Isolation and Biological Characterization of Muscle-Derived Stem Cells from Sheep Skeletal Muscle
Isolation and Biological Characterization of Muscle-Derived Stem Cells from Sheep Skeletal Muscle
Ping Zhang1,2, Yabin Pu1, Yu Zhang2, Jia Chen2, Kunfu Wang3, Qian Li3, Yujiao Sun3, Yuehui Ma1, Shuqing Jiao2,* and Weijun Guan1,*
1Institute of Beijing Animal Science and Veterinary, Chinese Academy of Agricultural Science, Beijing, 100194, China
2College of Pharmacy, Jiamusi University, Heilongjiang Province Key Laboratory of Biological Medicine Formulation, Jiamusi, 154007, Heilongjiang, China
3College of Wildlife Resources. Northeast Forestry University, No.26 Hexing Road, Xiangfang District, 150040, Harbin, China
Ping Zhang and Yabin Pu contributed equally to this work.
ABSTRACT
The Objective of this study was to investigate the isolation, cultivation, identification and differentiation potential of Muscle-derived stem cells (MDSCs) from sheep skeletal muscle and provide an experimental evidence for clinical application. The skeletal muscle tissue were studied from fetal sheep, and the stepwise digestion by XI collagenase and trypsin was used to isolate MDSCs and were purified by differential attachment technique. Cell morphology were observed using an inverted microscope, and MDSCs proliferation pattern were determined through growth curve analyses. MDSCs were identified by immunofluorescence and RT-PCR, and the immunofluorescence antibody invoved Sca-1, CD34, CD144, Desmin and CD45 which are the makers of MDSCs. Finally, MDSCs were induced into Adipocytes, osteoblasts, chondrocytes and neuron-like cells by the optimized inducing medium. After the two-step digestion and differential attachment technique, high purity MDSCs were successfully cultured. MDSCs were identified by round or short spindle cell, and gathered into clusters; The average doubling time and clone efficiency of the cells were 35.5h and 47.2%, respectively. The MDSCs makers, such as Sca-1, CD34, CD144 and Desmin, were positive expressed by immunofluorescence and RT-PCR detection, and CD45 was negative expressed. MDSCs were successfully induced into Adipocytes, osteoblasts, chondrocytes, myoblasts and neuron-like cells under the optimized inducing medium. To conclude, in vitro high purity MDSCs have multiple differentiation potential, and can play an important role in muscle tissue repair, and provide seed cells for tissue engineering research and clinical applications.
Article Information
Received 20 October 2017
Revised 02 December 2017
Accepted 16 December 2017
Available online 01 May 2019
Authors’ Contribution
PZ and YP carried out experimental operation and paper editing. YZ, JC, KW, QL, YS and YM supplied essential experimental materials and protocol. SJ and WG presentedt eh research idea and panned the experiments,
Key words
Sheep skeletal muscle, Muscle-derived stem cells, Biological differentiation, Clinical applications, Muscle derived stem cells.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.4.1259.1272
* Corresponding authors: [email protected];
0030-9923/2019/0004-1259 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Skeletal muscle may represent a convenient source of stem cells for cell-mediated therapies. A potentially new type of undifferentiated cell isolated from skeletal muscle without myogenic restrictions, the muscle-derived stem cells (MDSCs), may form the basis for such therapy (Deasy et al., 2001). Satellite cells, referred to by many as muscle stem cells, are myogenic precursors that are capable of regenerating muscle and demonstrate self-renewal properties; however, they are considered to be committed to the myogenic lineage. MDSCs, which may represent a predecessor of the satellite cell, are considered to be distinct in that they may not be restricted to the myogenic lineage or even to mesenchymal tissues (Wu et al., 2010). These cells display capacities of long-term proliferation, high self-renewal, immune-privileged behavior, and a superior capacity to regenerate skeletal muscle (Gharaibeh et al., 2008; Deasy et al., 2005). In addition, MDSCs can readily be transduced with a variety of different genes using viral vectors, which is a very important attribute in the development of tissue-engineering applications where the secretion of specific proteins is desired to aid in the regeneration of specific tissues. Several other types of muscle-derived stem cells including side population (SP) cells, mesangioblasts, and perivascular cells have been recently identified and purified by flow cytometry from adult skeletal muscle (Usas et al., 2011). A human counterpart to MDSCs has been recently isolated from human skeletal muscle using fluorescence- activated cell sorting (FACS) to select cells coexpressing myogenic and endothelial markers. These cells can retain the expression of surface markers and capacity of myogenic differentiation during long-term culture and exhibit multilineage developmental potential in vitro and in vivo at the clonal level (Zheng et al., 2007).
MDSCs, another adult pluripotent stem cells, have become a hot topic in the field of gene therapy and cell-based tissue engineering. It has wide prospect in treating stress incontinence by periurethral injection of patients’ MDSCs (Kwon et al., 2006), Muscular dystrophy (Gussoni et al., 1999; Ambrosio et al., 2009), Duchenne muscular dystrophy(DMD) (Qu-Petersen et al., 2002; Torrente et al., 2007), and muscle injury (Tamaki et al., 2005). Their multi-potentiality allows them to differentiate into different lineage cells, but their differentiation potential is not as strong as embryonic cells (Jankowski et al., 2002).
MDSCs expressed in pluripotent cells capable of differentiating into the three germ layers. such as endoderm liver cells (Vourc’h et al., 2004; Bellayr et al., 2010); mesoderm myoblast (Tamaki et al., 2002; Lu et al., 2010), adipocytes (Matsumoto et al., 2009; Wu et al., 2012), osteoblasts (Levy et al., 2001; Deasy et al., 2001), chondrocytes (Claros et al., 2008; Kuroda et al., 2006), and so on. restrictions can break blastoderm differentiation into neural ectoderm cells (Danisovic et al., 2008; Arsic et al., 2008).
Current research of stem cells focuses on humans, rabbits, mice, and other model animals (Oztopcu-Vatan et al., 2017). As the other animal model, the sheep possesses abundant dermal tissues. Moreover a wide range of MDSCs can provide a theoretical basis and experimental basis, can be widely used in clinical transplantation. In this study, we carried out a stud study on the isolation, culture, and differentiation potential of sheep MDSCs.
Materials and methods
Experimental animal
All animal procedures was approved by The Institutional Animal Care and Use Committee of Chinese Academy of Agricultural Sciences, sheep full-term placentas were provided by the Animal Husbandry Experimental Base Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing. The use of animals in research and all experimental procedures involving cattle were conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee at UMDNJ-RWJMS.
Experimental reagents
High glucose DMEM (Gibco), fetal bovine serum (Hyclone), horse serum(Sigma), trypsin 1:250 (Amresco), collagenase XI (Sigma America), EDTA(Gibco), rabbit anti sheep Sca-1, CD34, CD144 polyclonal primary antibody (Abcam, America), mouse to sheep Desmin, CD45 primary antibody (Santa cruz, America), rabbit to sheep NSE, NF primary antibody, FITC-conjugated goat to rabbit secondary antibody IgG, FITC-conjugated goat to mouse secondary antibody IgG (Zhongshan Golden Bridge , China), dexamethasone (Sigma), IBMX (Life Technologies, America), insulin, indometacin, β-glycerophosphate, ascorbate, glutamine (Sigma), B27, N2, ITS, TGF-β3 (Gibco), bFGF, EGF, GDNF(Life Technologies, America), L-proline (Sigma), Sodium pyruvate (Abcam), alizarin red, Alcian blue (Boster, China), Oil red O (Sigma).
Isolation of sheep MDSCs
Sheep full-term placentas were collected by cesarean section. The central placenta cotyledons were obtained after removing the blood and vessels by phosphate-buffered saline (PBS), the samples was cut into pieces (1 mm3) and were enzymatically dissociated by adding 0.25% trypsin for 1 h at 37°C and 0.2% collagenase XI for 1 h. adding equal quantity of H-DMEM contained 10 % Fetal Bovine Serum for neutralization. The digested tissue was passed through a 200 µm mesh filter and then centrifuged at 1200 r/min for 8 min at room temperature. The supernatant was discarded, and the pellet was resuspended with the complete culture medium. The muscle cell extract was preplated on collagen-coated petri dish.We isolated different populations of muscle-derived cells based on the number of preplates performed on collagen-coated, petri dish. Preplate (PP) 1 represented a population of muscle-derived cells that adhered in the first hour after isolation, PP2 in the next 2 h, PP3 in the next 18 h, and the subsequent preplates were obtained at 24 h intervals (PPs 4-6). The proliferation medium was H-DMEM with 20% FBS, 10% HS, 2% Chick embryo extract (CEE) and 1% penicillin/streptomycin. when cells reached 80 % confluence, adding 0.25 % trypsin and 0.04 % EDTA to dissociate cells from the plates, neutralized with complete medium for subculture, purified cells were gained after 3-4 passages. All experiments were conducted using at least three distinct sources of placenta.
Preparation of chick embryo extract
Currently, the market price of chicken embryo extract is expensive, therefore , this experiment chick embryo extract used in the preparation of their own , the steps are: (i) incubate the chicken eggs for 11 to 14 days at 37°C in a humidified incubator. The length of time that the eggs are incubated depends on the age of the eggs when they arrive at the lab. Normally, eggs that are ordered arrive within 2 to 3 days of being laid. Most universities and educational institutions have agreements with neighboring or local farms. Please check with your animal facility at your institution for a source for fertilized chicken eggs. (ii) wash the surface of the eggshells carefully with 75% ethanol. (iii) crack open the egg. Dissect out the embryos and place them in H-DMEM at 4°C. Use approximately 7mL of H-DMEM for every 3 dissected embryos. (iv) macerate approximately 10 embryos at a time by passing them through a 30 mL syringe into a 50 mL sterile centrifuge tube. This should produce approximately 25 mL of volume. (v) add an equal volume of H-DMEM at 4°C to the tube of embryos. Incubate with rotary shaking action for 45 min at 4°C. (vi) add 100 μL of sterile Hyaluronidase for every 50 mL of embryo and H-DMEM mix. (vii) centrifuge the mix at 12000 rpm for 4 h at 4°C. (viii) filter the supernatant through a 0.45 μm filter and then through a 0.22 μm filter using sterile technique. (ix) aliquot and store at -80°C.
Estimation of cell viability
MDSCs viability was detected using the Trypan blue exclusion test (Bai et al., 2010) before and after cryopreservation as previously described. Cells were digested and seeded in 6-well plates and 1000 cells were stained and checked for cell viability rate.
Table I.- Primer information for MDSCs identification.
|
Gene |
Primer sequence |
Tm (°C) |
Cycle |
Fragment size(bp) |
|
GAPDH |
F:5’-GAAGGTCGGAGTGAACGGATT-3’ R:5’-GGTCATAAGTCCCTCCACGAT-3’ |
60 |
30 |
517 |
|
Sca-1 |
F:5’-TCAGGGAAACCTTCACGAG-3’ R:5’-CCACTGTCTGTCTCAATACCACA-3’ |
59.4 |
30 |
310 |
|
CD34 |
F:5’-CGGCATCTTCTACCCTCATC-3’ R:5’-GCCTGTTCCTCCTGACACA-3’ |
59.7 |
30 |
304 |
|
CD144 |
F:5’-TGGTCACACGCAAGTCCTAA-3’ R:5’-TAACAACCCTCCTCGCCATA-3’ |
60 |
30 |
302 |
|
Desmin |
F:5’-GACCTTACCCTTCCCACCTC-3’ R:5’-CATCTCCTGCTCCCACATCT-3’ |
59.9 |
30 |
314 |
|
CD45 |
F:5’-GCTGGTCCTTTGGAGAGTGT-3’ R:5’-GCTGGTCCTTTGGAGAGTGT-3’ |
59.5 |
30 |
316 |
|
LPL |
F:5’-ACTATCCCCTGGGTAATGTGC-3’ R:5’-GGTTGGAAAGTGCCTCCGTTA-3’ |
60 |
30 |
283 |
|
PPAR-γ |
F:5’-CGGGAAAGACGACAGACAAAT-3’ R:5’-CGTAGAAGGTCCCCACAGTCA-3’ |
60 |
30 |
147 |
|
OPN |
F:5’-TTTCACTCCACCTTTCCCTAC-3’ R:5’-CCACCCTGCTTTAACATATCC-3’ |
58 |
30 |
489 |
|
ALP |
F:5’-CGAAGCACAAGCACTCTCACT-3’ R:5’-GGGAGCCAGACCAAAGATAGA-3’ |
60 |
30 |
419 |
|
COL-I |
F:5’-CCCAGTTGTCTTACGGCTATG-3’ R:5’-ACCTCTGTGTCCCTTCATTCC-3’ |
60 |
30 |
323 |
|
COL-II |
F:5’-TTCCTGCGCCTGATGTCC-3’ R:5’-CAGTTTGGGTTGTTTGTCTGTT-3’ |
58 |
30 |
416 |
|
SOX-9 |
F:5’-CCAAACTCAGCCAAGAAT-3’ R:5’-GAAAGAACATAGACTAAACCCT-3’ |
60 |
30 |
349 |
|
COL-X |
F:5’-GCGGAGACTACTGGATTGACC-3’ R:5’-TCTTGTCCTTGCTCTTGCTG-3’ |
60 |
30 |
150 |
|
aggrecan |
F:5’-CCACTCCCACGTCTTTACC-3’ R:5’-ACCCACCACCTTGTAGCC-3’ |
55 |
30 |
142 |
|
NSE |
F:5’-TGTGGCTTTGGATCTCGT-3’ R:5’-TTCTTCCGCTTGTGCTTG-3’ |
54 |
30 |
489 |
|
NF |
F:5’-TGGAAGTAGTGGTCGGAAGA-3’ R:5’-GGGAACTGGAGGGTAGAT-3’ |
56.5 |
30 |
460 |
Colony-forming cell assay
Cells from passage 3, passages 15 passages 27 and passages 40 were seeded in 24-well plates at a density of 1×104 cell/well and cultured for 7 days, the numbers of colony-forming units (CFU) were counted to calculate colony-forming rate through the formulation as colony-forming units number/seeding cell number per 24-well× 100 %.
Cell population growth dynamics
MDSCs at passages 3, 15, 27 and 40 were plated in 24-well plates at 1×104 cell/well, cultured for 7 days and counted every day (3 wells each time) after wards. Mean cell counts at each time point were then used to plot their growth curve, based upon which PDT (population doubling time) was calculated. PDT=(t-t0)lg2/(lgNt-lgN0), where, t0 is starting time of culture, t is termination time of culture; N0 is initial cell number of culture and Nt is ultimate cell number of culture.
Identification of sheep MDSCs markers
Reverse transcription PCR (RT- PCR)
Cells from Passage 3, passage 15, passage 27, and passage 40 were chosen as the low, middle, high passages for RT-PCR, the cells were collected and the total RNA were extracted with Trizol reagent (Invitrogen). Total RNA was reverse transcribed to cDNA using PrimeScript™ 1st Strand cDNA Synthesis Kit (TARAKA, China). Specific gene primer pairs were designed and were listed on in Table1. PCRs were performed in 25μL volumes containing 2.5mL 10 × RT-Buffer, 16.75μL of double-distilled H2O, 0.25 μL of Ex-Taq (Takara, Otsu, Japan), 1.0μL each of forward and reverse primers (Gene-specific primer pairs are listed in Table I), and 1.5μL of template cDNA, 2.0 μL dNTP (2.5mM). Reaction condition was consisted of an initial denaturation step at 94°C for 5 min, 30 cycles at 94°C for 30s, 55-60°C for 30s and 72°C for 30s, one more 72°C for 10 min at last, PCR products were visualized by 2.5% agarose gel electrophoresis (AGE).
Immunofluorescent analysis of cell surface antigens
MDSCs were washed three times with PBS and fixed in 4% paraformaldehyde for 30 min and then washed three times in PBS (5 min per wash) again. Cells were permeabilized by 0.25 % Triton X-100 (Sigma, Santa Clara, USA) for 15 min, then washed for three times, incubated with goat serum (diluted with PBS by 1:10, Zhongshan Golden Bridge, Beijing, China) for 30 min at room temperature. Added anti-Sca-1 (rabbit anti-sheep, 1:100, Abcam, San Francisco, USA), anti-CD34 (rabbit anti-sheep, 1:100, Abcam, San Francisco, USA), anti-CD144 (rabbit anti-sheep, 1:100, Abcam, San Francisco, USA), anti-Desmin (mouse anti-sheep, 1:100, Santa, cruz, USA), anti-CD45 (mouse anti-sheep, 1:100, Santa, cruz, USA), anti-neurofilament (NF) (rabbit anti-sheep, 1:100, Abcam, San Francisco, USA), anti-neuron-specific enolase (NSE) (rabbit anti-sheep, 1:100, Abcam, San Francisco, USA) and incubated overnight at 4°C or 1 h at room temperature. Next, the cells were washed three times (5 min each) with PBS and then incubated in PBS containing appropriate FITC-conjugated goat to mouse secondary antibody IgG or FITC-conjugated goat to rabbit secondary antibody IgG (1:100, Zhong- shan Golden Bridge, Beijing, China) for 1 h at 37°C in the dark. Slides were washed for three times, cells were incubated with 1 μg/mL DAPI (Sigma, Santa Clara, USA) for 15 min then washed for three times. Images were acquired using a laser-scanning confocal microscope. As technical controls, PBS was used in place of primary antibodies. Eight randomly selected non-overlapping fields of view from the four passages for each antigen were observed and photographed.
Karyotype analysis
Karyotype of P3, P15, P27 and P40 cells were analyzed as previously described (Baran and Ware, 2007). Cells were harvested when 80-90 % confluent, subjected to hypotonic treatment and fixed, and the chromosome numbers were counted from 100 spreads under an oil immersion objective upon Giemsa staining. Relative length refers to the ratio of a single chromosome length to total haploid chromosome length include the chromosome X (or Y). It can be formulated by percentage:
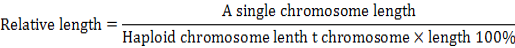
Arm ratio index refers to the ratio of the long arm to the short:
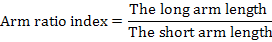
Centromere index refers to the ratio of the short arm length to the chromosome length and is formulated by percentage. It determines the relative position of centromere:

Induced differentiation of sheep MDSCs
Adipogenic differentiation of sheep MDSCs
Passage 3 which have higher differentiation potential were choosen for adipogenic differentiation. As the passage 3 MDSCs reached at the confluence degree of 70-80%, MDSCs were divided into two groups: induced and control group. Cells of the induced group were incubated in adipogenic medium containing 10% FBS, 0.5mM isobutylmethylxanthine (IBMX; LifeTechnologies),1μM dexamethasone (Sigma), 10µg/mL insulin (Sigma), and 60μM indometacin. Cells of the control group were incubated in complete medium without any induce factors. Medium were refreshed every three days. After 2 weeks of differentiation, Oil Red-O staining was used for detection of accumulated oil droplets. And the specific makers of adipogenic cell including lipoproteinlipase (LPL) and Peroxisome Proliferator Activated Receptor-γ (PPAR-γ) were detected by RT-PCR analysis.
Osteogenic differentiation of sheep MDSCs
The cells of passage 3 were divided into two groups: the induced group and the control group. When cultures reached 50%-60% confluence, cells of the induced group were incubated in osteogenic medium containing 0.1 mM dexamethasone (Sigma), 10 mM β-glycerophosphate (Sigma), and 50 mg/L vitamin C 1% FBS and 10% HS. Meanwhile, cells of the control group were cultured in complete medium without any inducer. The medium was refreshed every three days. After 2 weeks of differentiation, Alizarin Red staining was used to detected the presence of calcium node formation and the osteoblast- specific gene containing osteopontin (OPN), alkaline phosphatase (ALP) and collagen type I (COLI) were analyzed by RT-PCR.
Cartilaginous differentiation of sheep MDSCs
Passage 3 MDSCs were reseeded in the six orifice at the density of 3×104 cells/mL, when the confluence degree reached 70-80%, the cells spanided into inducted and control groups. Cells of the induced group were incubated in cartilaginous medium containing: 1% ITS, 50µg/mL L-proline, 0.1µM dexamethasone 0.9mM sodium pyruvate, 50µg/mL ascorbate and 10ng/mL transforming growth factor-beta3 (TGF-β3). Cells in the control group were cultured in complete medium. And the cell medium was changeed every 2 days. About three weeks later, Alcian blue staining was used to detected the presence of cartilage condensations (Bian et al., 2013) and The osteoblast-specific gene containing collagen type II (COLII), Sry related HMG box-9 (SOX-9), collagen type X (COL- X) and aggrecan were analyzed by RT-PCR.
Neuron-like cell differentiation of sheep MDSCs
The MDSCs were seeded in the 6-well plates for neuron-like cell differentiation, and the cells were separated into two groups: inducted and control groups. When the confluence of cells reached 70%, the medium was changed. After the cells were washed three times, which were incubated in neuron-like cell inducted medium containing pre induced liquid: 2% B27, 2mM glutamine, 40ng/mL bFGF, 20ng/mL EGF. This stage lasted for 6 days. induced liquid: pre induced liquid, 1% N2, 50μg/mL Vc, 10ng/mL GDNF and the stage lasted about two weeks (Yang et al., 2012). Cells in the control group were cultured in complete medium. And the cell medium was changed every 3 days. About 21 days later, the inducted and control cells were used to detect neuron-like cell markers including neuron-specific enolase(NSE) and neurofilament (NF). These were detected through immunofluorescence.
Results
Morphology observation of sheep MDSCs
We have observed that different populations of primary MDSCs isolated from hindlimb muscle using preplate technique. The isolated MDSCs were seeded in the 60 mm petri plate, The PP1-PP3 mostly were fibroblast-lik and myoblasts, by and large PP4 and PP5 were satellite cells, PP6 were main MDSCs but that have a small amount satellite cells. Various shapes of cells began to adhere to the culture plates, the cell type is in homogenous.The Most cells were round, there are a small number of shuttle type. The cells proliferated quickly, displaying obvious karyokinesis, after about 3 days, the cells confluence degree could reach 80-90%, then, the cells were digested by 0.125% trypsin and 0.02% EDTA. The passage cells began to adhere after 1 h and completely adhered after 12 h. The passage cells tachyauxesis, and the proportion of round-shaped cells also increased gradually. After three passages, the MDSCs were purified almost completely, and the shape was also equably. The cells were subcultured to passage 44 (Fig. 1), when the cells of passage 40 displayed representative senescent appearance as vacuole, tabular shape and karyopyknosis in most cells, and the generation time increased with the increase of passage.
Cryogenic preservation and resuscitation
The P3, P15, P27 and P40 generation cells were selected were frozen and recovery. P3, P15 and P27 no significant difference in the cell growth and morphology. Upon reaching P40, most cells displayed features representative of senescence such as blebbing and karyopyknosis. After resuscitation, the cells began to adhere at 30 h and grew slowly thereafter. But im allgemeinen there was no significant difference in growth and morphology between cells before and after cryopreservation (Table II).
Table II.- The viability of MDSCs before and after cryopreservation was determined using the Trypan blue exclusion test.
|
Category |
P3(%) |
P15(%) |
P27(%) |
P40(%) |
|
Before frozen |
96.56 |
97.68 |
94.34 |
91.2 |
|
After reviving |
93.2 |
94.38 |
90.63 |
85.47 |
The P3, P15 and P27 no significant difference in the cell growth and morphology. Upon reaching P40, most cells displayed features representative of senescence such as blebbing and karyopyknosis. But im allgemeinen there was no significant difference in growth and morphology between cells before and after cryopreservation.
Colony-forming cell assay
Colony formation was observed after cultured for 7 days by microscopy. The CFU were 50.57 ± 4.03, 52.38 ±5.30, 46.23 ± 1.43, 39.60 ± 1.65 for passage 3, passage 15, passage 27 and passage 40, respectively (Fig. 2), indicating the ability of cultured MDSCs for self-renewal.
Growth kinetics
Viability, detected using cell hemocytometer and trypan blue exclusion test of P3, P15, P27 and P40 was more than 97.68 % and dropped slightly to 85.47 % on average after recovery. Growth and proliferation of the sheep MDSCs was similar at P3, P15, P27 and P40 according to the growth curves (Fig. 3). After the latency phase of 1-3 days, cell growth entered the logarithmic phase, and reached plateau phase at about day 7. The population doubling time (PDT) were approximately 28.62 h, 35.85 h, 34.39 h and 43.34 h for passage 3, passage 15, passage 27, and passage 45, respectively on the base of the growth curve, the mean time of PDT was 35.55 h. The sheep MDSCs displayed a strong and steady proliferative potential when cultured in vitro.
Identification of sheep MDSCs
Reverse transcription PCR(RT-PCR) and immunofluorescence
We analyzed five gene expressions of the sheep MDSCs by RT-PCR. The five genes, included Sca-1, CD34, CD144, Desmin and CD45 these were all specific makers of sheep MDSCs. Experimental results showed that the sheep MDSCs were positive for Sca-1, CD34, CD144 and Desmin (Fig. 4). GADPH was used as an internal control.
RT-PCR revealed that sheep MDSCs expressed Sca-1, CD34, CD144, Desmin, but not CD45. The average positive rate of Sca-1, CD34, CD144 and Desmin was different in four passages (Table III), indicating that different passages had differential expression of surface markers. But the differences were non-significant (P>0.05). The immunofluorescence assays consistently showed that sheep MDSCs were Sca-1, CD34, CD144, Desmin positive and CD45 negative (Fig. 5).
Karyotype analysis
The karyotype of sheep MDSCs was evaluated. The chromosome number of sheep is 2n=54, including 26 pairs of euchromosomes and one pair of sex chromosomes, XX (♀)/XY (♂) (Fig. 6). The chromosome numbers per spread were counted for 100 spreads of the passage 3, passage 15, passage 27 and passage 40, and the ratio of cells with 2n=54 were 93.5%, 92.6% and 91.3%, 90.3%, respectively, implying that the cultured cells possessed of genetic stability. The results also demonstrated that the chromosome number of individual cells tended to alter. And in vitro culture affected the hereditary property of cells slightly, but the evidence showed that the cell line was reproducibly diploid (Liu et al., 2014).
Table III.- Surface marker expression of sheep MDSCs.
|
Sca-1(%) |
CD34(%) |
CD144(%) |
Desmin(%) |
CD45(%) |
|
|
P3 |
91.2±0.3 |
86.2±2.5 |
68.9±1.2 |
67.9±1.8 |
(-) |
|
P15 |
92.8±1.2 |
83.4±1.8 |
69.3±1.5 |
67.3±2.1 |
(-) |
|
P27 |
92.3±1.6 |
82.9±0.6 |
67.2±0.8 |
68.1±0.6 |
(-) |
|
P40 |
90.8±2.3 |
80.1±2.1 |
66.8±0.3 |
66.4±1.1 |
(-) |
Adipogenic differentiation of sheep MDSCs
Adipogenic differentiation of the sheep MDSCs was demonstraed by positive Oil Red O staining. After incubation in adipogenic medium for 2 weeks, the MDSCs changed their morphology from spindle cell to oblate, and there were many lipid droplets in the cells (Fig. 7A-C). The number of droplets increased in a time-dependent manner, and tiny lipid droplets aggregated to form larger ones (Fig. 7A-E, G). Control cells cultured in complete medium through the culture process were not stained by Oil Red O (Fig. 7A-D, F, H).
RT-PCR indicated that after incubation with isobutylmethylxanthine, dexamethasone, insulin and indometacin the adipocyte specific genes LPL and PPAR-γ were detected, and which did not occur in the control group (Fig. 7B). In addition, we could draw a conclusion that the successful differentiate depended to upregulation of LPL and PPAR-γ gene expression. Above all, MDSCs can be induced to differentiate into adipogenic cells in vitro successfully.
Osteogenic differentiation of sheep MDSCs
After incubation in osteogenic medium for 14 days, morphological changes of the MDSCs were obvious. The cells changed from fusiform to tridimensional and then aggregated and formed miner- alized nodules with increasing incubation time. Further, the nodules were Alizarin Red staining positive (Fig. 8A-C). In addition, as a result of the continuing effects of the inducers, the nodules increased and grew in size (Fig. 8A-E, G). The cells cultured in complete medium were not changed in morphology or stained by Alizarin Red (Fig. 8A-B, D, F, H).
Osteogenic differentiation of the MDSCs was analyzed by RT-PCR. The specific genes, including OPN, ALP and COLI, were all detected in the induced group, in sharp contrast to that shown in the control group (Fig. 8B).
Cartilaginous differentiation of sheep MDSCs
The ability of sheep MDSCs to differentiate into cartilaginous cells was detected, and the morphological and phenotypic analysis were carried on for the induced cells. The cell morphology was changed at three weeks after induction, the cells became compressed (Fig. 9A-C)
and were positive for Alcian blues taining (Fig. 9A-E). However, the cells which were cutured after 1 d, 14 d, 21 d in control group did not show the above effects (Fig. 9A-B, D, F).
The result of cartilaginous differentiation of MDSCs was also analyzed by RT-PCR. Osteogenic-specific genes COLII, SOX9, COL-X and aggrecan were expressed in the induced group, but not in the control group (Fig. 9B).
Neuron-like cell differentiation of sheep MDSCs
The capacity for sheep MDSCs to differentiate into neuron-like cells was tested by induction with induction medium, the morphological and phenotypic difference were observed for induced cells. After incubation in neural differentiation medium for 21 days, MDSCs exhibited neuritis from elongated shape. While, there were no obvious morphological change in the control group.
Furthermore, neuron-like cell specific makers were detected using immunofluorescence. The neuron-like cell markers NSE and NF were expressed by immunofluorescence (Fig. 10A) and RT-PCR (Fig. 10B). However, the cells in the control group did not express the neuron-like cell specific makers. These results indicate that MDSCs can differentiate into neuron-like cell.
Discussion
Development and maintenance of an abundant tissue such as skeletal muscle poses several challenges. Curiously, not all skeletal muscle stem cells are born alike, since diverse genetic pathways can specify their birth. Stem and progenitor cells that establish the tissue during development, those that maintain its homeostasis, as well as participate in its regeneration have generated considerable interest. The ability to distinguish stem cells from more committed progenitors throughout prenatal and postnatal life has guided researchers to identify stem cell properties and characterize their niche. These properties include markers that influence cell behaviour and mode of division during normal development, after trauma and cell transplantations (Sambasivan and Tajbakhsh, 2007).
In this study, we has isolated a population of sheep skeletal muscle-derived stem cells (MDSCs), characterized utilizing a variety of stem cell markers (e.g., Sca-1, CD34, CD144 and Desmin). MDSCs were cultured successfully in vitro and kept high activity for at least 44 passages. In addition, according to the regular program, the biological property of MDSCs containing growth dynamics, karyotype analysis, the detection of specific markers and differentiation potentiality were performed on respectively. These cells have been shown to be able of circumventing some of the limitations seen with myoblasts and could prove to be a superior alternative to myoblasts for the regeneration and repair of skeletal, cardiac and smooth muscles (Payne et al., 2007).
The preplate technique described herein isolates various populations of muscle cells from the skeletal muscle of sheep on the basis of their adhesion characteristics to type I collagen-coated flasks. The first cells to adhere during the early stages of the preplate technique within minutes to hours of seeding are known as RACs and have been shown by our group to be comprised of mostly fibroblastic-like and myoblast cells. Other populations of SACs, containing MDSCs, are obtained in later preplates within several days of seeding. The MDSCs population was characterized using immunostaining for certain stem cell markers in vitro and also by FACS. The MDSCs are distinct from satellite and other progenitor manipulation is critically important for translational research in medicine and will greatly facilitate clinical trials using autologous muscle biopsies for regenerative medicine (Sarig et al., 2006).
The ability of MDSCs to proliferate in vivo for an extended period of time combined with their cell survival, self-renewal and strong capacity to undergo multilineage differentiation. Results showed that MDSCs were differentiated into adipocytes, osteoblasts, chondrocytes and neuron-like cell, and then examined relevant gene expression of these cell types. Different inducing factors have different functions for cell differentiation. IBMX, indometacin and insulin were used to induce MDSCs to differentiate into adipocytes; β-glycerophosphate and dexamethasone were used to induce MDSCs to differentiate into osteoblasts; TGF-β3 L-proline and sodium pyruvate were used to induce MDSCs to differentiate into chondrocytes; GDNF, bFGF, EGF, B27 and N2 were used to induce MDSCs to differentiate into neuron-like cell. And the roles of the inducing factors mainly affected the cell signaling pathways, some of the genes expressing that are over-active or under-active are changed. In addition, MDSCs can carry out the cross layer differentiation, MDSCs derived from mesoblastema can be induced to differentiate into mesodermal and ectodermal cells.
Skeletal muscle, like skin, is found throughout the body of the organism. The above results suggested that sheep MDSCs had not only a strong self-renewal ability, but also had the potential to differentiate towards mesodermal and ectodermal cells. Therefore, MDSCs have become an ideal cell source in tissue engineering and clinical application.
Conclusions
In conclusion, an optimal method for isolation and culture of MDSCs was established in this study and cell morphology, surface markers and biological characteristics were observed and detected. We also demonstrate the pluripotent potential of MDSCs and can be induced to differentiate into adipocytes, osteoblasts, chondrocytes, and neuron-like cell. These results have not only provided a technological platform for the establishment of a sheep MDSCs line, but also proposes a new method to preserve the valuable genetic resources of livestock and poultry.
Acknowledgments
This research was supported by the Agricultural Science and Technology Innovation Program (ASTIP) (cxgc-ias-01); Project supported by the National Natural Science Foundation of China (Grant No. 31201765; 31272403; 31472064); Supported by the earmarked fund for Modern Agro-industry Technology Research System (nycytx-40-01); the project National Infrastructure of Animal Germplasm Resources (2014 year).
Statement of conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this article.
References
Ambrosio, F., Ferrari, R.J., Fitzgerald, G.K., Carvell, G., Boninger, M.L. and Huard, J., 2009. Functional overloading of dystrophic mice enhances muscle-derived stem cell contribution to muscle contractile capacity. Arch. Phys. Med. Rehab., 90: 66-73. https://doi.org/10.1016/j.apmr.2008.06.035
Arsic, N., Mamaeva, D., Lamb, N.J. and Fernadez, A., 2008. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp. Cell Res., 314: 1266-1280. https://doi.org/10.1016/j.yexcr.2008.01.009
Bai, C.Y., Li, C.Y., Jin, D.P., Guo, Y., Guan, W.J., Ma, Y.H. and Zhao, Q.J., 2010. Establishment and characterization of a fibroblast line from landrace. Artificial Cells Blood Substitutes Biotechnol., 38:129-135. https://doi.org/10.3109/10731191003670525
Baran, S.W. and Ware, C.B., 2007. Cryopreservation of rhesus macaque embryonic stem cells. Stem Cells Dev., 16: 339-344. https://doi.org/10.1089/scd.2007.900-de
Bellayr, I.H., Gharaibeh, B., Huard, J. and Li, Y., 2010. Skeletal muscle-derived stem cells differentiate into hepatocyte-like cells and aid in liver regeneration. Int. J. clin. exp. Pathol., 3: 681-690.
Bian, L.M., Guvendiren, M., Mauck, R.L. and Burdick, J.A., 2013. Hydrogels that mimic developmentallyrelevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. natl. Acad. Sci. USA, 110: 10117-10122. https://doi.org/10.1073/pnas.1214100110
Claros, S., Alonso, M., Becerra, J., Andrades, J.A., 2008. Selection and induction of rat skeletal muscle-derived cells to the chondro-osteogenic lineage. Cell. mol. Biol., 54: 1-10.
Danisovic, L., Varga, I., Polak, S., Ulicna, M., Bohmer, D. and Vojtassak, J., 2008. Morphology of in vitro expanded human muscle-derived stem cells. BioMed Pap., 152: 235-238. https://doi.org/10.5507/bp.2008.036
Deasy, B.M., Gharaibeh, B.M., Pollett, J.B., Jones, M.M,. Lucas, M.A., Kanda, Y. and Huard, J., 2005. Long-term self-renewal of postnatal muscle-derived stem cells. Mol. Biol. Cell, 16: 3323-3333. https://doi.org/10.1091/mbc.E05-02-0169
Deasy, B.M., Jankowski, R.J. and Huard, J., 2001. Muscle-derived stem cells: Characterization and potential for cell-mediated therapy. Blood Cells Mol. Dis., 27: 924-933. https://doi.org/10.1006/bcmd.2001.0463
Gharaibeh, B., Lu, A., Tebbets, J., Zheng, B., Feduska, J., Crisan, M., Peault, B., Cummins, J. and Huard, J., 2008. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat. Protoc., 3: 1501-1509. https://doi.org/10.1038/nprot.2008.142
Gussoni, E., Soneoka, Y., Strickland, C.D., Buzney, E.A., Khan, M.K., Flint, A.F., Kunkel, L.M. and Mulligan, R.C., 1999. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature, 401: 390-394. https://doi.org/10.1038/43919
Jankowski, R.J., Deasy, B.M. and Huard, J., 2002. Muscle-derived stem cells. Gene Therap., 9: 642-647. https://doi.org/10.1038/sj.gt.3301719
Kuroda, R., Usas, A., Kubo, S., Corsi, K., Peng, H.R., Rose, T., Cummins, J., Fu, F.H. and Huard, J., 2006. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheumatol., 54: 433-442. https://doi.org/10.1002/art.21632
Kwon, D., Kim, Y., Pruchnic, R., Jankowski, R., Usiene, I., De Miguel, F., Huard, J. and Chancellor, M.B., 2006. Periurethral cellular injection: Comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology, 68: 449-454. https://doi.org/10.1016/j.urology.2006.03.040
Levy, M.M., Joyner, C.J., Virdi, A.S., Reed, A., Triffitt, J.T., Simpson, A.H.R.M., Kenwright, J., Stein, H. and Francis, M.J.O., 2001. Osteoprogenitor cells of mature human skeletal muscle tissue: An in vitro study. Bone, 29: 317-322. https://doi.org/10.1016/S8756-3282(01)00585-3
Liu, Z.Z., Wang, W., Gao, J.F., Zhou, H.M. and Zhang, Y.R., 2014. Isolation, culture, and induced multiple differentiation of Mongolian sheep bone marrow-derived mesenchymal stem cells. In Vitro Cell. Dev. Anim. Biol., 50: 464-474. https://doi.org/10.1007/s11626-013-9725-y
Lu, S.H., Yang, A.H., Wei, C.F., Chiang, H.S. and Chancellor, M.B., 2010. Multi-potent differentiation of human purified muscle-derived cells: Potential for tissue regeneration. BJU Int., 105: 1174-1180. https://doi.org/10.1111/j.1464-410X.2009.08823.x
Matsumoto, T., Cooper, G.M., Gharaibeh, B., Meszaros, L.B., Li, G.G., Usas, A., Fu, F.H. and Huard, J., 2009. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheumatol., 60: 1390-1405. https://doi.org/10.1002/art.24443
Oztopcu-Vatan, P., Kus, G., Inan, E., Korkut, M.G., Kabadere, S. and Uyar, R., 2017. Licofelone inhibits proliferation of rat hepatoma cells. Pakistan J. Zool., 49: 811-817. http://dx.doi.org/10.17582/journal.pjz/2017.49.3.811.817
Payne, T.R., Oshima, H., Okada, M., Momoi, N., Tobita, K., Keller, B.B., Peng, H. and Huard, J., 2007. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J. Am. Coll. Cardiol., 50: 1677-1684. https://doi.org/10.1016/j.jacc.2007.04.100
Qu-Petersen, Z.Q., Deasy, B., Jankowski, R., Ikezawa, M., Cummins, J., Pruchnic, R., Mytinger, J., Cao, B.H., Gates, C., Wernig, A. and Huard, J., 2002. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J. Cell Biol., 157: 851-864. https://doi.org/10.1083/jcb.200108150
Sambasivan, R. and Tajbakhsh, S., 2007. Skeletal muscle stem cell birth and properties. Semin Cell Dev. Biol., 18: 870-882. https://doi.org/10.1016/j.semcdb.2007.09.013
Sarig, R., Baruchi, Z., Fuchs, O., Nudel, U. and Yaffe, D., 2006. Regeneration and trans-differentiation potential of muscle-derived stem cells propagated as myospheres. Stem Cells, 24: 1769-1778. https://doi.org/10.1634/stemcells.2005-0547
Tamaki, T., Akatsuka, A., Ando, K., Nakamura, Y., Matsuzawa, H., Hotta, T., Roy, R.R. and Edgerton, V.R., 2002. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol., 157: 571-577. https://doi.org/10.1083/jcb.200112106
Tamaki, T., Uchiyama, Y., Okada, Y., Ishikawa, T., Sato, M., Akatsuka, A. and Asahara, T., 2005. Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation, 112: 2857-2866. https://doi.org/10.1161/CIRCULATIONAHA.105.554832
Torrente, Y., Belicchi, M., Marchesi, C., D’Antona, G., Cogiamanian, F., Pisati, F., Gavina, M., Giordano, R., Tonlorenzi, R., Fagiolari, G., Lamperti, C., Porretti, L., Lopa, R., Sampaolesi, M., Vicentini, L., Grimo-ldi, N., Tiberio, F., Songa, V., Baratta, P., Prelle, A., Forzenigo, L., Guglieri, M., Pansarasa, O., Rinaldi, C., Mouly, V., Butler-Browne, G.S., Comi, G.P., Biondetti, P., Moggio, A., Gaini, S.M., Stocchetti, N., Priori, A., D’Angelo, M.G., Turconi, A., Bottinelli, R., Cossu, G., Rebulla, P. and Bresolin, N., 2007. Autologous transplantation of muscle-derived CD133(+) stem cells in Duchenne muscle patients. Cell Transplant, 16: 563-577. https://doi.org/10.3727/000000007783465064
Usas, A., Maciulaitis, J., Maciulaitis, R., Jakuboniene, N., Milasius, A. and Huard, J., 2011. Muscle-derived stem cells: Implications for cell-mediated therapies. Med. Lith., 47: 469-479.
Vourc’h, P., Romero-Ramos, M., Chivatakarn, O., Young, H.E., Lucas, P.A., El-Kalay, M. and Chesselet, M.F., 2004. Isolation and characterization of cells with neurogenic potential from adult skeletal muscle. Biochem. Biophys. Res. Commun., 317: 893-901. https://doi.org/10.1016/j.bbrc.2004.03.121
Wu, H.Q., Ren, Y., Li, S., Wang, W., Yuan, J.L., Guo, X.D., Liu, D.J. and Cang, M., 2012. In vitro culture and induced differentiation of sheep skeletal muscle satellite cells. Cell Biol. Int., 36: 579-587. https://doi.org/10.1042/CBI20110487
Wu, X.Y., Wang, S.L., Chen, B.L. and An, X.L., 2010. Muscle-derived stem cells: Isolation, characterization, differentiation, and application in cell and gene therapy. Cell Tissue Res., 340: 549-567. https://doi.org/10.1007/s00441-010-0978-4
Yang, J., Wang, X., Wang, Y., Guo, Z.X., Luo, D.Z., Jia, J. and Wang, X.M., 2012. Dopaminergic neuronal conversion from adult rat skeletal muscle-derived stem cells in vitro. Neurochem. Res., 37: 1982-1992. https://doi.org/10.1007/s11064-012-0819-9
Zheng, B., Cao, B.H., Crisan, M., Sun, B., Li, G.H., Logar, A., Yap, S., Pollett, J.B., Drowley, L., Cassino, T., Gharaibeh, B., Deasy, B.M., Huard, J. and Peault, B., 2007. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol., 25: 1025-1034. https://doi.org/10.1038/nbt1334
To share on other social networks, click on any share button. What are these?










