, Sargodha, Punjab, Pakistan.
Collection of chickpea grains
Grains of two desi (Punjab-2008 and Thal-2006) and two Kabuli (Punjab-2009 and CM-2008) varieties of chickpea were acquired from the Pulses Research Institute, Ayyub Agricultural Research Institute (AARI), Faisalabad, Punjab, Pakistan. Twenty kilograms of collected grains were fumigated with aluminum phosphide tablets to nullify any antecedent pest infestation on the grains. These fumigated grains were further used for the experimentation.
Collection and rearing of C. chinensis
Mixed age culture of pulse beetle was collected from the godowns and stores of Punjab Food Department, and was mass reared under controlled laboratory conditions. For each variety, 100 adult beetles (50 pairs) were released in 1.0 kg of fumigated chickpea grains in a plastic jars (15×20 cm) which were closed with fine mesh cloth tightened with rubber rings, and were placed at 32±2 °C temperature and 65±5% relative humidity. Adult beetles were left for 3 to 4 days for mating and oviposition. Chickpea grains with eggs were left over for 25 days in order to attain adult beetles. Pupae of same days were collected and introduced in a plastic jar on one kilogram of un-infested and clean chickpea grains to ensure homogenous population.
Determination of quantitative and qualitative losses incurred by pulse beetle
Experimental setup
One kg of fumigated chickpea grains of collected varieties was taken in plastic jars (20 × 15 cm). Jars were closed with fine mesh cloth and rubber band and were exposed to room temperature (25°C) for seven days until grain moisture content was stabilized at approximately 13%, suitable for insect growth. These jars were then infested with 20 uniformly sized and aged adult pairs of C. chinensis. The jars were put in an environmental chamber set at 65 ± 5% relative humidity and 32 ± 2 °C temperature. Growth chamber was opened for 30 min daily to ensure proper aeration. Five independent replications were maintained for each treatment. After 30 days, the sample from each treatment was drawn for the analysis of quantitative and qualitative losses induced by the pulse beetle in chickpea varieties. Data was recorded after 30, 60, 90 and 120 days post-infestation. Quantitative and qualitative losses were determined before and after the infestation of C. chinensis in storage.
Determination of quantitative parameters
Grain weight loss
About 50 g of grains were drawn from each treatment and were separated as damaged and undamaged grains. The grains were enumerated and weighed using an electronic balance. Percent weight loss was calculated using the formula given by Gwinner et al. (1996).
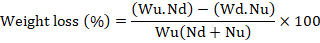
Where, Wu= Nu= Number of undamaged grains, Weight of undamaged grains, Nd= Number of damaged grains and Wd= Weight of damaged grains.
Determination of grain moisture content
For moisture determination, 20 g grains were drawn from each jar. The sample was grinded and mixed rapidly with a spoon and was transferred to Petri-dishes. Each sample was dried in an hot-air oven at 130 °C temperature for 60 min and Petri-dishes were weighed until a constant weight. Weight loss was calculated. Moisture content in each sample was determined by AACC (2000) Method 44-15a (Kalnina et al., 2015).
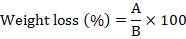
Where, A= grains moisture loss and B= original sample weight.
Germination percentage
Germination test was conducted after 30, 90, 60 and 120 days of infestation or storage. Ten grains from each replication were drawn and were washed with 5% sodium hypochlorite to remove any type of contamination. Selected grains were put on a moistened filter paper in Petri-plates for germination. Germination percentage was determined using following formula (Khanna et al., 2017).
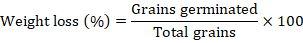
Determination of qualitative parameters
Determination of crude protein
Total nitrogenous compounds that were present in analyzed product are expressed as the total crude protein content and was calculated from nitrogen concentration of grains. The sample was digested by heating in digestion glass in the presence of catalyst and oxidizing agent. After digestion, neutralization of solution was carried in receiving flask and titration was carried out of ammonium borate formed with hydrochloric acid to calculate nitrogen content. Thereafter, nitrogen content was determined by Kjeldhal’s method using conversion factor i.e., percent protein = F (6.25) (Mariotti et al., 2008).
Determination of ash content
Ash content was measured from 20 g of sample from each treatment. The collected sample was charred on burner at 600 °C temperature and the sample was burned inside a muffle furnace for two hours (Technical, 2009). Formula given in AACC (2000) Method No. 08-01 was used to calculate ash content.
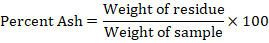
According to AACC Method No. 30-01 (Flores‐Silva et al., 2015), the crude fat content was determined by extracting dried sample through Soxhlet apparatus. About 10 g of the sample was dried in a vacuum oven at 95-100 °C temperature for about 5 h. Then sample was shifted to an extractor and was extracted using petroleum ether as extraction solvent for 4 h at a condensation rate 5-6 drops/sec. Ether was removed from the collection beaker at low temperature. Drying of the remaining was done in oven at 100 °C temperature and then sample was desiccated and cooled.
Determination of carbohydrates
The starch content of grains was determined after enzymatic decomposition with amyloglucosidase. In brief, sample was added in a glass centrifuge tube (16 × 120 mm; 17 ml capacity) and after addition of 5 ml of aqueous ethanol (80% v/v) it was stirred on vortex mixer as per procedure given in AACC Method No. 76-13.
Experimentation was done following completely randomized design (CRD). After correction by Abbott’s Formula (1925), data were subjected to factorial analysis of variance (ANOVA) using Statistix® (Version 8.1 V, Tallahassee, FL, USA) followed by Fischer’s least significant difference (LSD) post-hoc test (at α = 0.05) in order to differentiate between the treatment means.
Results and Discussion
Grains weight loss
Changes in weight loss of grains of different chickpea varieties infested by C. chinensis were determined at different post-infestation time intervals. Treatments, observation time periods and their interaction exerted a significant impact on the average grain weight loss (Table 1). Results showed that highest weight losses (9.07%) was recorded in Thal-2006 at 120 days of infestation, while relatively lower grain weight loss (6.95%) was recorded in case of Punjab-2009. Lowest grains loss value (0.75%) was recorded in untreated (control) treatment (Figure 1).
Table 1: Analysis of variance table regarding mean percent weight loss of grains of chickpea varieties infested with C. chinensis (see Figure 1).
|
Source |
DF |
SS |
MS |
F-value |
|
Time |
3 |
272.01 |
90.668 |
11.31** |
|
Treatment |
3 |
74.67 |
24.889 |
3.11* |
|
Time × Treatment |
9 |
95.20 |
10.578 |
2.73 * |
|
Error |
80 |
309.10 |
3.863 |
|
|
Total |
95 |
750.98 |
* p < 0.05 (significant), ** p < 0.001 (highly significant), ANOVA (analysis of variance) at α = 0.05. F, F-statistic; MS, Mean sum of squares; SS, Sum of squares; DF, Degree of freedom.
Moisture contents
Results revealed fluctuation in mean moisture contents of C. chinensis infested grains of all chickpea varieties stored for different time periods. Treatments, observation time and their interaction exerted a significant impact on average grain moisture contents (F = 11.05, p < 0.001, F = 9.90, p < 0.001 and F = 2.06 p < 0.05, respectively; Table 2). The highest moisture contents (16.32%) was noted in Thal-2006 after exposure period of 120 days, while relatively lower moisture content (14.25%) was recorded in Punjab-2009. Varieties Thal-2006 and Punjab-2008 were statistically at par. Relatively lower values of moisture losses were recorded at 30 days of storage. Lowest value (9.51%) was recorded in control (uninfested grains) (Figure 2).
Table 2: Analysis of variance table regarding mean percent moisture content of grains of chickpea varieties infested with C. chinensis (see Figure 2).
|
Source |
DF |
SS |
MS |
F-value |
|
Time |
3 |
82.964 |
27.654 |
9.90** |
|
Treatment |
3 |
92.670 |
30.890 |
11.05** |
|
Time × Treatment |
9 |
51.899 |
5.567 |
2.06 * |
|
Error |
80 |
223.570 |
2.795 |
|
|
Total |
95 |
451.103 |
* p < 0.05 (significant), ** p < 0.001 (highly significant), ANOVA (analysis of variance) at α = 0.05. F, F-statistic; MS, Mean sum of squares; SS, Sum of squares; DF, Degree of freedom.
Percent germination reduction
Results revealed a differential germination of C. chinensis-infested grains of all chickpea varieties stored for different time periods. Treatments, observation time periods and their interaction exerted a significant impact on the percent germination reduction (F = 3.11, p < 0.05, F = 11.31, p < 0.001 and F = 2.12 p < 0.05, respectively; Table 3). The highest germination reduction (23.67%) was noted in Thal-2006 after 120 days of storage followed by CM-2008 (16.83%). While relatively low germination reduction (10.32%) was recorded in case of Punjab-2009. Lowest value of germination reduction (2.10%) was recorded in untreated (control) treatment (Figure 3).
Table 3: Analysis of variance table regarding mean percent germination reduction of grains of chickpea varieties infested with C. chinensis (see Figure 3).
|
Source |
DF |
SS |
MS |
F-value |
|
Time |
3 |
1081.95 |
90.668 |
11.31** |
|
Treatment |
3 |
1578.28 |
24.889 |
3.11* |
|
Time × Treatment |
9 |
544.93 |
60.54 |
2.12* |
|
Error |
80 |
2290.10 |
28.62 |
|
|
Total |
95 |
* p < 0.05 (significant), ** p < 0.001 (highly significant), ANOVA (analysis of variance) at α = 0.05. F, F-statistic; MS, Mean sum of squares; SS, Sum of squares; DF, Degree of freedom.
Protein content of grains
The results showed variation in mean percent crude protein contents in C. chinensis infested chickpea varieties (Table 4). Protein contents reduced to 12.06% from 21.08% in Thal-2006 variety, 13.59% from 22.17% in Punjab-2008, while it was reduced to 10.56% from 23.02% in CM-2008 and to 12.71% from 22.86% in Punjab-2009 variety (Table 5).
Table 4: Analysis of variance table regarding mean crude protein contents of grains of chickpea varieties infested with C. chinensis (see Table 5).
|
Source |
DF |
SS |
MS |
F |
P-value |
F crit |
|
Treatment |
4 |
237.781 |
59.445 |
56.159 |
7.56E-09 |
3.055 |
|
Time |
15 |
15.877 |
1.058 |
|||
|
Total |
19 |
253.658 |
Ash content
C. chinensis infestation caused a significant and differential impact on mean ash content of chickpea varieties (Table 6). Ash content reduction after 120 days of infestation was from 2.77% to 1.96% and from 2.88% to 2.13% in Thal-2006 and Punjab-2008 varieties of desi chickpea, respectively. In CM-2008 and Punjab-2009 varieties, these ash contents reduced from of the 2.78% to 1.67% and 1.98%, respectively (Table 7).
Table 5: Mean crude protein content (%) of grains of different chickpea varieties incurred by the infestation of C. chinensis (see Table 4).
|
Types of chickpea |
Varieties |
Before infestation |
After 30 days |
After 60 days |
After 90 days |
After 120 days |
|
Desi Chickpea |
Thal-2006 |
21.08 |
18.48** |
16.87** |
15.74** |
12.06** |
|
Punjab-2008 |
22.17 |
20.37* |
17.75* |
15.94* |
13.59* |
|
|
Kabuli Chickpea |
CM-2008 |
23.02 |
19.61* |
17.30** |
14.58** |
10.56** |
|
Punjab-2009 |
22.86 |
21.21* |
18.78** |
16.86** |
12.71** |
* Significant at p ≤ 0.05, ** Significant at p ≤ 0.01, NS, Non-significant.
Table 6: Analysis of variance table regarding mean total ash contents of grains of chickpea varieties infested with C. chinensis (see Table 7).
|
Source |
DF |
SS |
MS |
F |
P-value |
F crit |
|
Treatment |
1.816 |
4 |
0.454 |
12.730 |
0.0001 |
3.055 |
|
Time |
0.535 |
15 |
0.035 |
|||
|
Total |
2.352 |
19 |
Table 7: Mean total ash content (%) of grains of different chickpea varieties incurred by the infestation of C. chinensis (see Table 6).
|
Types of chickpea |
Varieties |
Before infestation |
After 30 days |
After 60 days |
After 90 days |
After 120 days |
|
Desi Chickpea |
Thal-2006 |
2.77 |
2.59* |
2.42* |
2.14* |
1.96* |
|
Punjab-2008 |
2.88 |
2.78* |
2.67* |
2.52** |
2.13** |
|
|
Kabuli Chickpea |
CM-2008 |
2.78 |
2.37* |
2.17* |
1.92** |
1.67** |
|
Punjab-2009 |
2.78 |
2.57* |
2.58* |
2.20* |
1.98* |
* Significant at p ≤ 0.05, ** Significant at p ≤ 0.01, NS, Non-significant
Table 8: Analysis of variance table regarding mean crude fat contents of grains of chickpea varieties infested with C. chinensis (see Table 9).
|
Source |
DF |
SS |
MS |
F |
P-value |
F crit |
|
Treatment |
2.384 |
4 |
0.596 |
16.907 |
1.980 |
3.055 |
|
Time |
0.528 |
15 |
0.035 |
|||
|
Total |
2.913 |
19 |
|
Fat content
Results in Table 8 showed that in case of Thal-2006 and Punjab-2008 varieties, fat contents were reduced to 3.28 and 3.53 from 4.46 and 4.44%, respectively. While CM-2008 and Punjab-2009 varieties displayed reduction in fat contents to 3.23% from 4.39% and 3.50% from 4.42%, respectively (Table 9).
Table 9: Mean crude fat content (%) of grains of different chickpea varieties incurred by the infestation of C. chinensis (see Table 8).
|
Types of chickpea |
Varieties |
Before infestation |
After 30 days |
After 60 days |
After 90 days |
After 120 days |
|
Desi chickpea |
Thal-2006 |
4.46 |
4.14* |
3.98* |
3.72** |
3.28** |
|
Punjab-2008 |
4.44 |
4.32* |
4.20* |
3.94* |
3.53** |
|
|
Kabuli chickpea |
CM-2008 |
4.39 |
3.84* |
3.74* |
3.40** |
3.23* |
|
Punjab-2009 |
4.26 |
4.30* |
4.00* |
3.90* |
3.50* |
* Significant at p ≤ 0.05, ** Significant at p ≤ 0.01, NS, Non-significant.
Carbohydrate contents
Similarly, mean percent carbohydrate content were also considerable reduced by the infestation of C. chinensis in all chickpea varieties (Table 10). Carbohydrate contents reductions were statistically different in Punjab-2008, Thal-2006 and CM-2008. Among all the tested varieties, highest reduction in carbohydrates contents was for Thal-2006 (from 44.59% to 31.38%), whereas relatively lowest reduction was noted in Punjab-2008 variety (from 45.21% to 37.41%) (Table 11).
Table 10: Analysis of variance table regarding mean carbohydrate contents of grains of chickpea varieties infested with C. chinensis (see Table 11).
|
Source |
DF |
SS |
MS |
F |
P-value |
F crit |
|
Treatment |
249.685 |
4 |
62.421 |
8.150 |
0.001 |
3.055 |
|
Time |
114.8831 |
15 |
7.658 |
|||
|
Total |
364.569 |
19 |
Table 11: Mean carbohydrate content (%) of grains of different chickpea varieties incurred by the infestation of C. chinensis (see Table 10).
|
Types of chickpea |
Varieties |
Before infestation |
After 30 days |
After 60 days |
After 90 days |
After 120 days |
|
Desi chickpea |
Thal-2006 |
44.59 |
42.16* |
40.11* |
37.86* |
31.38* |
|
Punjab-2008 |
45.21 |
44.14* |
42.24* |
39.68** |
37.41** |
|
|
Kabuli chickpea |
CM-2008 |
46.24 |
39.58* |
36.61* |
34.57** |
35.78* |
|
Punjab-2009 |
47.54 |
46.20* |
44.27* |
41.25* |
38.44* |
* Significant at p ≤ 0.05, ** Significant at p ≤ 0.01, NS, Non-significant.
Discussion
The current research work was executed to investigate the qualitative and quantitative losses of protein, carbohydrate, ash and moisture loss of grains of different chickpea varieties due to C. chinensis infestation.
Weight loss
The results of weight loss disclosed that highest weight loss (9.07%) was noted in Thal-2006 at 120 days post-infestation, while relatively lower (6.95%) was recorded in case of Punjab-2009. These findings are in agreement with Pradhan et al. (2020) who reported a 37 to 64% loss in chickpea grains weight after 6 month of infestation by C. chinensis. The findings match with results of Phadtare et al. (2023) who found weight loss of up to 14.77 % in chickpea seeds after 240 days of infestation of C. chinensis. Siddiqa et al. (2013) also reported a weight loss in range of 4 to 70% by C. chinensis attack. Moreover, our findings are also consistent with Pokharkar and Chauhan (2010) who appraised the weight losses in different varieties of desi and Kabuli chickpea and noted greater losses in Kabuli varieties as compared to the desi ones. Likewise, Soumia et al. (2017) assessed vulnerability of certain chickpea varieties to the C. chinensis infestation and noted highest infestation in Kabuli grams, whereas no infestation of C. chinensis was noted in kidney shaped beans and in desi chickpea varieties. Our results are in line with Eker et al. (2018) who assessed susceptibility of some varieties of desi and Kabuli and noted that highest seed damage was observed in Kabuli type species as we noted in our research work. However, our results are somewhat different from the findings of Raghuwanshi et al. (2016). The difference may be due to different chickpea varieties used in this study than those tested in this study. Increased moisture content was also noted in infested chickpea varieties in our study. These results are corroborating the conclusions of Shaheen et al. (2006); Adetumbi et al. (2009) and Bhandari et al. (2017).
Moisture loss
Findings regarding percent grain moisture changes displayed that highest moisture contents (16.32%) was noted in Thal-2006 at 120 days of incubation or pest infestation, while relatively lower (14.25%) was recorded in case of Punjab-2009. The moisture content of different varieties may be different during the storage period. According to Rolania et al. (2021), 10.90, 10.15 and 10.12% moisture loss was recorded in chickpea varieties. Our results are in line with Verma et al. (2011) who found a positive association between the infestation of C. chinensis and grains moisture content.
Germination
The grains germination potential decreased significantly as the degree of infestation increased. Highest reduction in grain or seed germination was 23.67% in Thal-2006 at 120 days post-infestation followed by CM-2008 (16.83 %). These findings are according to Allali et al. (2020) who found germination reduction in chickpea by the infestation of C. chinensis. Previous work by Mukendi et al. (2018) revealed that germination potential is greatly reduced of infested grains. Our results corroborate the findings of Shaheen et al. (2006) and Dhakar et al. (2022) who demonstrated that pulse beetle infestation in stored chickpea grains minimized the seed germination.
Protein content
Results of qualitative parameters depicted that protein contents reduced from 10.56 to 23.02% in chickpea varieties. Deepika et al. (2020) noticed protein content from 15.33 from 22.70% in genotype JG 315 of chickpea. Saxena and Saxena (2011) depicted protein content losses from 18 to 21.22% incurred by C. chinensis infestation. The contents of crude protein and fat were significantly reduced after the infestation of C. chinensis for longer period during storage (Sarwar, 2012).
Ash content
Ash content reduction (2.77 to 1.96%) in Thal-2006 variety of desi chickpea. In Punjab-2008 (2.88 to 2.13), in CM-2008 and Punjab-2009 variety of the Kabuli chickpea (2.78 to 1.678% and 2.78 to 1.98%) was recorded. These results are in line with Saxena and Saxena (2011) who noticed significant reduction in ash content in different chickpea varieties. Values of ash content in this study are in line with those reported by Khandaitaray et al. (2023).
Crude fat
Crude fat percent in case of Thal-2006 and Punjab-2008 varieties, reduction was 3.28 from 4.46 % and 3.53 from 4.44%, respectively. While CM-2008 and Punjab-2009 varieties displayed reduction in fat contents, respectively from 3.32 to 4.42%. Saxena and Saxena (2011) demonstrated as well a reduction in crude fat from 4.8 to 5.40% after 6 months of infestation of C. chinensis. Siddiqa et al. (2013) studied different germplasms of chickpea and established that the fat content of different germplasms was significantly different in chickpea grains during the storage.
Carbohydrates
Highest reduction in carbohydrates contents (44.59 to 31.38%) was found in Thal-2006 variety, whereas relatively lowest reduction was noted in Punjab-2009 variety. Current findings are in agreement with Sharma et al. (2023) who found reduction in carbohydrates in chickpea grains after C chinensis infestation. Results of this study are similar to those of Deepika et al. (2020) who found carbohydrates loss from 37.67 to 48.65%. Current finding is partially consistent with the findings of Saxena and Saxena (2011) who stated 45 to 54% carbohydrate content reduction after 6 months of pest infestation.
Surface structure of the chickpea grains plays an important role in the selection of grams for insect pest infestation. Grains with smooth surface are more preferred by C. chinensis. Khana et al. (2017) assessed the susceptibility of desi and Kabuli chickpea varieties and noticed that desi chickpea showed resistance to damage than the Kabuli varieties. Due to wrinkled and rough shape, desi chickpea varieties experienced relatively less infestation compared to smooth shape of Kabuli varieties (Erler et al., 2009). Similar trend was observed in the current research work. Many researchers have worked on assessment of susceptibility and storage losses in different pulse varieties including chickpea by infestation of C. chinensis. Kabuli variety CM-2008 was found more susceptibly than the Punjab-2008 in the current study. Our results revealed that the germination of the attacked grains were reduced. A quantitative study was conducted by Jha (2002) and noticed that the variety BG-267 was highly preferred by the pulse beetle, while the variety BG-256 was not much preferred. Results of crude protein in our research revealed the reduction in nutritional contents. It has been found that the crude protein content is positively correlated with the grain moisture content and pest infestation (Akhtar et al., 2022).
Conclusions and Recommendations
Based on overall findings of the study, it is concluded that the infestation by C. chinensis has differential qualitative and quantitative loss in different chickpea varieties. C. chinensis resulted in considerable damage to stored chickpea grains of all varieties. Moreover, rough surface chickpea varieties (Punjab-2008 and Punjab-2009) were comparatively less preferred by the pest than smooth surface varieties (Thal-2006 and CM-2008). The later ones were found more susceptible to C. chinensis attack than earlier ones. Furthermore, phenology factor was also crucial as smooth surface of grains was favored by insect pest attack.
Acknowledgement
Authors acknowledge the technical help and valuable advice given by Dr. Muhammad Asam Riaz and Dr. Muhammad Irfan Ullah during the preparation and proofreading of the draft.
Conflict of interest
The authors have declared no conflict of interest.
References
AACC (American Association of Cereal Chemists), 2000. Approved Methods of the AACC, 10th ed. Methods 08-01, 44-16, 46-30, 56-10, 56-11, and 56-61A. The Association: St. Paul, MN.
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol., 18: 265-267. https://doi.org/10.1093/jee/18.2.265a
Adetumbi, J.A.A., Odiyi, A.C.B., Olakojo, S.A.C. and Adebisi, M.A.D., 2009. Effect of storage materials and environments on drying and germination quality of maize (Zea mays L) seed. Elect. J. Environ. Agric. Fd. Chem., 8: 1140-1149.
Ahmed, K., Itino, T. and Ichikawa T., 2003. Duration of developmental stages of Callosobruchus chinensis (Coleoptera: Bruchidae) on Adzuki bean and the effects of Neem and Sesameoils at different stages of their development. Pak. J. Biol. Sci., 6: 932-335. https://doi.org/10.3923/pjbs.2003.932.935
Akhtar, M., Khawar, K., Rafique, M., Hussain, K., Ashraf, S. and Hassan, M., 2022. Relative resistance of seed of advance genotypes of Cicer arietinum against Callosobruchus chinensis during storage. Sarhad J. Agric., 38: 1228-1234. https://doi.org/10.17582/journal.sja/2022/38.4.1228.1234
Allali, A., Rezouki, S., Louasté, B., Bouchelta, Y., El-Kamli, T., Eloutassi, N. and Fadli, M., 2020. Study of the nutritional quality and germination capacity of Cicer arietinum infested by Callosobruchus maculatus (Fab.). Pl. Cell Biotechnol. Mol. Biol., 21: 44-56. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.no1.008
Ashok, K., Aravinthraju, K. and Abirami, S., 2020. Evaluation of certain botanicals against pulse beetle, Callosobruchus chinensis (L.) on chickpea. J. Entomol. Zool. Stud., 8: 666-668.
Aslam, M., Shaheen, F.A., Abbas, M.A. and Saba, A., 2006. Management of Callosobruchus chinensis Linnaeus through use of resistance in stored chickpea varieties. World J. Agric. Sci., 2: 82-84.
Bhandari, G., Ghimire, T.B., Kaduwal, S., Shrestha, J. and Acharya, R., 2017. Effects of storage structures and moisture contents on seed quality attributes of quality protein maize. J. Maiz. Res. Dev., 3: 77-85. https://doi.org/10.3126/jmrd.v3i1.18924
Collins, P.J., 2006. Resistance to chemical treatments in insect pests of stored grain and its management. In: Proceedings of the 9th international working conference for stored-product protection, 15e18 October 2006. ABRAPOS, Campinas, Säo Paulo, Brazil, pp. 209-216.
Daglish, G.J., Nayak, M.K., Arthur, F.H. and Athanassiou, C.G., 2018. Insect pest management in stored grain. Rec. Adv. Stor. Prod. Prot., pp. 45-63. https://doi.org/10.1007/978-3-662-56125-6_3
Deepika, K.L., Singh, P.S., Singh, S.K. and Saxena, R.P.N., 2020. Biochemical basis of resistance against pulse beetle, Callosobruchus chinensis (L.) in stored chickpea genotypes. J. Exp. Zool. India., 23: 1175-1180.
Dhaker, N., Vaishampayan, S. and Sharma, N., 2022. Screening of chickpea varieties against pulse beetle (Callosobruchus chinensis L.) infestation in storage condition. Ann. Pl. Prot. Sci., 30: 34-42. https://doi.org/10.5958/0974-0163.2022.00027.1
Eker, T., Erler, F., Adak, A., Imrek, B., Guven, H., Tosun, H.S. and Ikten, C., 2018. Screening of chickpea accessions for resistance against the pulse beetle, Callosobruchus chinensis L. (Coleoptera: Bruchidae). J. Stored Prod. Res., 76: 51-57. https://doi.org/10.1016/j.jspr.2017.12.007
Erler, F., Ceylan, F., Erdemir, T., Toker, C. and Liu, T.X., 2009. Preliminary results on evaluation of chickpea, Cicer arietinum, genotypes for resistance to the pulse beetle, Callosobruchus maculatus. J. Insect Sci., 9: 58. https://doi.org/10.1673/031.009.5801
Flores-Silva, P.C., Rodriguez-Ambriz, S.L. and Bello-Pérez, L.A., 2015. Gluten-free snacks using plantain–chickpea and maize blend: Chemical composition, starch digestibility, and predicted glycemic index. J. Fd. Sci., 80: 961-966. https://doi.org/10.1111/1750-3841.12865
Gupta, L. and Srivastava, M., 2008. Effect of Withania somnifera extracts on the mortality of Callosobruchus chinensis L. J. Biopestic., 1: 190-192.
Gwinner, J., Harnisch, R. and Muck, O., 1996. Manual on the prevention of post-harvest seed losses, post-harvest project, GTZ, D-2000, Hamburg, FRG, pp. 294.
Herald, P., Tayde, A.R. and Kumar, A., 2022. In vitro studies on the life cycle and morphometrics of Callosobruchus chinensis (L.) in stored chickpea. Ann. Pl. Prot. Sci., 30: 114-118. https://doi.org/10.5958/0974-0163.2022.00041.6
Hirdyani, H., 2014. Nutritional composition of Chickpea (Cicer arietinum L) and value added products-a review. Indian J. Comm. Hlth., 26: 102-106.
Hossain, M.A., Alim, M.A., Ahmed, K.S. and Haque, M.A., 2014. Insecticidal potentials of plant oils against Callosobruchus chinensis (Coleoptera: Bruchidae) in stored chickpea. J. Entomol. Soc. Iran., 34: 47-56.
Islam, M.S., Haque, M.A., Ahmed, K.S., Mondal, M.F. and Dash, C.K., 2013. Evaluation of Some Spices Powder as Grain Protectant against Pulse Beetle, Callosobruchus chinensis (L.). Univers. J. Plant Sci., 1: 132-136. https://doi.org/10.13189/ujps.2013.010404
Jaiswal, D.K., Raju, S.V.S., Shyamrao, I.D., Singh, P. and Kumar, G.S., 2019. Effect of some seed protectants on adult emergence of pulse beetle, Callosobruchus chinensis (L.) in fresh chickpea grains under storage conditions. J. Pharmacogn. Phytochem., 8: 534-537.
Jha, A.N., 2002. Response of chickpea cultivars to Callosobruchus chinensis. Indian J. Entomol., 64: 434-437.
Kalnina, S., Rakcejeva, T., Kunkulberga, D. and Galoburda, R., 2015. Rheological properties of whole wheat and whole triticale flour blends for pasta production. Agron. Res., 13: 948-955.
Khalequzzaman, M. and Goni, S.O., 2009. Toxic potentials of some plant powders on survival and development of Callosobruchus maculatus (F.) and Callosobruchus chinensis L. J. Life Earth Sci., 1-6. https://doi.org/10.3329/jles.v3i0.7437
Khandaitaray, T., Mishra, P.R., Satapathy, S.N., Hosamani, G.B., Shankar, T., Badjena, T. and Soren, L., 2023. Biochemical changes of chickpea genotypes before and after infestation of pulse beetle, Callosobruchus Chinensis L. (Coleoptera: Bruchidae) during storage. J. Adv. Zool., 44: 629-637.
Khanna, N., Jain, P. and Teckchandani, C.K., 2017. Comparative study of quality and nutritive parameters of insect infested bengal gram under vacuum and modified atmosphere storage in laminated LDPE bags. Int. J. Curr. Microbiol. Appl. Sci., 6: 4303-4308. https://doi.org/10.20546/ijcmas.2017.612.494
Kim, S.I., Roh, J.Y., Kim, D.H., Lee, H.S. and Ahn, Y.J., 2003. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res., 39: 293-303. https://doi.org/10.1016/S0022-474X(02)00017-6
Kiradoo, M.M. and Srivastava, M., 2010. A comparative study on the efficacy of two lamiaceae plants on egg-laying performance by the pulse beetle Callosobruchus chinensis Linn. (Coleoptera: Bruchidae). J. Biopestic., 3: 590-596.
Kumar, A., Shukla, R., Singh, P., Singh, A.K. and Dubey, N.K., 2009. Use of essential oil from Mentha arvensis L. to control storage moulds and insects in stored chickpea. J. Sci. Fd. Agric., 89: 2643-2649. https://doi.org/10.1002/jsfa.3768
Kyogoku, D. and Nishida, T., 2013. The mechanism of the fecundity reduction in Callosobruchus maculatus caused by Callosobruchus chinensis males. Popul. Ecol., 55: 87-93. https://doi.org/10.1007/s10144-012-0344-3
Mariotti, F., Tomé, D. and Mirand, P., 2008. Converting nitrogen into protein beyond 6.25 and jones’ factors. Crit. Rev. Fd. Sci. Nutr., 48: 177-184. https://doi.org/10.1080/10408390701279749
Mukendi, R.T., Longanza, L.B. and Kanyenga, A., 2018. Response of twelve cowpea genotypes (Vigna unguiculata L. Walp) to pest attack pressures under field and controlled environmental conditions of Lomami province, central part of Democratic Republic of Congo. J. Entomol. Zool. Stud., 6: 13-21. https://doi.org/10.18488/journal.70.2019.61.33.46
Phadtare, P.R., Chaudhari, C.S., Aghav, S.T., Kadam, U.K., Chavan, T.R. and Patil, M.R., 2023. Management of pulse beetle (Callosobruchus chinensis L.) in chickpea using biorational products. Pharm. Innov. J., 12: 2768-2770.
Pokharkar, P.K. and Chauhan, N.R., 2010. Susceptibility of different varieties of chickpea to Callosobruchus chinensis (Linnaeus). Environ. Ecol., 28: 1792-1794.
Pradhan, L., Singh, P.S., Singh, S.K. and Saxena, R.P.N., 2020. Biochemical factors associated with resistance against pulse beetle, Callosobruchus chinensis (L.) in stored chickpea genotypes. J. Exp. Zool., 23: 1937-1942.
Purohit, P., Jayas, D.S., Yadav, B.K., Chelladurai, V., Fields, P.G. and White, N.D.G., 2013. Microwaves to control Callosobruchus maculatus in stored mung bean (Vignaradiata). J. Stored Prod. Res., 53: 19-22. https://doi.org/10.1016/j.jspr.2013.01.002
Raghuwanshi, P.K., Sharma, S., Bele, M. and Kumar, D., 2016. Screening of certain gram genotypes against Callosobruchus chinensis L. (Coleoptera: Bruchidae). Legum. Res., 39: 651-653. https://doi.org/10.18805/lr.v0iOF.11038
Righi-Assia, A.F., Khelil, M.A., Medjdoub-Bensaad, F. and Righi, K., 2010. Efficacy of oils and powders of some medicinal plants in biological control of the pea weevil (Callosobruchus chinensis L.). Afr. J. Agric. Res., 5: 1474-1481.
Rolania, K., Yadav, S.S., Singh, B., Yadav, J.L., Kumar, N. and Pilania, S., 2021. Assessment of losses due to pulse beetle in chickpea under stored conditions in Southern Haryana. J. Agric. Enomol., 12: 98-105. https://doi.org/10.53911/JAE.2021.12210
Sarwar, M., 2012. Assessment of resistance to the attack of bean beetle Callosobruchus maculatus (Fabricius) in chickpea genotypes on the basis of various parameters during storage. Songklanakarin J. Sci. Technol., 34: 287-291.
Saxena, B. and Saxena, R., 2011. Nutritional changes in s tored chickpea, Cicer arietinum in relation to bruchid damage. J. Stored Prod. Postharv Res., 2: 110-112.
Shaheen, F.A., Khaliq, A. and Aslam, M., 2006. Resistance of chickpea (Cicer arietinum L.) cultivars against pulse beetle. Pak. J. Bot., 38: 1237.
Sharma, P., Pandya, P. and Parikh, P. 2023. Elucidating the host preference by the pulse Callosobruchus chinensis (L). Indian J. Entomol., 1-4. https://doi.org/10.55446/IJE.2023.778
Siddiqa, A., Perveen, F., Naz, F. and Ashfaque, M., 2013. Evaluation of resistance in local chickpea varieties against the pulse beetle, Callosobruchus chinensis L. (Coleoptera: Bruchidae). Pak. Entomol., 35: 43-46.
Singh, A.K., Vikram, N. and Pandey R.K., 2016. Screening of different germplasm of chickpea against pulse beetle (Callosobruchus chinensis L.) and its relationship with quality parameters. Int. J. Pl. Prot., 9: 89-94. https://doi.org/10.15740/HAS/IJPP/9.1/91-96
Singh, B.U., Sharma, H.C. and Rao, K.V., 2012. Mechanisms and genetic diversity for host plant resistance to spotted stem borer, Chilopartellus in sorghum, Sorghum bicolor. J. Appl. Entomol., 136: 386-400. https://doi.org/10.1111/j.1439-0418.2011.01647.x
Soumia, P.S., Srivastava, C., Dikshit, H.K. and Pandi, G.G.P., 2017. Screening for resistance against pulse beetle, Callosobruchus analis (F.) in green gram (Vignaradiata (L.) Wilczek) accessions. Proc. Natl. Acad. Sci. India B Biol. Sci., 87: 551-558. https://doi.org/10.1007/s40011-015-0635-5
Srinivasan, T., Durairaj, C. and Kumar, B.V., 2008. Damage potential of bruchids in different edible legumes and interspecific competition between two species of Callosobruchus spp. (Bruchidae: Coleoptera). J. Life Sci., 2: 42-49.
Stejskal, V., Vendl, T., Aulicky, R. and Athanassiou, C., 2021. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects, 12: 590. https://doi.org/10.3390/insects12070590
Technical, A., 2009. Moisture-air-oven methods. AACC Int. sApprov. Methods. https://doi.org/10.1094/AACCIntMethod-44-15.02
Thakur, A.K. and Pathania, M., 2013. Biology of Pulse beetle (Callosobruchus chinensis) and its management through Plant products on Black Gram (Vigna mungo). Sci. Technol. Arts Res. J., 2: 18-21. https://doi.org/10.4314/star.v2i1.98838
Upadhyay, R.K., Yadav, N. and Ahmad, S., 2011. Assessment of toxic effects of solvent and aqueous extracts of Capparis decidua on biochemical and enzymatic parameters of Callosobruchus chinensis L. (Coleoptera: Bruchidae). Agric. Environ., 3: 68-92.
Verma, V.C., Singh, S.K. and Prakash, S., 2011. Biocontrol and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J. Basic Microbiol., 51: 550-556. https://doi.org/10.1002/jobm.201000155
To share on other social networks, click on any share button. What are these?







