Phytochemical and Toxicological Studies of Methanolic Extract of Lemon Verbena in Hamsters
Research Article
Phytochemical and Toxicological Studies of Methanolic Extract of Lemon Verbena in Hamsters
Oumaima Abouyaala, Soukaina Bougrine, Mohamed Yassine El Brouzi*, Radia Elgui, Sara Brikat, Khadija Elmotia, Abdelhalem Mesfioui, Aboubaker El-Hessni, Moulay Laarbi Ouahidi
Laboratory of Biology and Health, Neurosciences, Neuro-immunology and Behaviour Unit, Faculty of Science, Ibn Tofail University, Kenitra, Morocco.
Abstract | Lemon verbena, a member of the verbenacea family, originated from South America, and Africa, and is recognized for numerous biological activities and therapeutic effects. However, its potential toxicity and adverse effects necessitate thorough investigation. The present study investigated the acute and subacute toxicity of Lemon verbena, within 14- and 28-day exposure, respectively. In acute toxicity, female hamster was treated orally with methanolic extract of lemon verbena (MELV) at 1, 2, 3, and 5 g/kg bw, respectively. For subacute toxicity, doses of 125, 250, and 500 mg/kg bw, of MELV were administered daily for 28 days, in male and female hamsters. Changes in body weight as well as biochemical, hematological, and histopathological parameters were studied. In the present study, we demonstrated that the extract exhibits high levels of total polyphenols, catechins, and gallic tannins. Oral LD50 value was higher than 5 g/kg bw in female hamster. In Subacute toxicity, no significant pathological changes were detected in biochemical parameters related to glycemia, lipid profile, renal and hepatic function; hematological parameters including erythrocyte parameters and, differential leukocyte count in both sex of hamsters treated with MELV at different doses. A lymphocyte infiltrate, a reduction in nitric oxide level, and an increase in catalase activity were observed in liver and kidney of hamster treated with 500mg/kg bw of MELV. This result suggests that methanolic extract of lemon verbena is considered as a non-toxic substance by a single administration. However, prolonged treatment with high doses of MELV should be used carefully.
Keywords | Lemon verbena, Methanol extract, Acute toxicity, Subacute toxicity, LD50, Phytochemical screening
Received | September 03, 2024; Accepted | October 09, 2024; Published | December 30, 2024
*Correspondence | Mohamed Yassine El Brouzi, Laboratory of Biology and Health, Neurosciences, Neuro-immunology and Behaviour Unit, Faculty of Science, Ibn Tofail University, Kenitra, Morocco; Email: [email protected]
Citation | Abouyaala O, bougrine S, El Brouzi MY, Elgui R, Brikat S, Elmotia K, Mesfioui A, El-Hessni A, Ouahidi ML (2025). Phytochemical and toxicological studies of methanolic extract of lemon verbena in hamsters. Adv. Anim. Vet. Sci. 13(1): 115-124.
DOI | https://dx.doi.org/10.17582/journal.aavs/2025/13.1.115.124
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2025 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Since 1978, the World Health Organization (WHO) has been dedicated to researching and advocating traditional pharmacopeia to meet the primary healthcare and medication needs of African populations. The reason is that many people think medicinal plants can substitute for high-priced chemical drugs or special drugs that are hard to make in developing countries or are prohibitively expensive (WHO, 2013). The Moroccan population practices many of the traditional uses of medicinal plants for the treatment of various diseases, as reported by (Kachmar et al., 2021).
The plant kingdom represents a major source of an immense variety of bioactive molecules (Dhaval et al., 2016), known for their biological, pharmacological, and nutritional properties (Correia et al., 2012; Guaadaoui et al., 2014). Lemon verbena, belongs to the Verbenaceae family and is known as Aloysia or Lippia citriodora (Valentão et al., 2002; Stashenko et al., 2013), it is from South America but is also found in Central and North America and Africa (Carnat et al., 1999). recognized in the south of Morocco for its various biological effects (Valentão et al., 2002; Stashenko et al., 2013), including; antioxidant (Choupani et al., 2014; Farahmandfar et al., 2018), hypoglycemic (El-Ouady and Eddouks 2021), anxiolytic (Veisi et al., 2015), anti-depressive, and neuroprotective effects (Zhao et al., 2023). Phenes, flavonoids, and tannins components are responsible for these pharmacological activities (Quirantes-Piné et al., 2013; Hematian Sourki et al., 2021a).
However, utilization of these plant-based treatments often occurs without scientific knowledge or evidence regarding their toxicological effects. This lack of scientific understanding poses potential risks, as the safety and potential side effects of these traditional phytotherapies may not be well-established. Therefore, there is a need for further research, validation, and documentation to ensure the safe and effective use of traditional medicinal plants (Carneiro et al., 2014).
In addition, the data from the toxicological study of Lemon verbena are still limited. For this purpose, this study is conducted to assess the acute and subacute toxicity of the methanolic extract of lemon verbena in hamster and to determine a dose that is safe for the biological effects of the extract studied, while also avoiding the plant’s toxic effects, to determine the LD50 in acute toxicity test, evaluate the daily administration of this extract during 28 days, on renal and hepatic function, glycemia, lipid profile, hematological parameters, and oxidative stress in hepatocyte and renal cells.
MATERIALS AND METHODS
Plant Materials
The leaves of lemon verbena were collected in the Jamaat Aghmat area of El Haouz, Marrakech, Morocco, on 02 Jun 2023. The plant was authenticated by Assistant Professor Zidan, a botanist of the Department of Biology, Faculty of Sciences Kenitra, Ibn Tofail University.
Preparation of Extract
The collected leaves were dried away from the light and crushed to obtain a powder. The extract was prepared according to the traditional method, 40 grams of powder was extracted using a Soxhlet with 300 ml of methanol. The extract solution was separated from the solvent by a rotary evaporator. Then, the extraction yield in the methanolic extract of leaves of lemon verbena (MELV) was evaluated using the equation below:
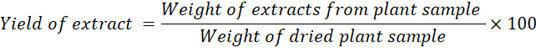
Phytochemical Screening
The detection of the presence of phytochemical constituents is based on staining and/or precipitation reactions (Harborne, 1998); using the method of Stiasny (Karumi, 2004) to detect catechins and gallo tannins, the Cyanidine reaction is used to detect flavonoids (Houghton and Raman, 1998) The polyphenols, flavonoids (Houghton and Raman, 1998), leuco anthocyanins and anthocyanin (Mibindzou Mouellet, 2004) are also detected.
Experimental Animals
Our experimental study was performed on female and male hamsters raised in the animal facility at the Faculty of Sciences, Ibn Tofail University. The hamsters were maintained at a controlled temperature of 22 C° and subjected to a light/dark cycle (LD: 12/12), to maintain their normal circadian cycle with free Access to water and Food (ad libitum). A five-day adaptation period was applied before the beginning of the experiment, according to the experimental conditions. The experiments were carried out according to the guidelines of the local committee.
Acute Oral Toxicity
According to the OECD 423 (OECD 423 2002), a total of 30 female hamsters (90±10 g) were distributed into 5 groups (6 Animals per group), the control group received distillate water and 4 treated groups received a single oral dose of MELV at different doses (1000, 2000, 3000, and 5000mg/kg bw). The signs of toxicity, mortality, body weight (bw), food intake, and water consumption were observed during 14 days.
Subacute Toxicity
For subacute toxicity, 24 female and 24 male hamsters were used to evaluate the subacute toxicity of MELV, according to OECD 407 (OECD 407, 2001). Hamsters of each sex were divided into 4 groups, the control group was given distilled water, while the treated groups were administered daily with the MELV at different doses (125, 250, and 500 mg/kg bw) for 28 days. The body weight, signs of toxicity, food intake, and water consumption were observed during the experiment.
At the end of the experiment, the animals were anesthetized and sacrificed. The blood sampling was performed by cardiac puncture to realize a hematological and biochemical parameter. The liver and kidney were dissected to realize a macroscopic and microscopic evaluation (histological sections). Additionally, the organ homogenates were prepared to measure oxidative stress markers.
Hematological and Biochemical Parameters
The blood samples were used immediately for the analysis of hematological parameters, such as erythropoietic analysis; Red blood cell (RBC), mean corpuscular hemoglobin concentration (MCHC), hemoglobin (Hb), hematocrit (HCT) and mean corpuscular volume (MCV). Additionally, the analysis of white blood cells and differential leukocyte count included, Neutrophils (NEUT), Lymphocytes (LYMPH), Monocytes (MONO), Eosinophils (EO), Basophils (BASO), as well as the platelets count.
The biochemical analyses carried out were: Glucose (Glu), the lipid profile: (total cholesterol (TC), triglycerides (TG)), the hepatic function (aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT)) and renal function (urea, creatinine (Crea)). The assays were performed under the manufacturing instructions, using a spectrophotometer to read the optical density (OD) at different wavelengths.
Relative Weight of the Organs
After blood sampling, the liver and kidney were weighed immediately to estimate the organ/body weight ratio. A relative weight ratio is defined by the formula:
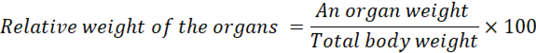
Histopathology Examination
The organs were carefully fixed in 10 % formalin. After fixation, tissue specimens were dehydrated in a graded series of ethanol (70–100%). At the end of the process, specimens were sectioned at 6μm thickness and were stained with Haematoxylin and Eosin (H&E) before microscopic examination.
Oxidative Stress Assay
Catalase activity: The antioxidant activity of MELV was evaluated by the measurement of Catalase activity in the liver and kidney homogenate, as described by (Clairbone, 1985). Specifically, 780 μl of phosphate buffer solution (pH 7.0) was added to 20 μl of kidney or liver homogenate, along with 200 μl of H2O2 (1 M). Subsequently, the absorbance was measured at 240 nm for 1 minute. The results were expressed in millimoles of decomposed H2O2/min/mg of proteins (Timoumi et al., 2019).
Measurement of nitric oxide: Nitric oxide (NO) measurement in the liver and kidney homogenate was determined by the Griess reaction, 500 μl of the homogenate tissue was added to 500 μl of Griess reagent (1% sulphanilamide (1 ml) and 0.1% N-1-naphthyl ethylenediamine dihydrochloride (1 ml) in 2.5% orthophosphoric acid). After incubation for 30 min at room temperature without light, absorbance was measured at 540 nm (Li et al., 2011).
Statistical Analysis
To determine the differences between the control and treated groups, statistical analysis was carried out using GraphPad Prism 8 software. The results, presented as mean ± SD (n = 6), were analyzed using unidirectional ANOVA followed by Tukey’s post hoc test. Differences were considered significant when: P<0.05, very significant when P<0.01, and highly significant when: P<0.001.
RESULTS AND DISCUSSION
Extract Yield
The yield of the methanolic extract of lemon verbena leaves is calculated as the ratio of the mass of the extract obtained to the initial mass of the plant subjected to extraction (powder), expressed as a percentage (Table 1).
Table 1: Extract Yield of methanolic extract of lemon verbena leaves.
|
Species |
Quantity of powder (g) |
Quantity of methanolic extract |
Extract Yield (%) |
|
Lemon verbena |
40g |
11,6 g |
R= (11.6/40) *100=29% |
Phytochemical Screening
Table 2, shows the results of the phytochemical analysis, where the presence of total polyphenols, catechins, gallo tannins, and flavonoids was detected, while the total absence of leuco anthocyanins and anthocyanins was detected.
Table 2: Main chemical constituents of methanolic extract of lemon verbena leaves. (-): negative Test, (+): weakly positive Test, (++): positive Test, (+++): strongly positive Test.
|
Chemical groups |
Methanolic extract of Lemon Verbena |
|
|
Total polyphenols |
+++ |
|
|
Flavonoids |
+ |
|
|
Tanins |
Catechic |
++ |
|
Gallic |
+++ |
|
|
Leuco anthocyanes |
- |
|
|
Anthocyanins |
- |
|
Acute Toxicity
A single oral administration of various doses of the MELV showed no signs of mortality and/or clinical signs during the 14-day test (Table 3), Also no significant difference was detected for variation in body weight, food consumption, and water intake between hamsters treated with MELV and control (P>0.05) (Table 4). Accordingly, the LD50 is considered to be greater than 5000mg/kg bw of the animal, So the extract studied can be classified in category 5 and considered as a non-toxic substance.
Table 3: Effect of MELV on clinical signs and mortality in acute toxicity in hamster.
|
Sign of toxicity observed |
Mortality |
|
|
Control : Distilled water |
None |
0/6 |
|
Dose 1 : 1000 mg/kg bw |
None |
0/6 |
|
Dose 2 : 2000 mg/kg bw |
None |
0/6 |
|
Dose 3 : 3000 mg/kg bw |
None |
0/6 |
|
Dose 4 : 5000 mg/kg bw |
None |
0/6 |
Table 4: Body weight gain, food consumption, and water intake of hamsters treated orally with MELV in acute toxicity. The results are represented by the mean ± SD (n = 6), significance levels were indicated as: * P <0.05, ** P <0.01, *** P <0.001.
|
Parameters |
Control |
1000mg/kg bw |
2000mg/kg bw |
3000 mg/kg bw |
5000mg/kg bw |
|
Initial weight (g) |
104 ± 2.58 |
102.75 ± 3.3 |
101.2 ± 4.14 |
98.6 ± 3.36 |
99±6.16 |
|
One week (g) |
106.1 ± 2.82 |
109.25 ± 1.5 |
104.6 ± 4.77 |
101.31 ± 1.84 |
105.19 ± 0.85 |
|
Two week (g) |
113.76 ± 4.29 |
112.47 ± 0.44 |
110.6 ± 3.36 |
107.92 ± 2.00 |
106.05 ± 0.82 |
|
BWG (g) |
9.76 |
9.72 |
9.4 |
9.32 |
7.05 |
|
Food consumption (g/day) |
13.64 ± 1.2 |
13.68 ± 1.15 |
13.06 ± 0.83 |
12.87 ± 1.05 |
13.10 ± 1.03 |
|
Water Intake (ml/day) |
11.17 ± 0.48 |
11.21 ± 0.47 |
10.84 ± 0.21 |
10.70 ± 0.41 |
10.93 ± 0.14 |
Table 5: Effect of subacute MELV toxicity on clinical signs and mortality.
|
Group |
Clinical sign |
Molarity |
|
Female |
||
|
Control |
None |
0/6 |
|
125 mg/kg bw |
None |
0/6 |
|
250 mg/kg bw |
Hair modification |
0/6 |
|
500 mg/kg bw |
Hair modification,diarrhea |
0/6 |
|
Male |
||
|
Control |
None |
0/6 |
|
125 mg/kg bw |
None |
0/6 |
|
250 mg/kg bw |
None |
0/6 |
|
500 mg/kg bw |
Hair modification |
0/6 |
Subacute Toxicity
Body weight, relative organ weight, and clinical observations in hamsters: A daily oral administration of the MELV at different doses (125, 250, and 500 mg/kg bw) for male and female hamsters has showed no mortality and no grave signs (Just Hair modification in the male and female hamsters treated with 500mg/kg bw, the female treated with 500mg/kg bw showed a Hair modification and a Diarrhea) of toxicity during 28-days of the experiment (Table 5), Also no significant difference was detected in body weight variation (Figure 1), food consumption, and water intake (Table 6), as well as for relative weight of the organs (Figure 2) between hamsters treated with MELV and control (P>0.05) for both sexes.
Table 6: Mean food consumption and water intake in female and male hamsters during subacute toxicity of MELV. The results are represented by the mean ± SD (n = 6), significance levels were indicated as: * P <0.05, ** P <0.01, *** P <0.001.
|
Control |
125 mg/kg bw |
250 mg/kg bw |
500 mg/kg bw |
|
|
Female |
||||
|
Food consumption (g/day) |
13.72 ± 0,28 |
15.23 ± 0,38 |
14.51 ± 0,30 |
13.49 ± 0,18 |
|
Water intake (ml/day) |
12.57 ± 0,26 |
12,30 ± 0,31 |
13.35 ± 0,27 |
11.80 ± 0,15 |
|
Male |
||||
|
Consumption food (g/day) |
14.58 ± 0.29 |
13.85 ± 0.23 |
14.24 ± 0.23 |
13.00 ± 0.18 |
|
Water intake (ml/day) |
12.33 ± 0.25 |
11.08 ± 018 |
12.05 ± 0.19 |
11.37 ± 0.16 |
Biochemical parameters: The biochemical parameters in male and female hamsters administered daily with MELV for 28 days in the subacute toxicity test showed no significant differences in the groups treated with the extract studied at different doses compared with control in both sex (P >0.05) for glucose levels (Figure 3a), lipid profile; cholesterol levels (Figure 3b) and serum triglyceride (Figure 3c) and renal function parameters; serum urea (Figure 3d) and creatinine levels (Figure 3e). Regarding a liver function tests, a significant increase in ALAT levels was observed in female hamsters treated with 500 mg of MELV per kg of bw compared with the control group (P <0.05) (Figure 3g). However, ASAT levels did not show a statistically significant difference between treated and control groups (P >0.05) (Figure 3f).
Hematological parameters: Concerning erythrocyte parameters represented in (Table 7), data analysis showed that daily administration of MELV has no significant effect on erythropoietic parameters in female hamsters. However, there was a significant decrease in hematocrit in the male hamsters treated with 500 mg/kg bw of MELV extract compared to the control group (P <0.01). In addition, thrombocytopenia, highly significant reduction in platelets, was observed in male treated-groups compared to the control group (P <0.001). The female hamsters treated with 500 mg/kg bw of MELV extract, showed an increase in WBC and neutrophil counts (P<0.01) and (P<0.05), respectively. Also, a highly significant increase in WBC of male hamsters treated with 500 mg/kg bw of MELV extract, compared to the control group (P<0.001) (Table 8).
Table 7: Effect of MELV on Variation of erythrocyte parameters and platelet levels of the subacute toxicity test in hamsters. The results are represented by the mean ± SD (n = 6), significance levels were indicated as: * P <0.05, ** P <0.01, *** P <0.001.
|
Control |
125mg/kg bw |
250mg/kg bw |
500mg/kg bw |
|
|
Female |
||||
|
"RBC [10^6/uL]" |
7,59±0,71 |
8,02±1,15 |
7,72±0,75 |
6,78±1,08 |
|
"HGB [g/dL]" |
15,71±0,91 |
15,52±0,78 |
14,52±0,90 |
13,51±1,76 |
|
"HCT [%] " |
43,25±1,39 |
46,11±2,07 |
42,56±2,30 |
38,27±2,15 |
|
"MCV - [fL]" |
56,90±1,26 |
57,77±2,75 |
55,01±2,06 |
54,02±3,08 |
|
"MCHC [g/dL]" |
36,26±1,57 |
33±1,80 |
34,28±2,48 |
35,81±3,08 |
|
"RDW-CV [%]" |
17,06±0,16 |
16,08±0,57 |
17,72±1,10 |
15,87±1,93 |
|
"PLT * [10^3/uL]" |
312±23,25 |
302,33 ± 8,73 |
304±15 |
293,66±8.5 |
|
Male |
||||
|
"RBC [106/uL]" |
7,34±1,06 |
7,66±0,86 |
8,42±0,51 |
5,72±0,71 |
|
"HGB [g/dL]" |
14,1±1,01 |
14,45±0,75 |
15,78±1,13 |
11,74±0,77 |
|
"HCT [%] " |
40,16±1,25 |
41,8±1,03 |
36,87±0,97 |
33,33 ± 0,69*** |
|
"MCV - [fL]" |
56,30±1,26 |
56,11±1,23 |
56,59±1,32 |
57,16±1,04 |
|
"MCHC [g/dL]" |
34,77±1,07 |
34,87±1,11 |
33,96±0,85 |
35,61±0,95 |
|
"RDW-CV [%]" |
34,29±0,63 |
32,61±0,67 |
30,37 ± 0,80** |
26,40 ± 1,49*** |
|
"PLT * [103/uL]" |
397±27,22 |
190,64 ± 4,07*** |
119,33 ± 3,51*** |
171,89 ± 1,16*** |
Effect of MELV on oxidative stress markers:
- Catalase activity: The data analysis of catalase activity in Liver and Kidney homogenates (Figure 4) in both sexes of hamsters showed a highly significant increase of catalase activity in liver homogenate of female hamsters treated with 250 and 500 mg/kg bw of MELV (P <0.001), and a very significant increase of catalase activity for the kidney of female hamsters treated with 250mg/kg bw of MELV (P <0.01). However, no significant difference was detected between the male hamsters treated with different doses of the MELV and the control group.
- Measurement of NO Concentrations:The concentration of NO level in the liver homogenate showed a significant decrease in the NO-level in male group treated with 125 mg/kg bw of MELV (P <0.05), a very significant decrease in male group treated with 250mg/kg bw (P <0.01) and a highly significant decrease in female hamsters treated with 250mg/ kg bw, and in male and female group treated with 500mg/kg bw of MELV (P <0.001). No significant difference was detected in the kidney level of NO, except a significant decrease in the male group treated with 500mg/ kg bw of MELV (Figure 5).
Table 8: Effects of MELV on white blood cells and differential leukocyte count during subacute toxicity in hamsters. The results are represented by the mean ± SD (n = 6), significance levels were indicated as: * P <0.05, ** P <0.01, *** P <0.001.
|
Control |
125mg/kg bw |
250mg/kg bw |
500mg/kg bw |
|
|
Female |
||||
|
"WBC *[103/uL]" |
2,47±0,55 |
2,63±0,40 |
1,66±0,43 |
4,02±0,18** |
|
"NEUT * [103/uL] " |
0,38±0,11 |
0,54±0,16 |
0,26±0,12 |
0,72±0,11* |
|
"LYMPH * [103/uL]" |
1,53±0,60 |
1,6±0,60 |
2,04±0,18 |
0,94±0,38 |
|
"MONO * [103/uL] " |
0,75±1,12 |
0,44±0,67 |
0,86±0,22 |
0,11±0,04 |
|
"EO * [103/uL] " |
0,11±0,07 |
0,06±0,09 |
0,10±0,15 |
0,78±1,10 |
|
"BASO * [103/uL] " |
0,28±0,17 |
0,38±0,11 |
0,37±0,09 |
0,28±0,07 |
|
Male |
||||
|
"WBC *[10^3/uL]" |
1,8±0,10 |
1,96±0,16 |
2,01±0,32 |
2,80± 0,15*** |
|
"NEUT * [10^3/uL] " |
0,28±0,17 |
0,48±0,17 |
0,45±0,13 |
0,43±0,05 |
|
"LYMPH * [10^3/uL] " |
1,25±0,43 |
1,06±0,20 |
1,56±0,50 |
2,00±0,33 |
|
"MONO * [10^3/uL] " |
0,33±0,19 |
0,11±0,06 |
0,22±0,14 |
0,49±0,73 |
|
"EO * [10^3/uL] " |
0,01± 0,0046 |
0,10±0,15 |
0,06±0,02 |
0,16±0,19 |
|
"BASO * [10^3/uL] " |
0,16±0,10 |
0,18±0,08 |
0,18±0,02 |
0,22±0,09 |
Histological studies: The histological studies on hamsters’ liver showed moderate necrosis for both male and female hamster treated with 250 and 500mg/kg bw of the MELV, the observation also showed a peri portal infiltration of lymphocytes in particular for female administered by 500mg/kg bw. For the Histological kidney section, a tubular atrophy was observed in the both sex of hamsters treated with 125mg/kg bw, a sinusoidal dilatation and a glomerular lymphocyte Infiltrate were detected in male and female treated with 250 and 500mg/kg bw (Figure 6).
For centuries, various cultures have utilized herbal medicines, often perceived as natural and safe (Maldonado Miranda, 2021). Medicinal herbs typically contain diverse components that can induce both beneficial and potentially adverse effects, such as Lemon verbena (Majewska et al., 2022). The identification of phytochemicals in the MELV is crucial because of the antioxidant and biological effects of its secondary metabolites, as well as their harmful effects (Muanda, 2010). The present study aims to investigate the main constituents of the MELV, to assess the acute toxicity of this extract and estimate its LD50, and the subacute toxicity to study its effects on biochemical, hematological, and histological parameters, in hamsters.
In the present study, the phytochemical screening of MELV indicated the presence of polyphenols, catechic, gallic tannins, and flavonoids, while anthocyanins and leuco anthocyanins were absent. Our results are similar to those of (Choupani et al., 2014), who reported that the methanolic solvent of lemon verbena contained a high level of polyphenols, measuring 25.94 mg GAE/g, there were 49.2 ± 0.11 mg GAE/g in the same extract (Hematian Sourki et al., 2021b). The total flavonoid content was 39.86 mg EC/g (mg of catechin per gram of extract) in the methanolic extract of lemon verbena (Tammar et al., 2021) and it ranged from 10.59 to 19.26 mg CE/g for the same extract, also, the total tannins were between 0.89 and 1.10 mg EC/g (Rezig et al., 2019), as well as, it was equal to 0.04 mg EC/g in the MELV (Tammar et al., 2021). These differences in the quantity of polyphenols, flavonoid, and tannins can be explained by harvest time (Hawary et al., 2011), growing conditions, environmental factors (Hematian Sourki et al., 2021b), growth stage, extraction technique (Aldeen et al., 2015), solvent types (Choupani et al., 2014) and harvest regions (Tammar et al., 2021).
The results of our study showed that a single oral administration of MELV, at variable doses (1000, 2000, 3000, and 5000mg/kg bw), did not cause any death or behavioural alterations in the treated hamsters. The absence of toxic effects in the acute toxicity assessment of MELV suggests that the NOAEL dose, the dose with no adverse effect, is 1000mg/kg bw. The LD50 in female hamsters is considered superior to 5000 mg/kg bw, based on these results. Furthermore, no significant variations were noted in body weight, food, and water consumption during the 14-day experiment in different treated groups compared to the control group.
Our results were in accordance with many studies. (Rashidian et al., 2016), showed that the LD50 of the methanolic extract of lippia citriodora is higher than 2000 mg/kg bw, without any adverse or toxic effects. The LD50 for the aqueous extract of Aloysia citriodora is reported as 5g/kg bw (Etemad et al., 2016), while the LD50 for the alcoholic extract of Egyptian lippia citriodora is noted as 5.9 g/kg bw according to (El-Hawary et al., 2012). Additionally, the LD50 for verbacoside, an active component of L. citriodora, is documented to be higher than 5g/kg bw (Etemad et al., 2015).
Concerning the subacute exposure, no mortality was detected in both sexes of hamsters administered orally at different doses 125, 250, and 500mg/kg bw of the MELV. In addition, no serious clinical signs, changes in body weight, food and water consumption, or relative organ weights were observed in the treated and control groups. According to many studies (Etemad et al., 2015, 2016; De Lima et al., 2018), the administration of lemon verbena extract or verbacoside did not exhibit any impact on the mentioned parameters, as confirmed by this result.
Medical assessments often include biochemical and hematological parameters to assess the body’s health and functioning. The information provided by these parameters is crucial when it comes to understanding different physiological processes, organ functions, and the overall health status of an individual, such as the toxicity of compounds (Olayode et al., 2019). For example, examination of the function of hepatocytes and kidney cells, which play crucial roles in detoxification, blood cleaning, and elimination of waste products from the body (Ozer et al., 2008).
Regarding biochemical parameters, no significant difference was detected between the groups treated with MELV and control groups in blood glucose, cholesterol, and triglyceride levels. Our results showed that the administration of MELV induced a significant increase in ALAT levels in females receiving 500mg/kg bw of the MELV, which was not associated with an increase in ASAT levels. However, no significant difference was detected between the treated and control groups in terms of renal function (urea, creatinine). No significant differences were shown after administration of the Verbascoside and aqueous extract of lemon verbena (Etemad et al., 2015, 2016). However, a decrease in LDL level induced by the verbascoside (Pastorelli et al., 2012), and in triglycerides levels by the aqueous extract of Lemon verbena (Etemad et al., 2016); which could be explained by the presence of polyphenols in the MELV (Casamassima et al., 2017).
Concerning the hematological parameters, this study showed a significant decrease in the platelets count in male hamsters treated at different doses of MELV. This reduction could have potential implications for blood coagulation and immune response (Garraud et al., 2011). A decrease in hematocrit was revealed in male hamsters treated with 500mg/kg bw of MELV; that could be a result of testosterone-stimulating erythropoiesis in male animals (Hershkovitz et al., 2015). The number of leucocytes increased in both sexes in hamsters treated with 500mg/kg bw of MELV, with an increase in neutrophils in the female group. The pathological significance of these variations is not significant due to their normal average hematological values in hamsters (Boussarie, 1999).
The histological studies showed sinusoidal dilatation, glomerular lymphocyte infiltrate, and moderate hepatocyte necrosis in male and female treated with 250 and 500mg/kg bw of MELV. Moreover, a peri portal lymphocyte infiltrate was observed in female hamster treated with 500mg/kg bw of MELV. which may be explained by the level of oestrogen present in females, a factor that modulates the immune response (Kovats, 2012). However, no abnormalities in all organs tested were observed after administration of Lemon verbena aqueous extract in 50, 100, and 200 mg/kg of body weight of male Wistar rats (Etemad et al., 2016), and,10, 30 and 60 mg kg b.w. of verbacoside in mice (Etemad et al., 2015), this absence of histopathological changes can be explained by the dose difference, species, or extract type.
In the liver homogenate, the level of NO was decreased in both sex hamsters, demonstrating a reduction in oxidant marker, which was approved by amelioration of antioxidants such as catalase activity in female treated with MELV at 250 and 500mg/kg bw showing the hepatoprotective effect of estrogen in antioxidant/oxidant balance (Hamden et al., 2007). However, in kidney homogenate, a significant decrease in NO level, and an increase in catalase activity were detected in the male group treated with 500mg/kg bw and the female group treated with 250mg/kg bw of MELV respectively, showing the antioxidant defense of MELV in the renal cells. These results were in line with other studies that demonstrated a decrease in the level of oxidant activities, and an increase in the concentration of the antioxidant enzymes, after the verbascoside administration (Casamassima et al., 2017), and the Aloysia citriodora extract (Habibi et al., 2021). Therefore, our result may be explained by the polyphenols contained in the MELV, such as Verbascoside, by reinforcing the antioxidant system (Veskoukis et al., 2019); which indicates that lemon verbena, acts as a potent antioxidant and antioxidant system modulator.
CONCLUSIONS AND RECOMMENDATIONS
The present study showed that MELV has high levels of total polyphenols, catechins, and gallic tannins. The estimated LD50 of the studied extract is superior to 5000mg/kg bw. For subacute toxicity, no significant pathological changes were observed in biochemical, hematological, and histological in male and female hamsters treated with different doses of MELV. The MELV induced a significant decrease in the NO level and an increase in catalase activity in the liver and kidney homogenate of male and female treated with 500mg/kg bw. According to these results, the MELV was non-toxic in acute oral administration. However, further analysis is essential to confirm these observations and to determine the mechanisms underlying their potential effects: such as studying long-term effects, different extraction methods, diverse animal models, gender-specific studies, and mechanistic studies of the antioxidant properties of MELV.
NOVELTY STATEMENT
To our knowledge, this is the first study assessing the toxicity of the methanolic extract of Moroccan lemon verbena, a plant traditionally used for its therapeutic properties in Moroccan population.
Highlights
- Lemon verbena, is recognized for numerous biological activities and therapeutic effects. However, its potential toxicity and adverse effects necessitate thorough investigation.
- Lemon verbena extract exhibits high levels of total polyphenols, catechins, and gallic tannins.
- Oral LD50 values were higher than 5 g/kg bw in female hamster.
- Oral Subacute exposure to MELV showed no pathological significance in biochemical and hematological analysis between the treated and control groups.
- The administration of MELV induced a lymphocyte infiltrate, a reduction in nitric oxide level, and an increase in catalase activity, in the liver and kidney of hamster treated with 500mg/kg bw of MELV.
AUTHOR’S CONTRIBUTIONS
Oumaima Abouyaala: Execution of the acute and subacute toxicity experiment, redaction of the manuscript.
Soukaina bougrine: assistance in animal euthanasia, data statistics, assistance in the analysis of antioxidants and toxicity.
Mohamed Yassine El Brouzi: hematological analyzes of animal blood, assistance in animal euthanasia, formatting of the manuscript in the norms of the journal.
Radia Elgui: preparation of histological sections, histological interpretation.
Sara Brikat: Assistance to prepare the methanolic extract of Lemon Verbena, Phytochemical screening, oxidative stress.
Khadija Elmotia: Language correction, data statistics.
Abdelhalem Mesfioui and Aboubaker El-Hessn: advisor of the work, acces to the annimalerie and laboratory.
Moulay Laarbi Ouahidi: Supervisor of this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Statement
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee (Local Institutional Research Committee).
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflict of Interest
No potential conflict of interest was declared by the authors.
REFERENCES
Aldeen MGN, Mansoor R, AlJoubbeh M (2015). Fluctuations of phenols and flavonoids in infusion of lemon verbena (Lippia citriodora). dried leaves during growth stages. Nutr. Food Sci., 45: 766-773. https://doi.org/10.1108/NFS-04-2015-0037
Boussarie D (1999). Hématologie des rongeurs et lagomorphes de compagnie. Bull. Acad. Vét. Fr., 152: 209-216. https://doi.org/10.4267/2042/62828 https://doi.org/10.4267/2042/62828
Carnat A, Carnat AP, Fraisse D, Lamaison JL (1999). The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia, 70: 44-49. https://doi.org/10.1016/S0367-326X(98)00016-1
Carneiro FM, Silva M da, Borges LL (2014). Tendências dos estudos com plantas medicinais no Brasil. Revista Sapiência: sociedade, saberes e práticas educacionais 3: 44-75.
Casamassima D, Palazzo M, Vizzarri F (2017). Dietary effects of plant extracts, based on verbascoside, lycopene and horseradish on several blood variables and plasma oxidative status in growing rabbits. Livestock Science 206: 148-153. https://doi.org/10.1016/j.livsci.2017.10.022 https://doi.org/10.1016/j.livsci.2017.10.022
Choupani M, Delouee SA, Alami M (2014). Antioxidant properties of various solvent extracts of lemon verbena (Lippia Citriodora). leaves. Int. J. Adv. Biol. Biomed. Res., 2: 1340-1346.
Clairbone A (1985). Catalase activity. Handbook of methods for oxygen radical research. In: CRC Press Boca Raton FL. 283-284
Correia RT, Borges KC, Medeiros MF, Genovese MI (2012). Bioactive compounds and phenolic-linked functionality of powdered tropical fruit residues. Food Sci. Technol. Int. 18: 539-547. https://doi.org/10.1177/1082013211433077
De Lima R, Guex CG, da Silva ARH (2018). Acute and subacute toxicity and chemical constituents of the hydroethanolic extract of Verbena litoralis Kunth. J. Ethnopharmacol., 224: 76-84. https://doi.org/10.1016/j.jep.2018.05.012
Dhaval A, Yadav N, Purwar S (2016). Potential Applications of Food Derived Bioactive Peptides in Management of Health. Int. J. Pept. Res. Ther., 22: 377-398. https://doi.org/10.1007/s10989-016-9514-z
El-Hawary SS, Yousif MF, Abdel Motaal AA, Abd-Hameed LM (2012). Bioactivities, phenolic compounds and in-vitro propagation of Lippia citriodora Kunth cultivated in Egypt. Bull. Fac. Pharm., Cairo Univ., 50: 1-6. https://doi.org/10.1016/j.bfopcu.2011.12.001
El-Ouady F, Eddouks M (2021). Antihyperglycemic and Antihyperlipidemic Effects of Lippia citriodora in Rats. Endocr. Metab. Immune Disord. Drug Targets, 21: 711-719 https://doi.org/10.2174/1871530320666200610153532.
Etemad L, Oskouei Shirvan Z, Vahdati-Mashhadian N (2016). Acute, Subacute, and Cell Toxicity of the Aqueous Extract of Lippia citriodora. Jundishapur J. Nat. Pharm. Prod. 11: https://doi.org/10.17795/jjnpp-32546
Etemad L, Zafari R, Mashhadian NV (2015). Acute, Sub-Acute and Cell Toxicity of Verbascoside. Res. J. Med. Plant, 9: 354-360. https://doi.org/10.3923/rjmp.2015.354.360
Farahmandfar R, Asnaashari M, Pourshayegan M (2018). Evaluation of antioxidant properties of lemon verbena (Lippia citriodora). essential oil and its capacity in sunflower oil stabilization during storage time. Food Sci. Nutr., 6: 983-990. . https://doi.org/10.1002/fsn3.637
Garraud O, Damien P, Berthet J (2011). Plaquettes sanguines, réponses aux signaux de danger infectieux et inflammation: vers un nouveau paradigme? Transfus. Clin. Biol., 18: 165-173. . https://doi.org/10.1016/j.tracli.2011.02.012
Guaadaoui A, Benaicha S, Elmajdoub N (2014). What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food Sci., 3: 174-179. https://doi.org/10.11648/j.ijnfs.20140303.16
Habibi E, Talebpour Amiri F, Mokhatari M, Shaki F (2021). Effects of Aloysia citriodora Hydroalcoholic Extract on Ethanol-induced Hepatotoxicity in Male Wistar Rats. PBR https://doi.org/10.18502/pbr.v7i3.7698.
Hamden K, Carreau S, Ellouz F (2007). Protective effect of 17beta-estradiol on oxidative stress and liver dysfunction in aged male rats. Journal of physiology and biochemistry 63: 195-201 https://doi.org/10.1007/BF03165782
Harborne AJ (1998). Phytochemical methods a guide to modern techniques of plant analysis. springer science and business media
Hawary SE, Yousif M, Motaal AA, Hameed LA-E (2011). Composition and Bioactivities of the Essential Oil from Leaves of Lippia citriodora Kunth Cultivated in Egypt. J. Biol. Act. Prod. Nat., 1: 112-119. https://doi.org/10.1080/22311866.2011.10719077
Hematian Sourki A, Ghani A, Kiani F, Alipour A (2021a). Phytochemical profiles of lemon verbena ( Lippia citriodora H.B.K.). and its potential application to cookie enrichment. Food Sci. Nutr., 9: 3100-3113. https://doi.org/10.1002/fsn3.2268
Hematian Sourki A, Ghani A, Kiani F, Alipour A (2021b). Phytochemical profiles of lemon verbena (Lippia citriodora H.B.K.). and its potential application to cookie enrichment. Food Sci. Nutr., 9: 1-14. https://doi.org/10.1002/fsn3.2268
Hershkovitz Y, Naveh S, Kessel B (2015). Elevated white blood cell count, decreased hematocrit and presence of macrohematuria correlate with abdominal organ injury in pediatric blunt trauma patients: a retrospective study. World J. Emerg. Surg., 10: 41. https://doi.org/10.1186/s13017-015-0034-5
Houghton P, Raman A (1998). Laboratory Handbook for the Fractionation of Natural Extracts. Springer Science and Business Media https://doi.org/10.1007/978-1-4615-5809-5
Kachmar MR, Naceiri Mrabti H, Bellahmar M (2021). Traditional Knowledge of Medicinal Plants Used in the Northeastern Part of Morocco. Evid. Based. Complement Altern. Med., 2021: 6002949. https://doi.org/10.1155/2021/6002949
Karumi Y (2004). Identification of Active Principles of M. balsamina (Balsam Apple). Leaf Extract Y. Karumi,” PA. Onyeyili and “VO Ogugbuaja. J. Med. Sci., 4: 179-182. https://doi.org/10.3923/jms.2004.179.182
Kovats S (2012). Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: Mechanisms and implications for immunity. Horm. Behav., 62: 254-262. https://doi.org/10.1016/j.yhbeh.2012.04.011
Li R, Yuan C, Dong C (2011). In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn-Schmiedeberg’s Arch. Pharmacol., 383: 437-445. https://doi.org/10.1007/s00210-011-0613-2
Majewska E, Kozłowska M, Tarnowska K (2022). Chemical Composition and Biological Activity of Lemon verbena (Lippia citriodora). Essential Oil - A Review. J. Essent. Oil Bear. Plants, 25: 796-810. https://doi.org/10.1080/0972060X.2022.2130015
Maldonado Miranda JJ (2021). Chapter 7 - Medicinal plants and their traditional uses in different locations. In: Bhat RA, Hakeem KR, Dervash MA (eds). Phytomedicine. Acad. Press, 207-223 https://doi.org/10.1016/B978-0-12-824109-7.00014-5
Mibindzou Mouellet A (2004). Screening phytochimique de deux espèces de plantes: crotalia retusa L (Papilionaceae). et hallea ciliata Aubrev et Pellegr.(Rubiaceae). récoltées au Gabon
Muanda FN (2010). Identification de polyphénols, évaluation de leur activité antioxydante et étude de leurs propriétés biologiques. Univ. Paul Verlaine-Metz 238:
OECD 407 (2001). OECD guideline for testing of chemicals. Organisation for Economic Co-Operation and Development: Paris, France
OECD 423 (2002). Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. Organisation for Economic Co-operation and Development, Paris
Olayode OA, Daniyan MO, Olayiwola G (2019). Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J. Tradit Complement Med., 10: 544-554. https://doi.org/10.1016/j.jtcme.2019.05.001
Ozer J, Ratner M, Shaw M (2008). The current state of serum biomarkers of hepatotoxicity. Toxicology 245: 194-205. https://doi.org/10.1016/j.tox.2007.11.021
Pastorelli G, Rossi R, Corino C (2012). Influence of Lippia citriodora verbascoside on growth performance, antioxidant status, and serum immunoglobulins content in piglets. Czech J. Anim. Sci., 57: 312-322. https://doi.org/10.17221/6006-CJAS
Quirantes-Piné R, Herranz-López M, Funes L (2013). Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine, 20: 1112-1118. https://doi.org/10.1016/j.phymed.2013.05.007
Rashidian A, Farhang F, Vahedi H (2016). Anticonvulsant Effects of Lippia citriodora (Verbenaceae). Leaves Ethanolic Extract in Mice: Role of GABAergic System. Int J Prev Med 7: 97. https://doi.org/10.4103/2008-7802.187251
Rezig L, Sadaa M, Trabelsi N (2019). Chemical composition, antioxidant and antimicrobial activities of Aloysia Triphylla L. essential oils and methanolic extract. Ital.J. Food Sci., 31: https://doi.org/10.14674/IJFS-1373
Stashenko EE, Martínez JR, Cala MP (2013). Chromatographic and mass spectrometric characterization of essential oils and extracts from L ippia (V erbenaceae). aromatic plants. J. Sep. Sci., 36: 192-202. https://doi.org/10.1002/jssc.201200877
Tammar S, Salem N, Aidi Wannes W (2021). Chemometric Profiling and Bioactivity of Verbena (Aloysia citrodora). Methanolic Extract from Four Localities in Tunisia. Foods, 10: 2912. https://doi.org/10.3390/foods10122912
Timoumi R, Amara I, Neffati F (2019). Acute triflumuron exposure induces oxidative stress responses in liver and kidney of Balb/C mice. Environ. Sci. Pollut. Res., 26: 3723-3730. https://doi.org/10.1007/s11356-018-3908-8
Valentão P, Fernandes E, Carvalho F (2002). Studies on the antioxidant activity of Lippia citriodora infusion: scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biol. Pharm. Bull.tin, 25: 1324-1327. https://doi.org/10.1248/bpb.25.1324
Veisi M, Shahidi S, Komaki A, Sarihi A (2015). Assessment of aqueous extract of Lemon verbena on anxiety-like behavior in rats. J. Pharm. Negat. Results, 6: 37. https://doi.org/10.4103/0976-9234.157390
Veskoukis A, Kerasioti E, Priftis A (2019). A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: The biomarker issue. Curr. Opin. Toxicol., 13: 99-109. https://doi.org/10.1016/j.cotox.2018.10.001
WHO (2013). Stratégie de l’OMS pour la médecine traditionnelle pour 2014-2023. WHO, Genève
Zhao Y, Wang S, Pan J, Ma K (2023). Verbascoside: A neuroprotective phenylethanoid glycosides with anti-depressive properties. Phytomedicine, 120: 155027. https://doi.org/10.1016/j.phymed.2023.155027
To share on other social networks, click on any share button. What are these?





