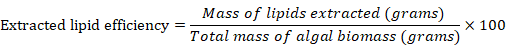Optimizing Biofuel Yields from Spirogyra Microalgae: The Role of Extraction Techniques and Environmental Factors
Optimizing Biofuel Yields from Spirogyra Microalgae: The Role of Extraction Techniques and Environmental Factors
Muhammad Waqas1*, Razia Bashir1, Haroon Ali2, Arslan Shahzad1 and Tehmina Saddique3
1Department of Zoology, Division of Science and Technology, University of Education, Lahore, Pakistan
2Department of Chemistry, Division of Science and Technology, University of Education, Lahore, Pakistan
3Institute of Chemical Sciences, Bahauddin Zakariya University, Multan, Pakistan
ABSTRACT
The increasing challenges of global warming, fossil fuel depletion and rising petroleum prices underscore the urgent need for renewable, eco-friendly, and sustainable biofuels. Fossil fuels, which currently meet over 80-85% of global energy demands, are rapidly depleting, with Pakistan heavily dependent on them for nearly 88% of its energy needs. This study aimed to evaluate the bioenergy potential of Spirogyra microalgae for biodiesel production, offering a sustainable solution to fossil fuel dependence and environmental degradation. Lipids from microalgae Spirogyra were extracted using n-Hexane as a solvent by both the hot plate magnetic stirrer method and simple solvent extraction method. The effects of habitat nature, n-Hexane to algal biomass ratio, algal biomass particle size, and contact time on the percentage yield of extracted lipids were studied and analyzed. The study showed that algae from agricultural runoff had the highest lipid extraction efficiency at 3.9%, while industrial effluent samples had the lowest at 3.56%. The hot plate magnetic stirrer method achieved 1.38% efficiency. An algae to n-Hexane ratio of 1:3 and smaller particle sizes yielded higher lipid extractions. The findings suggest that the Hot Plate Magnetic Stirrer method is more effective in lipid extraction from Spirogyra microalgae for biodiesel production. This underscores the importance of optimizing extraction methods for maximum lipid yield, contributing to the advancement of sustainable energy production. This study highlights the importance of nature-inspired solutions in addressing the complex challenges of energy security and environmental degradation. By harnessing the potential of Spirogyra microalgae for biodiesel production, it offers a pathway towards sustainable energy production and environmental conservation.
Article Information
The article was presented in 42nd Pakistan Congress of Zoology (International) held on 23-25th April 2024, organized by University of Azad Jammu & Kashmir, Muzaffarabad, Pakistan.
Authors’ Contribution
MW conducted the core research, formatted the manuscript, analysed and interpreted data, and presentated the work. RB conceived the research idea, supervised the study, and revised the manuscript. HA collected samples and assisted in conducting experiments. AS contributed to sample collection. TS provided support in manuscript writing.
Key words
Biofuels, Spirogyra microalgae, Biodiesel production, Sustainable energy, Environmental conservation
DOI: https://dx.doi.org/10.17582/ppcz/42.09.16
* Corresponding author: waqassiddique614@gmail.com, mtf2202199@ue.edu.pk
1013-3461/2024/0009 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Global warming, running out of fossil fuels, and the rising prices of petroleum are the main issues our world is facing today (Baig et al., 2018; Brennan and Owende, 2010). That is why there is a lot of focus on finding new ways to make renewable, eco-friendly and sustainable biofuels that would not harm the environment. Fossil fuels are a key source in meeting global energy needs, over 80-85%, from heating our homes to powering our automobiles (Dale, 2023; Friedemann, 2021; Ritchie et al., 2022; Keleş, 2011) especially Pakistan is heavily dependent on fossil fuels to meet nearly 88% of its energy requirements (Ali et al., 2021). In 2020, the distribution of world energy use was approximately 83.1% from fossil fuels, 12.6% from renewable sources, and 4.3% from nuclear power (Masson et al., 2021; Dale, 2023). It is true that the fossil fuels are still being formed, but at an entirely negligible rate compared to the rate at which we are consuming them. They will be run-off within 50 years if we continue our current usage due to their finite resources (Dale, 2023; Friedemann, 2021; Ritchie et al., 2022; Mitchell and Robinson, 2021). Predicting the exact timeline for the complete depletion of fossil fuels is challenging, but it is widely acknowledged that these resources are finite and face a future of diminishing availability.
The problems related to fossil fuels extend beyond depletion. The combustion of fossil fuels releases carbon dioxide, contributing significantly to global warming (UNEP, 2020) that leads to climate change (Keeling et al., 1995; Houghton, 1997; Houghton et al., 2001; Walther et al., 2002; Mann and Jones, 2003; Karl and Trenberth, 2003; Rogelj et al., 2018; Le Quéré et al., 2020). This is now considered the primary threat to the future of humanity by the leading scientists (Masson et al., 2021; IPCC, 2018; Ripple et al., 2017, 2021). When we burn fossil fuels (coal, oil, and natural gas) to generate energy, gases are released into the atmosphere. This atmospheric pollution is leading to health problems, even fatalities (Ristinen and Kraushaas, 2006). We tried in our study to focus on the replacement of fossil fuels with renewable and sustainable energy sources because this is considered to be the most practical single pathway to climate stabilization when physical, financial, political, and environmental factors are all considered (Masson et al., 2021; Dale, 2023; Heinberg and Friedly, 2016; Hickel and Kallis, 2019; IEA, 2021).
Bioenergy has received a significant attention in recent times due to the rapid growth of fuel prices, fast fossil fuel depletion, environmental deterioration caused by fossil fuels, and shifts in the global climate. It is considered a potent choice as a renewable energy source in order to reduce fossil fuel use and plays an important role in net-zero emission pathways (Seo et al., 2024; Ali et al., 2022). In the context of modern bioenergy, various products contribute to its diverse portfolio, including bioethanol, biodiesel, biomass energy, biogas energy, biohydrogen, biopower, biopropane, and biochar. However, among these, biodiesel and bioethanol emerge as the primary outputs (Rasool and Hamlatha, 2016). Notably, these two major bioenergy products serve as viable alternatives for traditional transportation fuels, hence are referred to as biofuels.
Within the context of bioenergy, microalgae emerge as a frontrunner due to their exceptional growth rates, high lipid content, and versatile biochemical composition (Chisti, 2007; Mata et al., 2010). Algal biofuel has garnered increasing attention in recent years because algae boast higher production yields than terrestrial crops, making them a promising alternative to gasoline, jet fuel, and diesel in the transportation industry. In terms of growth and oil content, the growth rate of algae is approximately 20-30 times faster than conventional food crops and the oil content of algae is around 30-40% and this value can rise up to 85% for many species (Khan et al., 2017; Ullah et al., 2015). Consequently, the per hectare cultivation yield of microalgae can be boosted up to ten times higher than any oil crop. Algal biofuel has the capacity to store and accumulate substantial amounts of triacylglycerides (TAGs), which serve as the primary feedstock for biodiesel production.
Microalgae have the innate ability to grow in all waters such as brackish, saline, waste waters, etc. (Tsai et al., 2015) and can multiply their mass several times a day, yielding more oil than other plant crops (Demirbas, 2010). Due to the simple cellular structure of microalgae, they can proficiently harness solar energy. The cultivation of algae decreases the CO2 concentration in the atmosphere by CO2 biofixation and converting it to oxygen (Laamanen et al., 2016). 183 tons of CO2 can be captured when 100 tons of microalgal biomass is produced (Chisti, 2008; Razeghifard, 2013). Additionally, photoautotrophic microalgae can metabolize inorganic carbon dioxide through photosynthesis and absorb environmental pollutants like nitrates and phosphates (Gouveia, 2009; Veillette et al., 2012). Moreover, after oil extraction the remnant algae can be used as fertilizers or as fish feed in fish and oyster farms (Ullah et al., 2015).
Among the diverse array of microalgae species, Spirogyra stands out as a particularly promising candidate for biofuel production (biodiesel), owing to its swift growth in various aquatic environments and favorable lipid accumulation characteristics (Becker, 2007; Khan et al., 2009; Dineshkumar et al., 2019). Spirogyra microalgae possess inherent advantages over terrestrial biomass feedstocks, such as rapid biomass generation, minimal land and water requirements, and the ability to thrive in nutrient-rich wastewater streams (Hu et al., 2020; Sharma et al., 2021). It produces lipids, carbohydrate, and proteins that can be utilized for the production of biodiesel, or bioethanol (Khan et al., 2009). Utilizing spirogyra microalgae for biodiesel production has the potential to significantly contribute to sustainable and environmentally friendly energy production. This approach could help alleviate the challenges associated with fossil fuel dependence and environmental degradation.
The purpose of the study was to evaluate the bioenergy potential of Spirogyra microalgae through lipid extraction using n-hexane as a solvent and to compare the efficacy of simple solvent extraction method and hot plate magnetic stirrer method. Effects of habitat, n-hexane to algal biomass ratio, algal biomass particle size and contact time on the percentage yield of oil extracted were studied.
MATERIALS AND METHODS
Chemicals
The laboratory experiment utilized analytical-grade chemicals including n-hexane, methanol, and ethanol without additional purification. The entire experimental procedure was carried out within the Zoology Laboratory at the University of Education, Lahore, Pakistan.
Algae sample collection
The algae samples were collected from three different locations from different habitats to assess the relationship between lipid quantities with habitat nature. These samples were collected from University of Education, Lahore (open pond), Kasur city (rainfall water) and Gujranwala City (agricultural runoff) by Muhammad Waqas, Haroon Ali and Arslan Shahzad, respectively.
Algae identification and preparation for oil extraction
The collected samples were examined under NOVEX HOLAND 86.025 Compound Microscope and identified as microalgae Spirogyra. The samples were spread under the sun for 4 days to dry them properly. The dried samples were grinded with the help of pestle and grinder and the fine powder was separated by passing it through different microsieves. The ground algae were dried in an incubator at 75 °C for 30 min. The algae powder was stored in sealed jars for later use.
Oil extraction from algae
In the simple solvent extraction method, n-hexane was mixed with algae powder in three different ratios (i.e., 1:1, 1:2 and 1:3) in a 250 ml separating funnel, thoroughly stirred for proper mixing, and left undisturbed for 24 h to allow for lipid extraction and settling of solid particles. The same procedure was repeated for all samples.
Similarly, in the hot plate magnetic stirrer method, n-hexane was mixed with algae powder in three different ratios (i.e., 1:1, 1:2 and 1:3) in a 250 ml round bottom flask, placed on a hot plate magnetic stirrer, and stirred for five h at a controlled temperature to enhance solvent penetration and mixing. The round bottom flask containing mixture was kept for 40 min after stirring for settlement of organic layers. After the extraction period, the round bottom flask was carefully opened to allow for phase separation, and the organic phase containing the dissolved algae oil was carefully transferred into pre-weighed 50 ml beakers using a glass pipette. To remove any remaining solid particles from the extracted oil, the organic phase was filtered using Whatman quantitative filter paper grade 40. The filtered algae oil was collected in pre-weighed beakers to determine the mass of the extracted oil accurately. Finally, to remove residual n-hexane from the extracted oil, the collected oil samples were subjected to evaporation in a water bath set at 30 °C, ensuring the evaporation of hexane while preserving the reliability of the extracted algae oil. All extraction procedures were performed in triplicates to ensure the reliability and reproducibility of the results, with statistical analysis conducted to compare the efficiency of the two extraction methods and validate the findings.
The lipid yield (wt. %) was then calculated by using the following equation (Arun et al., 2017; Chen et al., 2015).
RESULTS
Lipid extraction from algal samples by solvent extraction method
Table I described the results of lipids extraction by solvent extraction method from dried algal powder collected from different sources. The dried algal powder was mixed with n-hexane in different ratios. The results showed that the minimum lipids 0.78 g with extracted efficiency 2.6 were extracted by using algal to n-hexane in 1:1 ratio and maximum lipids 1.12 g with extracted efficiency 3.73 were extracted by using Algal to n-hexane in 1:3 ratio. The dried algal powder of sample from Kasur city was mixed with n-hexane in different ratios. The results showed that the minimum lipids 0.72 g were extracted with extracted efficiency 2.4 by using algal to n-hexane ratio 1:1 and the maximum lipids 1.07 g with extracted efficiency 3.56 were extracted with ratio 1:3. Likewise, the dried algal powder of samples from Gujranwala city were mixed with n-hexane in different ratios. The results showed that the minimum lipids 0.8 g with extracted efficiency 2.66 were extracted by using algal to n-hexane in 1:1 ratio and the maximum lipids 1.16 g with extracted efficiency 3.86 were extracted by using algal to n-hexane in 1:3 ratio.
Table I. Lipids extraction from samples collected from University of Education, Township, Kasur city and Gujranwala cities by solvent extraction method after 24 h of settling time.
|
Sample |
Algal biomass (g) |
n-Hexane (g) |
Algae to n-hexane ratio |
Lipids extracted (g) |
Extracted efficiency (%) |
|
University of Education sample |
|||||
|
1 |
30 |
30 |
1 |
0.78 |
2.6 |
|
2 |
30 |
60 |
2 |
0.92 |
3.06 |
|
3 |
30 |
90 |
3 |
1.12 |
3.73 |
|
Kasur city sample |
|||||
|
1 |
30 |
30 |
1 |
0.72 |
2.4 |
|
2 |
30 |
60 |
2 |
0.89 |
2.96 |
|
3 |
30 |
90 |
3 |
1.07 |
3.56 |
|
Gujranwala sample |
|||||
|
1 |
30 |
30 |
1 |
0.8 |
2.66 |
|
2 |
30 |
60 |
2 |
0.97 |
3.23 |
|
3 |
30 |
90 |
3 |
1.16 |
3.86 |
Lipid extraction from algal samples by hot plate magnetic stirrer method
Table II explained the results of lipids extraction by solvent extraction method by using hot plate magnetic stirrer, from dried algal powder collected from University of Education, Lahore. The mixture was stirred for 5 h with help of hot plate magnetic stirrer and then let settled for 40 min to separate the layers. The dried algal powder was mixed with n-Hexane in different ratios. The results showed that the minimum lipids 0.82 g with extracted efficiency 2.73 were extracted by using algal to n-hexane in 1:1 ratio and maximum lipids 1.14 g with extracted efficiency 3.8 were extracted by using algal to n-hexane in 1:3 ratio.
Table II. Lipids extraction from samples collected from University of Education, Township, Kasur city and Gujranwala cities by using hot plate magnetic stirrer.
|
Sample |
Algal biomass (g) |
n-hexane (g) |
Algae to n-hexane ratio |
Lipids extracted (g) |
Extracted efficiency % |
|
University of Education |
|||||
|
1 |
30 |
30 |
1 |
0.82 |
2.73 |
|
2 |
30 |
60 |
2 |
0.94 |
3.13 |
|
3 |
30 |
90 |
3 |
1.14 |
3.8 |
|
Kasur city sample |
|||||
|
1 |
30 |
30 |
1 |
0.74 |
2.46 |
|
2 |
30 |
60 |
2 |
0.92 |
3.06 |
|
3 |
30 |
90 |
3 |
1.15 |
3.83 |
|
Gujranwala sample |
|||||
|
1 |
30 |
30 |
1 |
0.82 |
2.73 |
|
2 |
30 |
60 |
2 |
0.98 |
3.26 |
|
3 |
30 |
90 |
3 |
1.17 |
3.9 |
Table II described the results of lipids extraction by solvent extraction method by using hot plate magnetic stirrer, from dried algal powder collected from Kasur city. The mixture was stirred for 5 h with the help of hot plate magnetic stirrer and then let settled for 40 min for settlement of the layers. The dried algal powder was mixed with n-Hexane in different ratios. The results showed that the minimum lipids 0.74 g with extracted efficiency 2.46 were extracted by using algal to n-hexane in 1:1 ratio and maximum lipids 1.15 g with extracted efficiency 3.83 were extracted by using algal to n-Hexane in 1:3 ratio. Table II shows the results of lipids extraction by solvent extraction method by using hot plate magnetic stirrer, from dried algal powder collected from Gujranwala city. The mixture was stirred for 5 h with help of hot plate magnetic stirrer and then let settled for 40 min to separate the layers. The dried algal powder was mixed with n-hexane in different ratios. The results showed that the minimum lipids 0.82 g with extracted efficiency 2.73 were extracted by using algal to n-hexane in 1:1 ratio and maximum lipids 1.17 g with extracted efficiency 3.9 were extracted by using algal to n-hexane in 1:3 ratio.
DISCUSSION
The lipid extraction efficiency varied significantly among the different sources of algal samples, revealing interesting insights into the influence of environmental conditions on lipid contents in algae. Our study showed that algae collected from agricultural runoff had the highest lipid extraction efficiency at 3.9%. This finding aligns with literature suggesting that agricultural runoff, which often contains nutrients such as nitrogen and phosphorus from fertilizers, can enhance algal growth and lipid accumulation. Previous studies have documented increased lipid content in algae due to nutrient-rich environments provided by agricultural runoff (Li et al., 2013; Tang et al., 2014). The elevated levels of these nutrients can stimulate lipid biosynthesis pathways, thereby increasing lipid content in algal cells.
In contrast, samples from industrial effluents exhibited the lowest lipid extraction efficiency at 3.56%. This lower efficiency may be attributed to the presence of toxic substances in industrial effluents, which can inhibit algal growth and lipid production. Similar observations have been reported in other studies, where industrial effluents negatively impacted algal lipid content due to the presence of heavy metals and other contaminants (Ahmad et al., 2017; Singh et al., 2019). Heavy metals, such as cadmium and lead, can interfere with cellular metabolism and reduce the overall lipid content by disrupting enzyme activities essential for lipid biosynthesis (Rai et al., 2020).
The hot plate magnetic stirrer method demonstrated a lipid extraction efficiency of 1.38%, outperforming other solvent extraction methods for Spirogyra microalgae. This method’s efficiency can be attributed to its ability to maintain uniform temperature and agitation, facilitating better solvent penetration and lipid solubilization. This finding is supported by existing research, which highlights the importance of controlled heating and agitation in enhancing lipid extraction efficiency (Wang et al., 2010; Lee et al., 2012). Uniform temperature control prevents the degradation of thermolabile lipids, while consistent agitation ensures that solvent molecules interact effectively with algal cell walls, promoting efficient lipid extraction (Sathish et al., 2013).
Our study identified an algal biomass to solvent (n-hexane) ratio of 1:3 as the most effective for lipid extraction. This ratio likely provides an optimal balance between solvent availability and algal biomass concentration, ensuring maximum lipid dissolution without excessive solvent use. Literature supports the use of n-hexane due to its non-polar nature, which efficiently extracts lipids from algal cells (Folch et al., 1957; Bligh and Dyer, 1959). Similar studies have shown that varying the solvent ratio can significantly impact lipid yield, with an optimal ratio often lying within the range of 1:2 to 1:4 (Zheng et al., 2013; Yoo et al., 2014). Excessive solvent use can lead to saturation and inefficiency, while insufficient solvent can result in incomplete lipid extraction (Johnson and Wen, 2009).
Our results indicate that smaller algal biomass particle sizes yield higher lipid extractions compared to larger particles. This outcome can be attributed to the increased surface area of smaller particles, which enhances solvent contact and lipid release. Previous research has similarly reported that reducing particle size improves lipid extraction efficiency by facilitating better solvent penetration and interaction with intracellular lipids (Park et al., 2015; Kim et al., 2016). The increased surface area allows for more effective disruption of cell walls and membranes, leading to greater lipid release into the solvent (Lee et al., 2010). Furthermore, mechanical pretreatment methods, such as milling or homogenization, can significantly reduce particle size and improve overall lipid recovery (Günerken et al., 2015).
Integrating our findings with the existing literature underscores the critical role of environmental factors, extraction methods, solvent ratios, and biomass particle size in optimizing lipid extraction from algae. The superior performance of samples from agricultural runoff suggests that nutrient enrichment plays a pivotal role in lipid biosynthesis. This insight is critical for selecting optimal cultivation conditions for algae intended for biodiesel production. Additionally, the effectiveness of the hot plate magnetic stirrer method emphasizes the need for precise control in extraction processes to maximize lipid yield and quality. Moreover, optimizing the biomass to solvent ratio and particle size can significantly enhance lipid yields, offering practical guidelines for improving biodiesel production from algal biomass.
These insights not only contribute to the body of knowledge on algal lipid extraction but also have practical implications for scaling up biodiesel production. Future research could focus on exploring other nutrient sources, such as wastewater from different industrial and domestic sources, and refining extraction techniques to further enhance lipid yields, ultimately supporting the development of sustainable biofuel technologies. Investigating the synergistic effects of combining different solvents and mechanical pretreatment methods could also provide valuable insights into further optimizing the lipid extraction process.
CONCLUSION
This study demonstrates that environmental conditions, extraction methods, solvent ratios, and biomass particle sizes significantly impact lipid extraction efficiency from algae. Algae from agricultural runoff showed the highest lipid extraction efficiency at 3.9%, likely due to nutrient-rich conditions, whereas industrial effluents had the lowest at 3.56% due to the presence of toxic substances. The hot plate magnetic stirrer method proved most effective for Spirogyra microalgae with an efficiency of 1.38%, benefiting from uniform temperature and agitation. An algal biomass to n-hexane solvent ratio of 1:3 was optimal for lipid extraction, and smaller particle sizes further enhanced lipid yields by increasing surface area and solvent contact. These findings offer practical guidelines for optimizing biodiesel production from algae and highlight the need for precise control over cultivation and extraction conditions to maximize lipid yields, suggesting future research should explore diverse nutrient sources and refine extraction techniques to support sustainable biofuel technologies.
Declarations
Acknowledgement
We acknowledge the Department of Zoology, University of Education, Lahore, and all team members for their valuable contributions.
Funding
No funding or grants were received for this study.
IRB approval
The Advance Study and Research Board at University of Education, Lahore approved the protocol of the present study.
Ethical statement
Permission from Ethical Committee, University of Education, Lahore, was taken for the research work.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Ahmad, A.L., Yasin, N.H.M., Derek, C.J.C. and Lim, J.K., 2017. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev., 58: 180-197.
Ali, S.S., Al-Tohamy, R., Mahmoud, Y.A.G., Kornaros, M., Sun, S. and Sun, J., 2022. Recent advances in the life cycle assessment of biodiesel production linked to azo dye degradation using yeast symbionts of termite guts: A critical review. Energy Rep., 8: 7557–7581. https://doi.org/10.1016/j.egyr.2022.05.240
Ali, Z., Liaquat, R., Khoja, A.H. and Safdar, U., 2021. A comparison of energy policies of Pakistan and their impact on bioenergy development. Sustain. Energy Technol. Assess., 46: 101246. https://doi.org/10.1016/j.seta.2021.101246
Anto, S., Mukherjee, S.S., Muthappa, R., Mathimani, T., Deviram, G., Kumar, S.S., Verma, T.N. and Pugazhendhi, A., 2020. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere, 242: 125079. https://doi.org/10.1016/j.chemosphere.2019.125079
Arun, J., Shreekanth, S.J., Sahana, R., Raghavi, M.S., Gopinath, K.P. and Gnanaprakash, D., 2017. Studies on influence of process parameters on hydrothermal catalytic liquefaction of microalgae (Chlorella vulgaris) biomass grown in wastewater. Bioresour. Technol., 244: 963-968. https://doi.org/10.1016/j.biortech.2017.08.048
Baig, R.U., Malik, A., Ali, K., Arif, S., Hussain, S., Mehmood, M. and Khan, M.N., 2018. Extraction of oil from algae for biodiesel production, from Quetta, Pakistan. IOP Conf. Ser. Mater. Sci. Eng., 414: 012022. IOP Publishing. https://doi.org/10.1088/1757-899X/414/1/012022
Becker, E.W., 2007. Microalgae as a source of protein. Biotechnol. Adv., 25: 207-210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Bligh, E.G. and Dyer, W.J., 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37: 911-917. https://doi.org/10.1139/o59-099
Brennan, L. and Owende, P., 2010. Biofuels from microalgae. A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev., 14: 557-577. https://doi.org/10.1016/j.rser.2009.10.009
Chen, H., Zhou, D., Luo, G., Zhang, S. and Chen, J., 2015. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev., 47: 427- 437. https://doi.org/10.1016/j.rser.2015.03.086
Chisti, Y., 2007. Biodiesel from microalgae. Biotechnol. Adv., 25: 294-306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Chisti, Y., 2008. Biodiesel from microalgae beats bioethanol. Trends Biotechnol., 26: 126–131. https://doi.org/10.1016/j.tibtech.2007.12.002
Dale, S., 2023. BP statistical review of world energy 2023, 72nd ed.; BP Plc: London, UK, 2023; Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 02 February 2024).
Demirbas, 2010. Use of algae as biofuel sources. Energy Convers. Manage. 51: 2738. https://doi.org/10.1016/j.enconman.2010.06.010
Dineshkumar, R., Sivaramakrishnan, R., Ramalingam, S., Hemalatha, A., Ananth, D.A., Arumugam, M. and Rajasekar, A., 2019. Microalgae and cyanobacteria: A sustainable alternative for biofuels. Bioresour. Technol. Rep., 5: 166-173.
Folch, J., Lees, M. and Sloane, S.G.H., 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. biol. Chem., 226: 497-509. https://doi.org/10.1016/S0021-9258(18)64849-5
Friedemann, A.J., 2021. Life after fossil fuels: A reality check on alternative energy. Springer International Publishing. https://doi.org/10.1007/978-3-030-70335-6
Gouveia, L.A.C., 2009. Oliveira, microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol., 36: 269–274. https://doi.org/10.1007/s10295-008-0495-6
Günerken, E., D’Hondt, E., Eppink, M.H., Garcia-Gonzalez, L., Elst, K. and Wijffels, R.H., 2015. Cell disruption for microalgae biorefineries. Biotechnol. Adv., 33: 243-260. https://doi.org/10.1016/j.biotechadv.2015.01.008
Heinberg, R. and Fridley, D., 2016. Our renewable future. In: Laying the path for one hundred percent clean energy (eds. R. Heinberg and D. Lerch), Island Press, Washington, DC, USA. https://doi.org/10.5822/978-1-61091-780-3
Hickel, J. and Kallis, G., 2019. Is green growth possible? New Polit. Econ., 25: 469–486. https://doi.org/10.1080/13563467.2019.1598964
Houghton, J., 1997. Global warming: The complete briefing (2nd ed.). Cambridge University Press, Cambridge. pp. 251. https://doi.org/10.1017/CBO9781139164252
Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K. and Johnson, C.A., 2001. Climate change 2001: The scientific basis, IPCC. Cambridge University Press, Cambridge. pp. 881.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M. and Darzins, A., 2020. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Pl. J., 54: 621-639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
IEA, 2021. Net zero by 2050: A roadmap for the global energy sector. International Energy Agency, Paris, France.
IPCC, 2018. Global warming of 1.5 °C. International Panel on Climate Change, Geneva, Switzerland.
Johnson, M.B. and Wen, Z., 2009. Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels, 23: 5179-5183. https://doi.org/10.1021/ef900704h
Karl, T.R., and Trenberth, K.E., 2003. Modern climate change. Science, 302: 1719-1721. https://doi.org/10.1126/science.1090228
Keeling, C.D., Whorf, T.P., Wahlen, M. and van der Plicht, J., 1995. Interannual extremes in the rate of rise of atmospheric carbon dioxide since 1980. Nature, 375: 666-670. https://doi.org/10.1038/375666a0
Keleş, S., 2011. Fossil energy sources, climate change, and alternative solutions. Energy Sour. A: Recovery, Util. Environ. Effects, 33: 1184-1195. https://doi.org/10.1080/15567030903330660
Khan, S., Siddique, R., Sajjad, W., Nabi, G., Hayat, K.M., Duan, P. and Yao, L., 2017. Biodiesel production from algae to overcome the energy crisis. Hayati J. Biosci., 24: 163. https://doi.org/10.1016/j.hjb.2017.10.003
Khan, S.A., Hussain, M.Z., Prasad, S. and Banerjee, U.C., 2009. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev., 13: 2361-2372. https://doi.org/10.1016/j.rser.2009.04.005
Kim, D.Y., Vijayan, D., Praveen-Kumar, R., Han, J.I., Lee, K. and Oh, Y.K., 2016. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol., 199: 300-310. https://doi.org/10.1016/j.biortech.2015.08.107
Laamanen,C.A., Ross, G.M. and Scott, J.A., 2016. Flotation harvesting of microalgae. Renew. Sustain. Energy Rev., 58: 75. https://doi.org/10.1016/j.rser.2015.12.293
Le Quéré, C., Jackson, R.B., Jones, M.W., Smith, A.J., Abernethy, S., Andrew, R.M. and G.P. Peters. 2020. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Change, 10: 647-653. https://doi.org/10.1038/s41558-020-0797-x
Lee, J.Y., Yoo, C., Jun, S.Y., Ahn, C.Y. and Oh, H.M., 2010. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol., 101: S75-S77. https://doi.org/10.1016/j.biortech.2009.03.058
Lee, S.Y., Cho, J.M., Chang, Y.K. and Oh, Y.K., 2012. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol., 140: 120-127.
Leong, S., Tan, G., Hao, O.S. and Lim, M., 2011. What is algae biofuel? In: Oil of the future algae.
Li, X., Hu, H.Y., Zhang, Y.P. and Xu, X.Q., 2013. Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol., 150: 586-592.
Mann, E.M. and Jones, P.D., 2003. Global surface temperatures over the past two millennia. Geophys. Res. Lett., 30: 1820. https://doi.org/10.1029/2003GL017814
Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S. and Gomis, M.I., 2021. IPCC climate change 2021: The physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK.
Mata, T.M., Martins, A.A. and Caetano, N.S., 2010. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev., 14: 217. https://doi.org/10.1016/j.rser.2009.07.020
Mitchell, J. and Robinson, P., 2021. The implications of future fossil fuel availability for global development pathways. Energy Strategy Rev., 34: 100677.
Park, J.Y., Kim, D.K., Lee, J.S., Park, S.C., Kim, Y.J. and Lee, J.S., 2015. Blending effects of alternative diesels on oxidation stability and low temperature flow properties. Renew. Energy, 79: 21-26.
Rai, P.K., Lee, S.S., Zhang, M., Tsang, Y.F. and Kim, K.H., 2020. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int., 134: 105-111.
Rasool, U. and Hmalatha, S., 2016. A review on bioenergy and biofuels: Sources and their production. Braz. J. biol. Sci., 3: 3-22. https://doi.org/10.21472/bjbs.030501
Razeghifard, R., 2013. Algal biofuels. Photosynth. Res., 117: 207-219. https://doi.org/10.1007/s11120-013-9828-z
Ripple, W.J., Wolf, C., Newsome, T.M., Galetti, M., Alamgir, M., Crist, E. and Laurance, W.F., 2017. World scientists warning to humanity: A second notice. BioScience, 67: 1026–1028. https://doi.org/10.1093/biosci/bix125
Ripple, W.J., Wolf, C., Newsome, T.W., Gregg, J.W., Lenton, M., Barnard, P. and Moomaw, W.R., 2021. World scientists warning of climate emergency 2021. BioScience, 71: 894–898. https://doi.org/10.1093/biosci/biab079
Ristinen, R.A. and Kraushaas, J.P., 2006. Energy and the environment. John Wiley.
Ritchie, H., Roser, M. and Rosado, P., 2022. Fossil fuels. Our world in data. Retrieved from https://ourworldindata.org/fossil-fuels (accessed on 02 February 2024).
Rogelj, J., Shindell, D., Jiang, K., Fifita, S., Forster, P., Ginzburg, V. and Masson-Delmotte, V., 2018. Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change. Retrieved from https://www.ipcc.ch/sr15/
Saifullah, A.Z.A., Karim, M.A. and Ahmad-Yazid, A., 2014. Microalgae: An alternative source of renewable energy. Am. J. Eng. Res., 3: 330-338.
Sathish, A., Smith, B.R. and Sims, R.C., 2013. Effect of moisture on in situ transesterification of microalgae for biodiesel production. J. Chem. Technol. Biotechnology, 88: 820-824.
Seo, B., Brown, C., Lee, H. and Rounsevell, M., 2024. Bioenergy in Europe is unlikely to make a timely contribution to climate change targets. Environ. Res. Lett., 19: 044004.
Sharma, N., Chisti, Y. and Banerjee, U.C., 2021. Commercial-scale production of microalgae for biofuels and bioproducts. Renew. Sustain. Energy Rev., 135: 110233.
Singh, B., Guldhe, A., Rawat, I. and Bux, F., 2019. Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew. Sustain. Energy Rev., 29: 216-245. https://doi.org/10.1016/j.rser.2013.08.067
Tang, D., Han, W., Li, P., Miao, X. and Zhong, J., 2014. CO2 bio-fixation and biomass production from marine red algae Galdieria sulphuraria under various culture conditions. Appl. Microbiol. Biotechnol., 98: 2919-2928.
Tsai, D.D.W., Chen, P.H., Chou, C.M.J., Hsu, C.F. and Ramaraj, R., 2015. Carbon sequestration by alga ecosystems. Ecol. Eng., 84: 386-389. https://doi.org/10.1016/j.ecoleng.2015.09.024
Ullah, K., Ahmad, M., Kumar, V., Lu, P., Harvey, A., Zafar, M. and Sultana, S., 2015. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel, 143: 414e423. https://doi.org/10.1016/j.fuel.2014.10.064
UNEP, 2020. Global environment outlook GEO-6: Healthy planet, healthy people. https://www.unep.org/resources/global-environment-outlook-geo-6 (accessed on 02 February 2024). https://doi.org/10.1017/9781108627146
Veillette, M.M., Chamoumi, J., Nikiema, N. and Faucheux, M., 2012. Heitz, production of biodiesel from microalgae. Adv. Chem. Eng., 10: 245. https://doi.org/10.5772/31368
Walther, G.R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T.J.C., Fromentin, J.M., Hoegh-Guldberg, O. and Bairlein, F., 2002. Ecological responses to recent climate change. Nature, 416: 389-395. https://doi.org/10.1038/416389a
Wang, L., Min, M., Li, Y., Chen, P., Chen, Y., Liu, Y., Wang, Y., and Ruan, R., 2010. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol., 162: 1174-1186. https://doi.org/10.1007/s12010-009-8866-7
Yoo, G., Park, W.K., Kim, C.W., Choi, Y.E. and Yang, J.W., 2014. Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresour. Technol., 163: 228-232.
Zheng, Y., Chen, H. and Shen, Y., 2013. Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem. Rev., 113: 5914-5933.
To share on other social networks, click on any share button. What are these?