Nitrite Level and Rehydration Capacity of Edible Bird’s Nest Heated at Commercial Sterilization Temperature
Research Article
Nitrite Level and Rehydration Capacity of Edible Bird’s Nest Heated at Commercial Sterilization Temperature
Satya Fahriani1, Hadri Latif2*, Herwin Pisestyani2, Zhang Shuqi3, Suyanto Chen3
1Graduate Student of Veterinary Biomedical Science, School of Veterinary Medicine and Biomedical Science, IPB University, Bogor, Indonesia 16680; 2Division of Veterinary Public Health and Epidemiology, School of Veterinary Medicine and Biomedical Science, IPB University, Bogor, Indonesia 16680; 3Animal Quarantine Installation, Batam City, Indonesia 29444.
Abstract | Indonesia is the world’s largest producer and exporter of edible bird’s nest (EBN). One of the importing countries requires that EBN be heated minimum 100 °C, with a minimum F0 value of 2.8, commercial sterilization with retort method. Indonesia’s government does not have data on the effect of retorts on the safety and quality of EBN. This study aimed to examine the nitrite level and rehydration capacity of EBN after undergoing a retort process. The samples were 24 raw uncleaned white EBN from 4 swiftlet farmhouses (SFHs) collected using purposive sampling. Two SFH from Sanggau District, West Kalimantan Province, Indonesia. Two SFH from Pekanbaru City, Riau, Indonesia. 12 pieces of samples were not heated, while the other 12 pieces were heated at retort temperature. The sample’s nitrite level was measured using a spectrophotometer, while the rehydration capacity was measured by soaking in aquabides for 3 hours. Data were analyzed using paired T test. The average nitrite level of non-heated EBN is 11.203 ± 2.692 ppm, while retort EBN 15.695 ± 5.630 ppm. The average rehydration capacity of the non-heated EBN bloomed 11.303±1.278 times the initial size. In comparison, the retort EBN bloomed 8.412 ± 1.257 times the initial size. This is the first study that reports retort EBN’s nitrite level and rehydration capacity. The retort sterilization method did not significantly change EBN nitrite levels. However, the distribution range of nitrite levels between samples became more extensive. The retort sterilization affects EBN quality by decreasing its rehydration capacity.
Keywords | Edible bird nest, Nitrite, Rehydration capacity, Retort
Received | May 17, 2023; Accepted | June 20, 2023; Published | July 23, 2023
*Correspondence | Hadri Latif, Division of Veterinary Public Health and Epidemiology, School of Veterinary Medicine and Biomedical Science, IPB University, Bogor, Indonesia 16680; Email: hadrilatif@apps.ipb.ac.id
Citation | Fahriani S, Latif H, Pisestyani H, Shuqi Z, Chen S (2023). Nitrite level and rehydration capacity of edible bird’s nest heated at commercial sterilization temperature. Adv. Anim. Vet. Sci., 11(9):1411-1416.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.9.1411.1416
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Edible bird’s nest (EBN) is a food commodity of animal origin with high economic value. Indonesia is the largest producer and exporter of EBN in the world, which produces 85% of EBN, followed by Malaysia at 13% and other countries, such as Thailand, Vietnam, the Philippines, and the People’s Republic of China (PRC) (Huang et al., 2020). According to Indonesian Quarantine Full Automation System (IQFAST) data for 2022, Indonesia exported 1502.289 tons of EBN to several main destination countries, like Hong Kong, the People’s Republic of China, Vietnam, Malaysia, Singapore, the United States, Macau, Taiwan, Australia, and Canada. This number increased by 13.24% from 2021, which amounted to 1324.696 tons (IAQA, 2023).
The destination country stipulates several technical requirements for EBN import. One is that EBN has undergone a heating process to a minimum core temperature of 100°C with a minimum F0 value of 2.8, which is commercial heating (IAQA, 2021). Commercial sterilization with method retort is a heating process for food with low acid content and susceptible to microbial contamination and then packaged in an airtight container (hermetically sealed) (Chantakun and Benjakul, 2020). Commercial sterilization is one of the preservation techniques to extend the shelf life of products, especially ready to eat food products changes (Majumdar et al., 2015). The heating temperature in the retort process is between 110–121 °C, depending on the food product characteristics. Generally, the temperature in the retort process for heat-sensitive food products is 115 °C (IAQA, 2023). Thermal conditions cause side effects on the product, affecting taste, appearance, nutrition, and product bioactivity (Benjakul et al., 2018). Pressure heating could change the product’s texture, chemical composition, and organoleptic changes (Majumdar et al., 2015).
Food safety standards prioritized in the EBN trade are microbial contamination and chemical content. EBN safety and quality standards set by the Indonesian government, as producer and exporter, are sensory index, water content, microbial contamination, and nitrite residue. At the same time, importing countries focus on the sensory index, nitrite, size, moisture, protein, and sialic acid (Chen et al., 2015). The maximum threshold for EBN nitrite levels refers to the requirements of the PRC, which is 30 ppm (IAQA, 2013). While in Indonesia, based on National Standar of Indonesia number 8998:2021,the maximum threshold for EBN nitrite levels is 80 ppm (NSAI, 2021) addition, EBN consumers also have criteria for selecting good quality EBN, including shape, volume, colour, density, rehydration capacity, aroma, and dryness. Generally, consumers soak EBN in water for 30 minutes to 1 hour, and then the EBN is cooked into soup or drink. Soaking in water is necessary to prepare EBN as a food ingredient. The soaking process expands and increases EBN size until 7 to 9 times larger than its initial size (GN, 2021).
So far, commercial sterilization using a minimum temperature of 100°C with a minimum F0 value of 2.8 on EBN has never been done in Indonesia. So in-depth data and studies about the commercial sterilization effect on the safety and quality of EBN are needed. The Indonesian government and EBN producers need this information to formulate policies on determining and negotiating EBN treatment methods for export purposes. Furthermore, business actors could use this scientific data regarding the safety and quality of EBN to improve quality and for export purposes.
MATERIALS AND METHODS
Study period and location
This study was conducted from June to November 2022. EBN rehydration capacity was tested in an Animal Quarantine Installation at Batam City. The nitrite test was conducted at The Center of Diagnostic Standard of Agricultural Quarantine, Jakarta.
Population and sample collection
This research is an experimental study using a purposive sampling. EBN samples came from 4 swiftlet houses (SFHs), purposively determined with the criteria such as symmetrical size, weight uniformed at a range of ± 7–8 g/sample, light to medium uncleaned feathers (less than 5–7 feathers on the EBN surface), and originate from the same fin also harvested at the same time. The samples were collected from the empty nest after the eggs hatched and the young swiflet (Aerodramus fuchipagus) left. 12 samples were used for the rehydration capacity test and the other 12 for the nitrite level test with 3 times repetition. The 1st and 2nd SFHs were in Sanggau Regency, West Kalimantan, while the 3rd and 4th were in Pekanbaru City, Riau, Indonesia.
Edible bird nest washing method
The sample preparation process begins by dividing the EBN into two symmetrical parts. The EBN was cleaned from the dirt and remaining feathers. The edges of the EBN attached to the fins are filled with an iron grinder to remove wood chips. After that, according to the method of Wahyuni et al. (2022), the EBN was washed with reverse osmosis (RO) water flowing for 10±2 seconds. The sample was dried with a particular tissue for food processing (hygiene food processing) and aired for 150±5 minutes. The soft feathers and other impurities left on EBN are cleaned again using food-grade stainless steel tweezers and assisted with a soft brush. Samples were rinsed with flowing RO water for 15 ± 2 seconds. The samples were again air-dried at room temperature for 120 minutes and then printed. The samples were again aerated to dry to a 10–12% moisture content. After obtaining a clean EBN sample, each half of the sample was continued to the heating process.
Commercial sterilization method
The EBN heating process was carried out using a heater that has been verified and calibrated. The results of verification of the heating device to achieve the required minimum commercial sterilization temperature of 100 °C with a minimum F0 value of 2.8, namely at 121 °C for 6 minutes 35 seconds. The cumulative value of F0 = 3.006 proves that the sterilization stage F0 ≥ 3 has been reached on the equipment used. F0 is heat adequacy measurement for commercial sterilization expressed as the equivalent of heating time (in minutes). The heat distribution process was done by filling the water at the bottom of the autoclave with a depth of 7 cm. When the autoclave temperature reached 95 °C, EBN was arranged on a tray and waited until the EBN reached the commercial sterilization temperature. The heater was turned off, followed by a decrease in pressure and temperature. It is safe to open the autoclave at 90 °C, and the pressure has reached a value of 0.
Nitrite levels measurement
Nitrite levels measurement refers to Widiyani et al. (2022). Analysis of nitrite levels used several reagents, namely: standard nitrite (Merck, Germany), sulfanilamide (Merck, Germany), and N-(1-naphthyl) ethylenediamine dihydrochloride (NED) solutions (Merck, Germany). The standard solution of nitrite (Merck, Germany) was diluted and mixed with 0.6 mL of saturated NaCl (Merck, Germany) and 9.4 mL of ion-free water (Millipore, Ireland). Next, 1 mL of sulfanilamide (Merck, Germany) was added and allowed to stand for 5 min, followed by 1 mL of NED (Merck, Germany). This solution was allowed to stand for 15 min, after which the absorbance was measured using a spectrophotometer (Thermo Fisher Scientific, USA). The EBN sample was homogenized for nitrite determination, and then 40 mL of ion-free water (Millipore) and 3 mL of saturated NaCl solution (Merck, Germany) were added. The mixed solution was heated in an ultrasonic digester (Thermo Fisher Scientific, USA) at 40°C for 30 min and filtered using Whatman paper no. 41 (Whatman, UK). Next, 2.5 mL of sulfanilamide (Merck, Germany) was added and allowed to stand for 5 min. Subsequently, 2.5 mL of NED (Merck, Germany) was added, homogenized, and allowed to stand for 15 min. Then, the absorbance was measured using a spectrophotometer at a wavelength of 541 nm. Moreover, the concentration of nitrite levels is calculated according to the calculation formula:
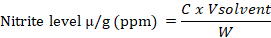
Where; C = the amount of nitrite in the sample obtained from the calibrated curve (µg/L); V = sample solvent volume (mL); W = sample weight (g).
Edible bird nest rehydration capasity measurement
The dry weight of the EBN samples was weighed before soaking. The EBN was soaked in a clean container with 500 mL of aquabides at room temperature. EBN soaked for 1 hour, 2 hours, and 3 hours. The soaked samples were weighed again and compared with the weight of the sample before the soaking process.
Data analysis
The data were analyzed using a paired t test with average ± standard deviation data values. The presentation of the analysis results was described descriptively in tabular form.
RESULTS AND DISCUSSION
Nitrite level
Generally, the nitrite (NO2-) level of non-heated EBN and retort EBN from this study were below 80 ppm as stipulated in the Indonesian National Standard Number 8998:2021 concerning raw clean Edible Bird Nest (NSAI, 2021). The average nitrite levels in retort EBN was 15.695 ± 5.630 ppm, and non-heated EBN was 11.203 ± 2.692 ppm. These results showed that there was no significant difference between non-heated EBN and retort EBN (P ≥ 0.05) (Table 1).
Table 1: The Nitrite levels of non-heated and retort EBN.
|
Treatment |
SHFs |
n |
Nitrit concentration (ppm) in SFHs |
Nitrit concentration (ppm) each treatment |
|
Non heated |
1 |
3 |
13.234 ± 3.323 |
11.203 ± 2.692a |
|
2 |
3 |
8.331 ± 0.0668 |
||
|
3 |
3 |
12.790 ± 0.558 |
||
|
4 |
3 |
10.476 ± 2.180 |
||
|
Retort |
1 |
3 |
9.802 ± 1.150 |
15.695 ± 5.630a |
|
2 |
3 |
21.867 ± 1.501 |
||
|
3 |
3 |
16.687 ± 7.421 |
||
|
4 |
3 |
14.425 ± 1.606 |
Numbers in the same column followed by the same letter are not significantly different based on the T test (P≥ 0.05).
Nitrite is a compound that dissolves in water. So, the EBN washing process can reduce EBN nitrite levels (Susilo et al., 2016). According to Yeo et al. (2021), EBN nitrite levels are affected by colour, SFHs cleanliness, age at harvest (harvest time), contamination from the environment during harvesting, and the cleaning process.
Environmental conditions can affect nitrite levels, including pH, humidity, environmental cleanliness, weather, decomposition of organic matter, and nitrifying bacteria in EBN (Susilo et al., 2016; Widiyani et al., 2022). Swiftlet farmhouses with poor sanitation can increase nitrite levels caused by decomposing faeces in the nest, so the ammonia levels (NH4-) are high. According to Widiyani et al. (2022), nitrite formation in EBN comes from natural processes of nitrogen in the SFH environment, like contamination from the oxidation of swiftlet faeces in the air. The decomposition of swiftlet faeces produces ammonia which will be oxidized to nitrite through the nitrification process (Yulianti et al., 2019) and then oxidized again to nitrate (NO3-) (Chan et al., 2018). The bacteria that play a role in the nitrification process are Nitrosomonas. For the nitration process, the bacteria that play a role are Nitrobacter (Leonanda and Zolanda, 2018). Based on Widiyani et al. (2022), the nitrifying bacteria found in dirt samples of SFH from the Sumatra Island, Indonesia, comes from the genus Aeromonas. Aeromonas sp. bacteria are found in environments such as water and soil. The nitrite content is already present in the EBN shortly after the swiftlet forms the nest. The nitrite might come from insects that are consumed by swiflet. Insects eat plants that may containing nitrogen-fixing bacteria, so nitrites and nitrates can accumulate in swiftlet saliva and faeces (Chan et al., 2013).
In the food industry, nitrites are used as food additives and preservatives. Nitrites can turn into nitrogen oxides which are beneficial to the body but can also turn into nitrosamines which can harm health (IFDA, 2019). According to The European Food Safety Authority (EFSA), the maximum limit for daily nitrite consumption is 0–0.07 mg/kg body weight per day (Yeo et al., 2021). The maximum consumption limit for nitrite in Indonesia is 0–0.06 mg/kg body weight per day, regulated in the Food and Drug Supervisory Agency Regulation Number 11 of 2019 concerning Food Additives (IFDA, 2019). Nitrites turn into nitrogen oxides in the body (signalling molecule), but high nitrites levels can cause dilatated blood vessels and decreased blood pressure. This situation results in blue skin (cyanosis), shortness of breath, vomiting, and death due to reduced oxygen levels in the blood (hypoxia). Consumption of nitrites that exceed the limit will cause carcinogenic compounds, nitrosamines. Nitrosamines are formed from nitrites that react with secondary amino acids if heated at high temperatures (Yeo et al., 2021).
The EBN nitrite levels in this study showed varying distribution diversity values (Figure 1). The nitrite distribution range of retort EBN is more extensive than the non-heated EBN. The extensive nitrite distribution range of retort EBN is probably due to the high variation in nitrate level in EBN before heating. The heating process could change nitrate to nitrite by slowly reducing the compound components. Changes in this component occur due to a collision process between substances that releases nitrate bonds with other compounds in a material (Starsburg et al., 2007). The changes in nitrate components can be caused by physical changes, such as temperature changes (Estiasih et al., 2016).
Rehydration capacity
The EBN rehydration capacity was determined by measuring the blooming capacity and EBN’s ability to retain water before and after the soaking process. The water-holding capacity of retort EBN and non-heated EBN is presented in Table 2.
Table 2: The rehydration capacity of non-heated and retort EBN after the soaking process.
|
n |
Water-binding capacity (times) |
|||
|
1 hour |
2 hour |
3 hour |
||
|
Non-heated |
12 |
9.510±0.851a |
||
|
Retort |
12 |
|||
Numbers in the same column followed by different letters are significantly different based on the T test (P <0.05).
After 1 hour of soaking, the average retort EBN bloom was 7.035 ± 1.247 times, while the average non-heated EBN bloom was 9.510 ± 0.851 times from the size before soaking (Table 2). The rehydration capacity of retort EBN was lower and significantly different (P≤ 0.05) than non-heated EBN. Furthermore, Table 2 shows that the retort EBN rehydration capacity after soaking for 2 hours and 3 hours became 7.969 ± 1.203 and 8.412 ± 1.257 times from the initial size. It was significantly different (P≤ 0.05) with the non-heated EBN rehydration capacity, which is 10.907 ± 1.475 and 11.303 ± 1.278 times from the initial size before soaking. The rehydration capacity of retort EBN is lower than non-heating EBN, with a significant difference (P≤ 0.05).
The EBN proteins denatured when treated with a specific temperature and pressure on commercial sterilization. Supported by research of Elviani (2013), EBN heated at 45, 55, 65, 75, 85 and 95 °C heated for 15, 30, 45, and 60 minutes, with an increase in temperature every 10 °C, from 45 °C to 95 °C there was a change in protein content from 47.56% to 30.88% at 15 minutes of heating. At 30 minutes of heating, the protein content changes from 41.69% to 30.50%; 45 minutes of heating changes the protein content from 45.50% to 30.06% and 60 minutes of heating changes the protein content from 42.94% to 29, 50%. This study showed that the higher temperature and longer heating time, the more significant total protein changes. The high heat treatment on EBN changes the original structure and protein exposure. Denatured protein affects the nutritional value and bioactive properties (Teodorowicz et al., 2017).
Protein denaturation is protein structural change without breaking the covalent bonds. So, the proteins no longer have secondary, tertiary, and quaternary structures. Only the protein’s primary structure remains. The change in protein structure causes changes in the physical-chemical properties of the protein, such as loss of solubility and biological activity (Basuki et al., 2019). Protein denaturation is caused by physical and chemical treatment. Physical treatment includes temperature, pressure, and stirring, while chemical treatment is pH, organic solvents, ultraviolet irradiation, ionizing radiation, organic compounds, and ionic detergents (Estiasih et al., 2016). The temperature range for denaturation and coagulation of most proteins is around 55–75 °C.
In foodstuffs, protein denaturation can change the food ingredient texture because of the protein’s ability to bind water due to the hydrophilic compounds. The main factors affecting the water-binding capacity (WBC) of proteins are pH, salt, and temperature. The higher the protein concentration, the WBC also increases. The higher the temperature, WBC decreases (Basuki et al., 2019).
The protein water-binding capacity process decreases due to a reduction in hydrogen bonding and a decrease in the hydration of ionic compounds. The WBC of denatured protein is generally 10% greater than the undenatured protein. An increased surface area ratio to mass causes this process. Denaturation also causes protein aggregation, so the WBC decreases due to increased protein interactions and causes the solubility of denatured proteins to decrease (Estiasih et al., 2016).
The EBN rehydration capacity after soaking affects the quality of the EBN, the imbibition capacity of the water, and the mixed ingredients. Large quantities of water trapped in EBN will increase the blooming capacity of EBN. Water trapped in EBN is included in the type of water in foodstuffs found in the form of imbibed water. Imbibition water is water that comes from outside the material, which is added to the material, causing an increase in volume. Imbibition water is not a constituent of the foodstuffs. The water binds to the components of the foodstuffs through hydrogen bonds. Water in food serves as a substance that can disperse various compounds. Water can dissolve multiple ingredients such as salt, water-soluble vitamins, minerals, and flavour compounds (Estiasih et al., 2016).
An edible bird nest is a food product with low water content. The WBC in EBN affects rehydration capacity as a critical point in determining the consumer’s product acceptability. In this research, the longer EBN soaking time will increase its rehydration capacity. But, consumers EBN soaking procedures and habits before cooking are only 30 minutes to a maximum of 1 hour. Based on information from EBN exporters, consumers prefer EBN that is soaked for a short time because EBN that is soaked for a long time shows low blooming ability and low quality. EBN consumers do not like it (Suyanto and Eni 28 July 2022, personal communication). It is necessary to reconsider heating with commercial sterilization in EBN so the heating process does not affect the EBN quality.
CONCLUSIONs and Recommendations
Heating with commercial sterilization does not affect EBN nitrite level but affects EBN rehydration capacity. The retort EBN rehydration capacity is lower than the non-heated EBN, decreasing the EBN quality. The retort sterilization method in EBN can be suggested for evaluation.
ACKNOWLEDGEMENTS
This study was supported by funding from the Agency of Agricultural Counseling and Human
Resources Development, Ministry of Agriculture Indonesia, with study assignment scholarship number 491/KPTS/KP.320/A/08/2021. The authors are thankful to Indonesia Agriculture Quarantine Agency and The Center of Diagnostic Standard of Agricultural Quarantine, Jakarta, Ministry of Agriculture Indonesia, for the facilities provided during the research. The authors are also thankful to Quarantine Officer at Batam Class I Agricultural Quarantine Center and Entikong Class I Agricultural Quarantine Station for their help and support.
Novelty Statement
This is the first study that reports the effect of retort sterilization on the nitrite level and rehydration capacity of EBN. This heating method decreases rehydration capacity, which is the main quality parameter of raw cleaned EBN so it needs to be evaluated as a trade requirements.
AUTHOR’S CONTRIBUTION
Satya Fahriani contributed to collect samples, data analysis and preparing the original manuscript. Hadri Latif and Herwin Pisestyani contributed to the research design, revised the manuscript and supervision. Zhang Suqi and Suyanto Chen supervised the method in this research. All authors read and approved the final version of the manuscript in the present journal.
Ethical approval
This study did not involve live swiftlets, so it did not require ethical approval.
Conflict of interests
The authors have declare no conflict of interest.
REFERENCES
Basuki E, Widyastuti S, Prarudiyanto A, Saloko S, Cicilia S, Amaro M (2019). Textbook of food chemistry. Mataram University Press, Mataram.
Benjakul S, Chantakun K, Karnjanapratum S (2018). Impact of retort process on characteristics and bioactivities of herbal soupmbased on hydrolyzed collagen from seabass skin. J. Food Sci. Tech., 55: 3779–3791. https://doi.org/10.1007/s13197-018-3310-z
Chan G, Zhu K, David JC, Ava JG, Tina TD (2013). Surveillance of nitrite level in cubilose: Evaluation of removal method and proposed origin of contamination. Food Contr., 34(2): 637–644. https://doi.org/10.1016/j.foodcont.2013.06.010
Chan GKL, Wu KQY, Fung AHY, Poon KKM, Wang CY, Gridneva E, Huang RRH, Fung SYZ, Xia YT, Hu WWH, Wong ZCF, Tsim KWK (2018). Searching for active ingredients in edible bird’s nest. J. Complement. Med. Altern. Health, 6(2): 1–5. https://doi.org/10.19080/JCMAH.2018.06.555683
Chantakun K, Benjakul S (2020). Effect of pretreatments and retort process on characteristics and sensory quality of edible bird’s nest beverage. J. Food Sci. Tech., 55(1): 2863-2871. https://doi.org/10.1111/ijfs.14542
Chen CX, Yang S, Li LQ (2015). Comparative study of domestic and foreign quality standards for edible bird’s nest. J. Food Saf. Qual., 6(7): 2603–2609.
Elviani Y (2013). The effect of temperature and duration of heating on the protein content contained in the cleaned edible bird’s nest (Collocalia fuciphagus). Thesis, Maranatha Christian University, Jakarta, Indonesia.
Estiasih T, Harijono WE, Fibrianto K (2016). Food chemistry and physics. Jakarta: Bumi Aksara.
Golden Nest Inc. (GN) (2021). Fake bird’s nests: How to tell if your bird’s nest soup is authentic. Partners available at: https://goldennest.com/blogs/news/fake-bird-s-nests-how-to-tell-if-you-re-bird-s-nest-soup-is-authentic (accessed 01 Febr 2023).
Huang X, Li Z, Xiaobo Z, Shi J, Tahir HE, XuY, Zhai X, Hu X (2020). Geographical origin discrimination of edible bird’s nests using smart handheld device based on colorimetric sensor array. J. Food Measuret. Charact. Component. Anal. Food Qual. Saf., 1(1): 83-92. https://doi.org/10.1007/s11694-019-00251-z
Indonesia Agricultural Quarantine Agency (IAQA) (2013). Guidelines for animal quarantine requirements and measures for exportation of edible bird’s nest from republic of indonesia to the people’s republic of China. Indonesia Agricultural Quarantine Agency, Jakarta.
Indonesia Agricultural Quarantine Agency (IAQA) (2021). Guidelines for animal quarantine measures for exportation of edible bird’s nest from republic of Indonesia to countries other than the people’s republic of China. Indonesia Agricultural Quarantine Agency, Jakarta.
Indonesia Agricultural Quarantine Agency (IAQA) (2023). List of public information of the agricultural quarantine agency: Edible bird nest export data. Indonesia Agricultural Quarantine Agency, Jakarta.
Indonesian Food and Drug Authority (IFDA). (2019). Food additives. Indonesian Food and Drug Authority, Jakarta.
Leonanda BD, Zolanda Y (2018). Biofilter nitrification reactor for residual wastewater food and fish feces. Met. J. Sist. Mek. Termal., 2(1): 9. https://doi.org/10.25077/metal.2.1.9-14.2018
Majumdar RK, Dhar B, Roy D, Saha A (2015). Optimization of process conditions for Rohu fish in curry medium in retortable pouches using instrumental and sensory characteristics. J. Food Sci. Tech., 52(9): 5671–5680. https://doi.org/10.1007/s13197-014-1673-3
National Standardization Agency of Indonesia (NSAI) (2021). Clean edible bird nest standar. National Standardization Agency of Indonesia, Jakarta.
Starsburg G, Xiong YL, Chiang W (2007). Physiology and chemistry of edible muscle tissues. In: (eds. S. Damodaran, K.L. Parkin, OR Fennemas). Fennemas’s food chemistry 4th edn. CRC Press, Boca Raton. pp. 924–968.
Susilo H, Latif H, Ridwan Y (2016). Application of washing method with water flow to reduce nitrite levels in edible bird nest nests. J. Kedokt Hewan Indones., 10(2): 95–97. https://doi.org/10.21157/j.ked.hewan.v10i2.5021
Teodorowicz M, VanNeerven J, Savelkoul H (2017). Food processing: The influence of the maillard reaction on immunogenicity and allergenicity of food proteins. J. Nutr., 9(835): 1–18. https://doi.org/10.3390/nu9080835
Wahyuni DS, Latif H, Sudarwanto MB, Basri C, Thong D (2022). An investigation of heavy metals in edible bird’s nest from Indonesia using inductively coupled plasma mass spectrometry. Vet. World, 15(2): 509-516. https://doi.org/10.14202/vetworld.2022.509-516
Widiyani P, Sudarwanto MB, Latif H, Lukman DW, Thong D, Rahayu P (2022). A preliminary metagenomics study of bacteria present in the dirt of swiftlet farmhouses based on nitrite levels in edible bird’s nest on sumatera island, Indonesia. Vet. World, 15(7): 1798–1803. https://doi.org/10.14202/vetworld.2022.1798-1803
Yeo BH, Tang TK, Wong SF, Tan CP, Wang Y, Cheong LZ, Lai OM (2021). Potential residual contaminants is edible bird’s nest. Front. Pharmacol., 12(136): 1-15. https://doi.org/10.3389/fphar.2021.631136
Yulianti I, Sukainah A, Caronge MW (2019). Effect of washing using lime on the quality of edible bird’s nest. J. Pendidik. Teknol. Pertan., 5(5 Supl): S158-168.
To share on other social networks, click on any share button. What are these?






