Micronucleus Assay as a Biomarker to Diagnose Lead, Chromium and Cadmium Induced Genotoxicity in Erythrocytes of Carnivorous Fish, Wallago attu
Micronucleus Assay as a Biomarker to Diagnose Lead, Chromium and Cadmium Induced Genotoxicity in Erythrocytes of Carnivorous Fish, Wallago attu
Sajid Abdullah1*, Huma Naz2*, Zunera Abbas1, Uzma Nazir1, Mehrunisa Basharat1, Tanveer Ahmed3, Adnan Ahmad Qazi2
1Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad, Pakistan.
2Department of Zoology, Cholistan University of Veterinary and Animal Sciences Bahawalpur, Pakistan.
3Department of Life Sciences, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan.
Abstract | Heavy metals discharged from agriculture and industries into water bodies are a significant cause of water contamination. This increase in heavy metals cause genetic damage in exposed species like fish. Therefore, this study focused on the genotoxic effects of lead (Pb), chromium (Cr) and cadmium (Cd) on nuclear abnormalities in peripheral erythrocytes of Wallago attu were assessed by micronucleus assay. Fish were exposed to different concentrations (1/3rd, 1/4th, 1/5thand 1/7th of LC50) of Pb, Cr and Cd, separately, for three weeks. Blood sample of fish was collected to see the micronuclei (MN) and deshape nuclei (DN) in peripheral erythrocytes of fish after one-week interval. Fish W. attu showed significant induction of MN and DN in blood due to metals (Pb, Cr and Cd) exposure. It was noted that Pb and Cd exposure caused significant formation of MN and DN in RBCs of fish throughout the experimental period. However, Cr treated fish showed higher formation of MN and DN during initial two weeks of exposure after that both were decreased. The toxic potential of different concentration of Pb, Cr and Cd induced genotoxicity followed order: 1/3rd>1/4th>1/5th>1/7th of LC50. The metal specific response of fish followed the trend: Pb>Cr>Cd. Statistical analysis revealed that different concentrations and duration of Pb, Cr and Cd have significant effect on the frequency of micro-nucleated erythrocytes. The inferences of this study will be helpful in monitoring aquatic ecosystems using fish biomarkers.
Novelty Statement | The study is novel for detecting the genetic damage at cellular level of fish (Wallago attu) caused by heavy metals and industrial toxicants in water.
Article History
Received: February 22, 2021
Revised: July 19, 2021
Accepted: August 13, 2021
Published: October 08, 2021
Authors’ Contributions
SA supervised the study and guided the author in planning the research work. HN helped in laboratory work. ZA, TA and UN executed the research work. MB helped in statistical analysis. AAQ assisted in manuscript writing.
Keywords
Blood, Nuclear abnormalities, Metal toxicity, Carnivorous fish
Corresponding author’s: Sajid Abdullah and Huma Naz
humanaz@cuvas.edu.pk, uaf_sajidabdullah@yahoo.com
To cite this article: Abdullah, S., Naz, H., Abbas, Z., Nazir, U., Basharat, M., Ahmed, T. and Qazi, A.A., 2021. Micronucleus assay as a biomarker to diagnose lead, chromium and cadmium induced genotoxicity in erythrocytes of carnivorous fish, Wallago attu. Punjab Univ. J. Zool., 36(2): 153-158. https://dx.doi.org/10.17582/journal.pujz/2021.36.2.153.158
Introduction
Metal pollution of freshwater bodies such as streams, rivers, and lakes has been recorded around the world due to its harmful effects not only on humans but also on other living species. Heavy metal pollution severely deteriorates the quality of environment by interfering with ecological equilibrium of an aquatic ecosystem (Farombi et al., 2007). In aquatic bodies, fish are the animal which cannot escaped from harmful impact of these heavy metals and that’s the reason fish is commonly used as bio-indicator of metals toxicity in water (Agah et al., 2009). The direct contact of fish with environment makes them more vulnerable to all chemical and physical modifications occurred due to pollution in water (Ip et al., 2005). Fish shows behavioral, physiological and molecular alteration upon exposure to contaminants (Navarro and Martinez, 2014; Prado et al., 2014).
Chromium (Cr) is known to be a toxic metal for health and environment due to its high mobility and solubility (Xu and Wang, 2012). Due to its non-biodegradable and bioaccumulation property, it is toxic to living organisms. The indiscriminate discharge of Cr from industries to aquatic bodies severely affects the growth and survival of aquatic life particularly fish (Mishra and Mohanty, 2008). Lead (Pb) is a common, growing and subtle environmental waste material that provokes a wide range of behavioral, biochemical and physiological impairments (Patra et al., 2001; El-Magd et al., 2016). Lead toxicity is associated with the formation of reactive oxygen species (ROS) that cause reduction in antioxidant defense systems of cell and damage to DNA (El-Ashmawy et al., 2006). According to Hong et al. (2007) Pb+2 can directly bind with DNA by a covalent bond. Some non-essential heavy metals like cadmium (Cd) can induce harmful effects in aquatic animals even at very low dose (Bertin and Averbeck, 2006; Cambier et al., 2010). Cd have ability to bio-accumulate and induce toxicity in fish by changing their physiology, histology, osmoregulation, reproduction, immune and enzyme response (Dang and Wang, 2009; Garcia-Santos et al., 2011; Guardiola et al., 2013; Li et al., 2014).
The genotoxic (DNA damage) affects of metals on aquatic animals can effectively be examined by the application of simple and reliable techniques such as Micronucleus (Frenzilli et al., 2009; Bolognesi and Hayashi, 2011) and nuclear abnormalities (notched nuclei, blebbed, lobbed, budding, fragmenting nuclei and bi-nucleated cells) assays both are most commonly used tests due to their verified suitability for aquatic species (Kirschbaum et al., 2009). The presence of both MN and NAs in RBCs has been widely used as a biomarker of environmental genotoxicity in fish exposed to complex mixtures and single substances (Carrola et al., 2014; Seriani et al., 2015).
Wallago attu is a valuable fish species for studying the toxicological effects due to its higher adaptation and easy reproduction. It is native species of Pakistan and can be used as sentinel organisms to evaluate the geno-toxic effects of metals even at a very low dose. Information regarding metal induced genotoxicity in carnivorous fish is very limited. Therefore, the main objective of current research was to assess the metal-, concentration-, and time-specific response of nuclear anomalies in Wallago attu erythrocytes exposed to chronic metals.
Materials and Methods
Fish sampling and experimental layout
Carnivore fish, Wallago attu were acquired from natural habitat, and were shifted to the Fisheries Research Farm, University of Agriculture Faisalabad. Fish were acclimatized to laboratory conditions for 14-day. A group of fish (n=10) were kept in each glass aquarium having 100-liter water capacity. Total five aquaria were used for each metal. Fish were exposed to different sub-lethal concentrations viz. 1/7th, 1/5th, 1/4th and 1/3rd of LC50 (96-h) of lead (Pb), chromium (Cr) and cadmium (Cd), separately, for three weeks. The LC50 (96-h) values of Pb, Cr and Cd for W. attu were calculated as 25.08, 61.38 and 32.94 mgL-1, respectively (Batool et al., 2014, 2018). The pure chloride compound of cadmium and lead, and nitrate compound of chromium were used to make metals solutions. The cyclophosphamide was injected into the positive control (PC) fish however, the negative control (NC) fish were kept in water without any treatment. Some water quality parameters including water temperature (30°C), hardness (250mg/L) and pH (7.5) were kept stable during the experimental trail. Fish caudal vein was ruptured to collect the blood sample after 7 days of exposure.
Micronucleus assay
Fish caudal vein was ruptured and blood was instantly smeared on slide and dried in air for few minutes. Methanol was applied to fix the smear and allowed to dry for 10 minutes. The blood smear was stained with wright-giemsa (Sigma Aldrich) stain for 8 minute (Barsiene et al., 2004). The erythrocytes with micronuclei and de-shaped nuclei were counted (per 1,000 cell) by using binocular microscope (1000 X magnification) for PC, NC and metals (Pb, Cr and Cd) stressed fish. Blind counting of abnormal nuclei was done by adopted the criteria of Fenech et al. (2003).
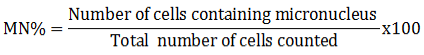
Data analysis
Data obtained from this experiment was subject to statistical analyses. Analysis of variance was done to determine the significance of the effect of concentration and period of treatment on NAs formation. All statistical analyses were performed by using statistix 8.0 version software.
Results and Discussion
According to Souza and Fontanetti (2006) any biological, chemical and physical agent can stimulate the formation of micronuclei by interacting with non-genomics constituents (mitotic apparatus) of cell and, resulting in failure of segregation of chromosome. The micronuclei assay is used to visualise and quantify all the abnormalities induced by these agents. At specific concentration, metals ions are also noted as geno-toxicants because they can bind with thiol groups and cause disturbance if the formation of spindle in cells (Patra et al., 2004).
Fish W. attu showed significant induction of MN and DN in blood due to metals (Pb, Cr and Cd) exposure. It was noted that Pb and Cd exposure caused significant formation of MN and DN in RBCs of fish throughout the experimental period while in case of Cr higher formation were noted during initial two weeks of treatment after that same were decreased in third week. The concentration specific response of MN and DN in RBCs of fish exposed to metals followed the order: PC>1/3rd>1/4th>1/5th>1/7th>NC (Tables 1, 2 and 3). The metal specific response of fish followed the trend: Pb>Cr>Cd. The variation in genotoxic response to different chemicals is primarily related to metabolism and other pharmacokinetic factors in animals (Al-Sabti and Metcalfe, 1995). While each metal has its own toxicity mechanism but some are common including mimicry, DNA or protein adduct formation, and oxidative stress. Heavy metals forms produce reactive oxygen species (ROS), which cause oxidative changes in DNA, resulting in abnormal gene expression and carcinogenesis (Ballatori, 2002). Razzaq et al. (2021) reported the concentration and duration dependent induction of MN and DN in RBCs of L. rohita due to cobalt+chromium mixture exposure.
Table 1: Nuclear abnormalities in erythrocytes of Wallago attu expose to cadmium.
|
Parameters |
Sampling time |
NC |
PC |
Sub-lethal concentrations |
|||
|
1/3rd |
1/4th |
1/5th |
1/7th |
||||
|
MN |
1st Week |
0.0 |
15 |
13 |
10 |
6 |
5 |
|
2nd Week |
0.0 |
18 |
15 |
11 |
7 |
7 |
|
|
3rd Week |
0.0 |
20 |
18 |
14 |
10 |
8 |
|
|
Frequency (%) |
1st Week |
0.00±0.00Cf |
0.75±0.05Ca |
0.65±0.04Cb |
0.50±0.01Cc |
0.30±0.02Cd |
0.25±0.01Ce |
|
2nd Week |
0.00±0.00Bf |
0.90±0.07Ba |
0.75±0.01Bb |
0.55±0.06Bc |
0.35±0.01Bd |
0.35±0.02Be |
|
|
3rd Week |
0.00±0.00Af |
1.00±0.01Aa |
0.90±0.05Ab |
0.70±0.01Ac |
0.50±0.06Ad |
0.40±0.03Ae |
|
|
DN |
1st Week |
0.0 |
19 |
17 |
10 |
6 |
5 |
|
2nd Week |
0.0 |
26 |
25 |
18 |
12 |
9 |
|
|
3rd Week |
0.0 |
28 |
28 |
22 |
16 |
11 |
|
|
Frequency (%) |
1st Week |
0.00±0.00Cf |
0.95±0.06Ca |
0.85±0.08Cb |
0.50±0.05Cc |
0.30±0.03Cd |
0.25±0.01Ce |
|
2nd Week |
0.00±0.00Bf |
1.30±0.08Ba |
1.25±0.07Bb |
0.90±0.01Bc |
0.90±0.07Bd |
0.45±0.06Be |
|
|
3rd Week |
0.00±0.00Af |
1.40±0.08Aa |
1.40±0.08Ab |
1.10±0.07Ac |
0.80±0.06Ad |
0.55±0.04Ae |
|
Small alphabet superscripts show the difference between treatments within the same row While capital alphabet shows significant (P < 0.05) among different durations of exposure within the same column.
Table 2: Nuclear abnormalities in erythrocytes of W. attu exposed to lead.
|
Parameters |
Sampling time |
NC |
PC |
Sub-lethal concentrations |
|||
|
1/3rd |
1/4th |
1/5th |
1/7th |
||||
|
MN |
1st Week |
0.00 |
20 |
22 |
18 |
13 |
11 |
|
2nd Week |
0.00 |
23 |
27 |
19 |
15 |
9.0 |
|
|
3rd Week |
0.00 |
28 |
33 |
25 |
20 |
17 |
|
|
Frequency (%) |
1st Week |
0.00±0.01Af |
2.0±0.1Cb |
2.2±0.1Ca |
1.8±0.1Cc |
1.3±0.01Cd |
1.1±0.01Be |
|
2nd Week |
0.00±0.1Af |
2.3±0.1Bb |
2.7±0.01Ba |
1.9±0.1Bc |
1.5±0.1Bd |
0.9±0.01Ce |
|
|
3rd Week |
0.00±0.01Af |
2.8±0.01Ab |
3.3±0.01Aa |
2.5±0.1Ac |
2.0±0.01Ad |
1.7±0.1Ae |
|
|
DN |
1st Week |
0.00 |
25 |
35 |
26 |
18 |
14 |
|
2nd Week |
0.00 |
29 |
28 |
26 |
19 |
10 |
|
|
3rd Week |
0.00 |
33 |
40 |
31 |
25 |
16 |
|
|
Frequency (%) |
1st Week |
0.0±0.01Af |
2.5±0.01Cc |
3.5±0.01Ba |
2.6±0.01Bb |
1.8±0.10Cd |
1.4±0.01Be |
|
2nd Week |
0.0±0.10Af |
2.9±0.01Ba |
2.8±0.10Cb |
2.6±0.01Bc |
1.9±0.01Bd |
1.0±0.10Ce |
|
|
3rd Week |
0.0±0.01Af |
3.3±0.10Ab |
4.0±0.01Aa |
3.1±0.01Ac |
2.5±0.10Ad |
1.6±0.10Ae |
|
Small alphabet superscripts show the difference between treatments within the same row While capital alphabet shows significant (P < 0.05) among different durations of exposure within the same column.
Table 3: Nuclear abnormalities in erythrocytes of Wallago attu exposed to chromium.
|
Parameters |
Sampling time |
NC |
PC |
Sub-lethal concentrations |
|||
|
1/3rd |
1/4th |
1/5th |
1/7th |
||||
|
MN |
1st Week |
0.0 |
10 |
8 |
6 |
6 |
4 |
|
2nd Week |
0.0 |
15 |
14 |
7 |
8 |
5 |
|
|
3rd Week |
0.0 |
11 |
10 |
8 |
6 |
5 |
|
|
Frequency (%) |
1st Week |
0.00±0.01Cf |
0.50±0.04Ca |
0.40±0.04Cb |
0.30±0.03Cc |
0.30±0.02Cd |
0.20±0.01Ce |
|
2nd Week |
0.00±0.03Af |
0.75±0.01Aa |
0.70±0.01Ab |
0.35±0.04Ac |
0.40±0.05Ad |
0.25±0.05Ae |
|
|
3rd Week |
0.00±0.02Bf |
0.55±0.01Ba |
0.50±0.07Bb |
0.40±0.06Bc |
0.30±0.05Bd |
0.25±0.04Be |
|
|
DN |
1st Week |
0.00 |
12 |
11 |
9 |
7 |
6 |
|
2nd Week |
0.00 |
20 |
13 |
12 |
10 |
8 |
|
|
3rd Week |
0.00 |
14 |
18 |
15 |
13 |
10 |
|
|
Frequency (%) |
1st Week |
0.0±0.01Af |
0.60±0.03Ba |
0.55±0.01Cb |
0.45±0.02Cc |
0.35±0.01Cd |
0.30±0.01Ce |
|
2nd Week |
0.0±0.10Af |
2.00±0.01Aa |
1.30±0.01Bb |
0.60±0.03Bc |
0.50±0.02Bd |
0.40±0.02Be |
|
|
3rd Week |
0.0±0.01Af |
0.70±0.02Bb |
1.80±0.03Aa |
0.75±0.03Ac |
1.30±0.01Ad |
0.50±0.01Ae |
|
Small alphabet superscripts show the difference between treatments within the same row While capital alphabet shows significant (P < 0.05) among different durations of exposure within the same column.
Similar results were observed by Kousar and Javed (2015) who noted the copper induced concentration specific formation of MN and NAs frequency in RBCs of three fish species exposed for 30-days. Similarly, Oreochromis niloticus showed higher frequency of MN and NAs in RBCs during initial hours of Cd and Zn exposure after that both were decreased in duration dependent manner (Abu-Bakar et al., 2014). Rasal et al. (2011) also reported the Cr induced MN in blood of Labeo rohita. Kousar and Javed (2016) also documented the dose specific induction of MN and NAs in four species of fish exposed to arsenic for a period of 30 days. Al-Tamimi et al. (2015) noted the progressive increase in MN formation in erythrocytes of Cyprinus carpio as the concentration of copper exposure increased. Alimba et al. (2015) also noted the time dependent formation of MN and NAs frequency in blood of Clarias gariepinus exposed to effluent from Bodija, Nigeria for 7-, 14- and 28-day. Ashmawy et al. (2015) also documented the formation of MN in RBCs of Oreochromis niloticus exposed to different concentrations of cadmium. Hussain et al. (2018) observed the 96% reduction in Labeo rohita population due to MN and NA induction captured from polluted River Chenab, Bhawana, Faisalabad. Mahboob et al. (2014) also confirmed the concentration dependent increase in MN frequency of Clarias gariepinus exposed to mercuric chloride for 7-day.
The different concentrations of lead nitrate have ability to induce of MN in Channa punctatus erythrocytes (Choudhary et al., 2012). According to Jindal and Verma (2015) RBCs of Labeo rohita showed significant formation of MN and NAs exposed to Cd for different time. Ahmed et al. (2013) noticed the dose reliant increase in MN frequency in RBCs of Heteropneustes fossilis during initial hours of Cr exposure after that MN formation was decreased. Monteiro et al. (2011) also reported the lead induced NAs in blood of Prochilodus lineatu.
Conclusions and Recommendations
It was concluded that metals have ability to induce geno-toxicity in erythrocytes of W. attu. It was also concluded that the measurement of genotoxic damage by micronucleus assay is the most promising method for evaluating the pollution load in aquatic ecosystem which effects the population of fish species like W. attu. More research is needed, with a focus on priority pollutants and other ecological receptors, especially those used as human food.
Conflict of interest
The authors have declared no conflict of interest.
References
Abu-Bakar, S.N.N., Ashriya, A., Shuib, A.S. and Razak, S.A., 2014. Genotoxic effect of zinc and cadmium following single and binary mixture exposures in Tilapia (Oreochromis niloticus) using micronucleus test. Sains Malaysiana, 43: 1053-1059.
Agah, H., Leermakers, M., Elskens, M., Fatemi, S.M.R. and Baeyens, W., 2009. Accumulation of trace metals in the muscles and liver tissues of five fish species from the Persian gulf. Environ. Monit. Assess., 157: 499-514. https://doi.org/10.1007/s10661-008-0551-8
Ahmed, M.K., Kundu, G.K., Al-Mamun, M.H., Sarkar, S.K., Akter, M.S. and Khan, M.S.,Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol. Environ. Saf., 92: 64-70. https://doi.org/10.1016/j.ecoenv.2013.02.008
Alimba, C.G., Ajayi, E.O., Hassan, T., Sowunmi, A.A. and Bakare, A.A., 2015. Cytogenotoxicity of abattoir effluent in Clarias gariepinus (Burchell, 1822) using Micronucleus Test. Chinese J. Biol., pp. 1-6. https://doi.org/10.1155/2015/624524
Al-Sabti, K. and Metcalfe, C.D., 1995. Fish micronuclei for assessing genotoxicity in water. Mutat. Res., 343: 121-135. https://doi.org/10.1016/0165-1218(95)90078-0
Al-Tamimi, A.H., Al-Azzawi, A.J. and Al-Adhmi, M.A., 2015. Chronic toxicity assessment of histological changes and micronuclei in fish Cyprinus carpio L. after exposed to copper. Am. Scient. Res. J. Eng. Technol. Sci., 13: 194-210.
Ashmawy, A.A., Rashed, M.A., Atta, A.H., Ibrahim, A.G. and Abdel-Gawad, F.K., 2015. Genotoxic effect of cadmium on NileTilapia (Oreochromis niloticus). Int. J. Scient. Eng. Res., 6: 971-980.
Ballatori, N., 2002. Transport of toxic metals by molecular mimicry. Environ. Health Perspec., 110: 689. https://doi.org/10.1289/ehp.02110s5689
Barsiene, J., Lazutka, J., Syvokiene, J., Dedonyte, V., Rybakovas, A., Bjornstad, A. and Andersen, O.K., 2004. Analysis of micronuclei in blue mussels and fish from the Baltic and North seas. Environ. Toxicol., 19: 365-371. https://doi.org/10.1002/tox.20031
Batool, M., Abdullah, S. and Abbas, K., 2014. Antioxidant enzymes activity during acute toxicity of chromium and cadmium to Channa marulius and Wallago attu. Pak. J. Agri. Sci., 51: 1017-1023.
Batool, M., Abdullah, S., Ijaz, M.U., Kousar, S., Fatima, M., Ilyas, R., Ambreen, F. and Mughal, K.T., 2018. Heavy metals (cadmium and lead) induced oxidative stress in Channa marulius and Wallago attu during acute toxicity experiments. Pakistan J. Zool. Suppl. Ser., 13: 74-79.
Bertin, G. and Averbeck, D., 2006. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie, 88: 1549-1559. https://doi.org/10.1016/j.biochi.2006.10.001
Bolognesi, C. and Hayashi, M., 2011. Micronucleus assay in aquatic animals. Mutagenesis, 26: 205-213. https://doi.org/10.1093/mutage/geq073
Cambier, S., Gonzalez, P., Durrieu, G. and Bourdineaud, J.P., 2010. Cadmium-induced genotoxicity in zebra fish at environmentally relevant doses. Ecotoxicol. Environ. Saf., 73: 312-319. https://doi.org/10.1016/j.ecoenv.2009.10.012
Carrola, J., Santos, N., Rocha, M.J., Fontainhas-Fernandes, A., Pardal, M.A., Monteiro, R.A. and Rocha, E., 2014. Frequency of micronuclei and of other nuclear abnormalities in erythrocytes of the grey mullet from the Mondego, Douro and Ave estuaries-Portugal. Environ. Sci. Poll. Res., 21: 6057-6068. https://doi.org/10.1007/s11356-014-2537-0
Choudhary, J. and Abha, Jha, A.M., 2012. Genotoxic testing of lead nitrate in air-breathing teleost Channa punctatus (BLOCH). Int. J. Plant Anim. Environ. Sci., 2: 229-234.
Dang, F. and Wang, W.W., 2009. Assessment of tissue-specific accumulation and effects of cadmium in a marine fish fed contaminated commercially produced diet. Aquat. Toxicol., 95: 248–255. https://doi.org/10.1016/j.aquatox.2009.09.013
El-Ashmawy, I.M., Ashry, K.M., El-Nahas, A.F. and Salama, O.M., 2006. Protection by turmeric and myrrh against liver oxidative damage and genotoxicity induced by lead acetate in mice. Basic Clin. Pharmacol. Toxicol., 98: 32-37. https://doi.org/10.1111/j.1742-7843.2006.pto_228.x
El-Magd, M.A., Kahilo, K.A., Nasr, N.E., Kamal, T., Shukry, M. and Saleh, A.A., 2016. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia, 49: 1-9. https://doi.org/10.1111/and.12750
Farombi, E.O., Adelowo O.A. and Ajimoko Y.R., 2007. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African Cat fish Clarias gariepinus from Nigeria Ogun River. Int. J. Environ. Res. Public. Health, 4: 158-165. https://doi.org/10.3390/ijerph2007040011
Fenech, M., Chang, W.P., Kirsch-Volders, M., Holland, N., Bonassi, S. and Zeiger, E., 2003. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte culture. Mut. Res., 534: 65-75. https://doi.org/10.1016/S1383-5718(02)00249-8
Frenzilli, G., Nigro, M. and Lyons, B.P., 2009. The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mut. Res., 681: 80-92. https://doi.org/10.1016/j.mrrev.2008.03.001
Garcia-Santos, S., Vargas-Chacoff, L., Ruiz-Jarabo, I., Varela, J.L., Mancera, J.M., Fontainhas-Fernandes, A. and Wilson, J.M., 2011. Metabolic and osmoregulatory changes and cell proliferation in gilt head seabream (Sparus aurata) exposed to cadmium. Ecotoxicol. Environ. Saf., 74: 270–278. https://doi.org/10.1016/j.ecoenv.2010.08.023
Guardiola, F.A., Cuesta, A., Meseguer, J., Martinez, S., Martinez-Sanchez, M.J., Perez- Sirvent, C. and Esteban, M.A., 2013. Accumulation, histopathology and im muno toxicological effects of waterborne cadmium on gilt head seabream (Sparus aurata). Fish Shellfish Immunol., 35: 792–800. https://doi.org/10.1016/j.fsi.2013.06.011
Hong, F., Wu, C., Liu, C., Wang, L., Gao, F., Yang, F., Xu, J., Liu, T., Xie, Y. and Li, X., 2007. Direct evidence for interaction between lead ions and kidney DNA from silver Crucian carp. Chemosphere, 68: 1442-1446. https://doi.org/10.1016/j.chemosphere.2007.04.020
Hussain, B., Sultana, T., Sultana, S., Masoud, M.S., Ahmed, Z. and Mahboob, S., 2018. Fish eco-genotoxicology: Comet and micronucleus assay in fish erythrocytes as in situ biomarker of freshwater pollution. Saudi J. Biol. Sci., 25: 393-398. https://doi.org/10.1016/j.sjbs.2017.11.048
Ip, C.C.M., Li, X.D., Zhang, G., Wong, C.S.C. and Zhang, W.L., 2005. Heavy metal and Pb isotopic compositions of aquatic organisms in the Pearl River Estuary, South China. Environ. Pollut., 138: 494-504. https://doi.org/10.1016/j.envpol.2005.04.016
Jindal, R. and Verma, S., 2015. In vivo genotoxicity and cytotoxicity assessment of cadmium chloride in peripheral erythrocytes of Labeo rohita (Hamilton). Ecotoxicol. Environ. Saf., 118: 1-10. https://doi.org/10.1016/j.ecoenv.2015.04.005
Kirschbaum, A.A., Seriani, R., Ranzani-Paiva, M.J.T., Abessa, D.M.S. and Pereira, C.D.S., 2009. Cytogenotoxicity biomarkers in fat snook Centropomus parallelus from Cananeiaand São Vicente estuaries, SP. Brazil. Gen. Mol. Biol., 32: 151-154. https://doi.org/10.1590/S1415-47572009005000007
Kousar, S. and Javed, M., 2015. Studies on induction of nuclear abnormalities in peripheral blood erythrocytes of fish exposed to copper. Turk. J. Fish. Aquat. Sci., 15: 879-886. https://doi.org/10.4194/1303-2712-v15_4_11
Kousar, S. and Javed, M., 2016. Determination of arsenic induced nuclear abnormalities in peripheral blood erythrocytes of carps using micronucleus test. J. Anim. Plant Sci., 26: 1501-1506.
Li, Z.H., Chen, L., Wu, Y.H., Li, P., Li, Y.F. and Ni, Z.H., 2014. Effects of waterborne cadmium on thyroid hormone levels and related gene expression in Chinese rare minnow larvae. Comp. Biochem. Physiol., 161: 53–57. https://doi.org/10.1016/j.cbpc.2014.02.001
Mahboob, S., Al-Balwai, H.F.A., Al-Misned, F. and Ahmad, Z., 2014. Investigation on the genotoxicity of mercuric chloride to freshwater Clarias gariepinus. Pak. Vet. J., 34: 100-103.
Mishra, A.K. and Mohanty, B., 2008. Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch). Environ. Toxicol. Pharmacol., 26: 136- 141. https://doi.org/10.1016/j.etap.2008.02.010
MonteiroCavalcante, D.G.S.M., Vilela, M.B.F.A., Sofia, S.H. and Martinez, C.B.R.,In vivo and in vitro exposures for the evaluation of the genotoxic effects of lead on the Neotropical freshwater fish Prochilodus lineatus. Aquat Toxicol., 104: 291-298. https://doi.org/10.1016/j.aquatox.2011.05.002
Navarro, C.D. and Martinez, C.B., 2014. Effects of the surfactant polyoxyethylene amine (POEA) on genotoxic, biochemical, and physiological parameters of the freshwater teleost Prochilodus lineatus. Comp. Biochem. Physiol. Comp. Toxicol. Pharmacol., 165: 83-90. https://doi.org/10.1016/j.cbpc.2014.06.003
Patra, M., Bhowmik, N., Bandopadhyay, B. and Sharma, A., 2004. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot., 52: 199-223. https://doi.org/10.1016/j.envexpbot.2004.02.009
Patra, R., Swarup, D. and Dwivedi, S., 2001. Antioxidant effects of α tocopherol, ascorbic acid and Lmethionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology, 162: 81-88. https://doi.org/10.1016/S0300-483X(01)00345-6
Prado, L.R., Felix, C., Abessa, D.M., Buruaem, L.M., Abujamara, L.D., Kirschbaum, G.C.R., Turatti, A.T., Ranzani-Paiva, M.J.T., Correia, A.A. and Seriani, R., 2014. Hematological parameters and nuclear abnormalities in peripheral erythrocytes of Achirus lineatus (Pleuronectiformes: Achiridae). Comp. Clin. Pathol., 361: 1-7. https://doi.org/10.1007/s00580-014-1880-3
Rasal, K., Rasal, A. and Nayan, M., 2011. Micronucleus test as a cytogenetic marker for evaluation of genotoxicity in fish, Labeo rohita. Asian J. Anim. Sci., 6: 32-34.
Razzaq, A., Abdullah, S., Naz, H., Abbas, K., Shafique, L. and Liu, Q., 2021. Micronuclei assay: A suitable tool for evaluating the heavy metals induced genotoxicity in fish, Labeo rohita. Pakistan J. Zool., 5: 1997-2000. https://doi.org/10.17582/journal.pjz/20190325130338
Seriani, R., Franca, J.G., Lombardi, J.V., Brito, J.M. and Ranzani-Paiva, M.J.T., 2015. Hematological changes and cytogenotoxicity in the tilapia Oreochromis niloticus caused by sub-chronic exposures to mercury and selenium. Fish. Physiol. Biochem., 41: 311-322. https://doi.org/10.1007/s10695-014-9984-x
Souza, T.S. and Fontanetti, C.S., 2006. Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Gene. Toxicol. Environ. Mutagen., 605: 87-93. https://doi.org/10.1016/j.mrgentox.2006.02.010
Xu, G.R., Wang, J.N. and Li, C.J., 2012. Preparation of hierarchically nanofibrous membrane and its high adaptability in hexavalent chromium removal from water. Chem. Eng. J., 1: 310-317. https://doi.org/10.1016/j.cej.2012.05.104
To share on other social networks, click on any share button. What are these?








