Isolation of Pseudomonas aeruginosa Phages from Wastewaters
Research Article
Isolation of Pseudomonas aeruginosa Phages from Wastewaters
Stephen Chijioke Emencheta1, Chinelo Charity Eze1, Anthony Amaechi Attama2, Damian Ejike Agbo1 and Ebele Benedette Onuigbo1*
1Department of Pharmaceutical Microbiology and Biotechnology, University of Nigeria, Nsukka, 410001, Nsukka, Enugu State, Nigeria; 2Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Nsukka, Enugu State, Nigeria.
Abstract | This research was designed to isolate and test the lytic action of a bacteriophage specific to Pseudomonas sp. Both Pseudomonas spp. and bacteriophages were isolated from residential wastewaters. The isolated Pseudomonas spp. were confirmed through biochemical tests. Antibiogram was done with nine (9) different antibiotics, including;aztreonam (ATM), chloramphenicol (CPL), gentamicin (GTN), tetracycline (TTE), sulphamethoxazole/trimethoprim (SXT), amoxicillin/clavulanic acid (AMC), meropenem (MEM), ciprofloxacin (CIP), ceftriaxone (CRO)). Plaque assay was done to determine lytic action of the bacteriophage on the lawns of the Pseudomonas sp. The host range of the isolated bacteriophages was also assessed. Cultural characteristics and biochemical tests confirmed the Pseudomonas aeruginosa isolates. The bacterial isolates were sensitive to only ciprofloxacin. The Multi Antibiotic Resistance Indexes (MARI) of the isolated strains were greater than 0.8. The formation of plaques (clear zone) on the lawn of Pseudomonas spp confirmed lytic action. The bacteriophage showed plaques on Pseudomonas sp. isolated from other sites also. The isolation of Pseudomonas aeruginosaphages from residential wastewaters is a promising molecular tool in combating the global antimicrobial resistance threat.
Received | December 23, 2021; Accepted | December 26, 2021; Published | December 28, 2021
*Correspondence | Ebele Benedette Onuigbo, Department of Pharmaceutical Microbiology and Biotechnology, University of Nigeria, Nsukka, 410001, Nsukka, Enugu State, Nigeria; Email: [email protected]
DOI | https://dx.doi.org/10.17582/journal.hv/2021/8.6.1.6
Citation | Emencheta, S.C., C.C. Eze, A.A. Attama, D.E. Agbo and E.B. Onuigbo. 2021. Isolation of Pseudomonas aeruginosa phages from wastewaters. Hosts and Viruses, 8(6): 1-6.
Keywords: Bacteriophage, Pseudomonas aeruginosa, Plaques, Lytic, Antibiotic, Resistance
Introduction
On the 13th of October 2020, the World Health Organization (WHO) announced that one of the top ten global health threats is antimicrobial resistance (AMR) (WHO, 2020). It has been forewarned that by 2050 antibiotic resistance will be responsible for the death of 10 million people (Sharma et al., 2021). In another study, AMR was projected to drain the global economy of US$100 trillion in the same period (Mogasale et al., 2021). Among the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumanii, Pseudomonas aeruginosa, Enterobacteriaceae) pathogens, Pseudomonas aeruginosa has been classified as one of the critical priority pathogens (WHO, 2017). Pseudomonas aeruginosa, a frontline nosocomial pathogen is a motile, aerobic, Gram-negative rod that produces water-soluble fluorescent yellow and blue pigments. It is versatile and is found widely in soil, water, plants, and animals. It is implicated in about 18-61 % of deaths in hospitals especially in immunocompromised patients (Zhang et al., 2020). Pseudomonas aeruginosa uses different adaptive metabolic pathways, quorum sensing to regulate virulence factor production, resistance, and the formation of biofilms (Diggle and Whiteley, 2020). It is the leading proven cause of mortality in cystic fibrosis patients. Recent trends of failures of first-line drugs in the treatment of Pseudomonas aeruginosa infection have led to the re-exploration of bacteriophages as an alternative in humans as well as in animals. Bacteriophages (phages) are natural eaters of bacteria and are ubiquitous in the environment. The use of host-specific bacteriophages has been promoted as a cost-effective and adaptable approach to control bacteria (Attama et al., 2017). Phages have unique advantages over antibiotics. They replicate only on the specific strain of bacteria, avoiding the imbalance of commensal gut flora (dysbiosis) often caused by broad-spectrum antibiotics. Additionally, they only replicate as long as the targeted bacterium is present and so are naturally self-limiting (Attama et al., 2017). The first step in a bacteriophage lytic cycle is the adsorption of the phage on the specific cell surface receptor of the bacteria. This attachment determines the phage host range. Some phages of Gram-negative bacteria interact with various specific lipopolysaccharides (LPS, endotoxin) components, some with outer membrane proteins (OMP), and others have complex adhesions that recognize specific receptors (Brzozowska et al., 2018; Kulikov et al., 2019). In this research, we attempted to isolate Pseudomonas aeruginosa bacteriophages in residential areas for further characterization.
Materials and Methods
Study area and sample collection
The study was conducted in Nsukka, Enugu State, Nigeria. Residential wastewater was obtained from residences in Nsukka community. A total of fifty-eight samples were collected from different residences over a period of three weeks. A 10 ml sample was collected in sterilized Bijou bottles and taken to the research laboratory for bacteriological analysis. Samples were randomly collected.
Isolation of Pseudomonas sp. from the wastewater
The wastewater samples were diluted 10-fold with distilled water. A 0.1 ml of the diluted samples was dropped at the center of different Petri dishes containing MacConkey agar. A spreader was used to spread the sample on the surface under aseptic conditions. The inoculated plates were incubated at 37 oC for 24 h in an autoclave.
Purification of isolated Pseudomonas sp.
The overnight culture was purified by picking a colony from each plate and sub-culturing using the streak plate method on MacConkey agar and incubating for 24 h at 37 oC. Distinct colonies from each colony were then sub-cultured on cetrimide agar and incubated for 24 h at 37 oC to identify the Pseudomonas sp. Pure Pseudomonas sp. isolates were then stocked in double strength nutrient agar pending further tests.
Biochemical tests
Citrate and Oxalase tests were carried out on 24 h broth culture to confirm the presence of Pseudomonas aeruginosa.
Antibiogram of pseudomonas isolates
Suspension of the bacterial culture was swabbed on the surface of the Mueller Hinton agar plates with the aid of sterile swab sticks and the antibiotic disks (aztreonam (ATM), chloramphenicol (CPL), gentamicin (GTN), tetracycline (TTE), sulphamethoxazole/ trimethoprim (SXT), amoxicillin/clavulanic acid (AMC), meropenem (MEM), ciprofloxacin (CIP), ceftriaxone (CRO)) were placed on the surface of the swabbed Mueller Hinton agar plates and was incubated at 37 oC for 24 h. The zone of inhibition was measured and recorded.
Multiple antibiotic resistance index (MARI)
Multiple Antibiotic Resistance Index (MARI) was determined using the procedure as described by Krumperman (1983). A MARIfor an isolate was calculated as:
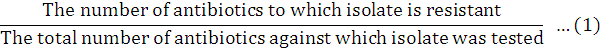
Isolation of bacteriophages
The Pseudomonas spp. phages were isolated from the residential wastewater by centrifuging at 15,000 rpm for 10 min to separate the phage from other particles. The supernatant was filtered through 0.45µm syringe filter units. The filtrates were stored in the freezer at the appropriate temperature.
Phage enrichment and filtration
The pure stocked isolates of Pseudomonas sp. were inoculated into 10 ml nutrient broth and incubated overnight. For the enrichment, the reaction mixture in a sterile test tube consisted of 0.4 ml of the overnight broth culture, 0.5 ml of 5x Luria Bertani (LB) broth, 0.04 ml calcium chloride (CaCl2), and 1.2 ml of the phage filtrate. They were incubated for 48 h. After incubation, the mixture was centrifuged for 15 min at 15,000 rpm. The supernatant was filtered using 0.45µm syringe filter units and the filtrates were stored at 4 oC.
Spotting and plaque assay
A suspension of the 24 h bacterial culture, adjusted to 0.5 McFarland was swabbed on the surface of Mueller-Hinton agar plates. A drop of the enriched phages was spotted on the lawn of the bacterial isolate. The plates were incubated at 37 oC for 24 h. After incubation, the plates were observed for the presence of plaques. A sterile needle was used to pick the plaques containing the phages after which they were put inside SM buffer and stored at 4 oC.
Phage purification
A 0.5 ml of 5x LB broth was prepared in a sterile bottle. 1.2 ml of the stored phages, 0.04 ml of sterile CaCl2, and 0.4 ml of overnight broth culture of the bacteria were added into the sterile bottle. The mixture was incubated for 48 h. After incubation, the medium was centrifuged at 15,000 rpm for 20 min. The supernatant was collected and passed through the membrane syringe filter under aseptic conditions. This was repeated two more times. The filtrate was put into SM buffer and adequately stored at 4 oC.
Host range of the phage
The host range of the phages was determined based upon the ability to form plaques on the lawns of eight (8) isolates of P. aeruginosa. For this, a suspension of the 24 h P. aeruginosa cultures, adjusted to 0.5 McFarland was swabbed on the surface of Mueller-Hinton agar plates. A drop aliquot of the phage isolates was spot inoculated onto each P. aeruginosa lawn. After the spots were dried the plates were incubated in an inverted position for 24 h at 37 oC. The presence of plaques was checked after 24 h incubation.
Results and Discussion
Fifty-eight samples were collected from different residential wastewater. Eight pure isolates of Pseudomonas sp. on cetrimide agar showed fluorescent yellow or greenish-blue pigmented colonies, respectively. The colonies had a sweet smell. The biochemical tests were catalase and oxidase-positive with no gas production in all eight isolates. Identification was based on the colonial morphology, oxidase positivity, the presence of blue-green or yellow pigments. P. aeruginosa is common in soils and water, especially in urban and rural communities with congested living conditions. It has been found also in deionized and distilled water due to its simple nutritional requirements and versatile adaptation mechanisms. This adaptation mechanism enables them to withstand oxidative, heat, and salt stress as well as equips them with virulence and resistance factors (Moradali et al., 2017). Sinks, water baths, showers, hot tubs, and other wet areas need to be decontaminated regularly since Pseudomonas thrives in moist environments especially in houses inhabited by persons with low immunity. The isolated strains of P. aeruginosa were screened against aztreonam (ATM), chloramphenicol (CPL), gentamicin (GTN), tetracycline (TTE), amoxicillin/clavulanic acid (AMC), sulphamethoxazole/ trimethoprim (SXT), ciprofloxacin (CIP), meropenem (MEM), and ceftriaxone (CRO). There was 100 percent susceptibility of the strains to ciprofloxacin (CIP), according to (CLSI, 2020) as shown in Table 1. CLSI (2020) states that antibiotics with a minimum inhibitory concentration greater than 1 µg/ml and with an inhibition zone diameter less than 7 mm show resistance. However, there was 100 percent resistance to amoxicillin/ clavulanic acid, meropenem, ceftriaxone, and sulphamethoxazole/ trimethoprim. This shows that these isolates are from high-risk sources of antibiotic resistance. This observed resistance of Pseudomonas aeruginosa from different residential wastewater to all the above antibiotics including gentamicin may have occurred through the production of multicellular biofilms making it difficult for targeted antibiotics to act. It has also been reported that the MexAB-OprM efflux pump of Pseudomonas aeruginosa has the widest antibiotic specificity and confers resistance to macrolides, aminoglycosides, sulfonamides, fluoroquinolones, tetracyclines, and many β-lactams (Poole, 2001). The deletion of the outer membrane protein has been associated with increased resistance to imipenem and meropenem. Most recent studies have shown that methylation of 16S rRNA confers high-level resistance against aminoglycosides in P. aeruginosa (Hirsch and Tam, 2010). The overexpression of chromosomally encoded cephalosporinase, AmpC is equally prevalent in P. aeruginosa. Multiple antibiotic resistance index (MARI) helps analyze health risks, as well as check the antibiotic resistance. Organisms that have MARI greater than 0.2 confirm the presence of multidrug-resistant genes originating from the environment where there is an abuse of these drugs. Also, that the plasmids contain one or more resistance genes each encoding a single antibiotic resistance phenotype (Afunwa et al., 2020). The widespread of multidrug-resistant Pseudomonas aeruginosa in the community reveals that there is a proliferation of patent vendors illegally and unprofessionally prescribing and dispensing antibiotics. This resistance to frontline antibiotics could also be connected to indiscriminate use of antibiotics in poultry breeding and the subsequent consumption of such poultry may have transferred the resistance to humans (Afunwa et al., 2020). This is worrisome because the multidrug-resistant isolates were from residential areas and not from the hospitals. There is then a serious need for antimicrobial stewardship for fostering and monitoring judicious use of antimicrobials to preserve their future effectiveness (Rahman et al., 2020; Murray, 2020).
Following phage enrichment and purification, two phages, δ274 and Ψ102 were isolated from a total of fifty-eight residential samples. Only bacteriophages from residential (R3) and (R4) wastewaters showed distinct plaques on the bacterial lawn of the Pseudomonas aeruginosa isolates with approximately 5±0.42 mm diameter. Some of the phages present in the wastewaters were possibly temperate phages. In many lytic phages, host lysis is achieved by single-gene lyses protein or through the holin-endolysin system (Attama et al., 2017). Phages can also produce enzymes when biofilms are present to dissolve the biofilm matrix produced by P. aeruginosa (Holger et al., 2021). Gupta and Prasad (2011) successfully purified an endolysin from phage P-27/HP and showed its ability (about 99.9%) to decolonize the spleens of treated mice from S. aureus 27/HP. Witzenrath et al. (2009) demonstrated the therapeutic potential of endolysin Cp-l in the systemic treatment of fatal S. pneumoniae-induced pneumonia in mice. Some plaques showed regrowth of the bacteria which may be a sign of growing resistance. Nevertheless, the discovery of phages in the same wastewater in which the MDR Pseudomonas aeruginosae were found shows the enormous potentials of bacteriophages, though, requires further purification and standardization (Qin et al., 2020; Petrovic and Lin, 2020; Patil et al., 2021; El-Haddad et al., 2019). This is a welcome development in drug development research especially in this time of AMR threat.
Based on the lytic spectra data of δ274 and Ψ102 screened against eight different Pseudomonas aeruginosa isolates from the abattoir and residential wastewaters and done in quadruplicates, there were differences in the number of distinct plaques as shown in Table 2. These differences in the number of plaques against specific Pseudomonas strains for δ274 and Ψ102 could be attributed to their different phenotypes, hence the need to characterize the phages. Though, for phage therapy to be useful, it has to be done under in-vivo conditions mimicking real-life situations and challenging the models with MDR isolates. In-vitro tests may not be a reliable indicator of its activity in-vivo. It has been said that phages are inhibited by blood, serum, bile, white cells, and tissue debris. Ψ102 was ineffective against RES 144 and ABA 41 which could have resulted from a failure to be adsorbed to the cell surfaces of the Pseudomonas strains. This is usually determined by the host density in the environment and the adsorption rate constant of the phage. A way to solve this setback is the use of phage cocktails, as this widens the range of action (Domingo-Calap and Delgado-Martinez, 2018).
Table 1: Antibiogram showing inhibition zone diameter (IZD) of different antibiotic disks.
|
Pseudomonasaeruginosa strain from residential wastewater |
Inhibition zone diameter (mm) |
||||||||
|
ATM 30 µm |
CPL 30 µm |
GTN 10 µm |
TTE 30 µm |
SXT 25 µm |
AMC 30 µm |
MEM 10 µm |
CIP 5 µm |
CRO 30 µm |
|
|
BS201 |
12(R) |
5(R) |
4(R) |
0(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS221 |
10(R) |
5(R) |
5(R) |
2(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS432 |
7(R) |
8(R) |
5(R) |
10(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS453 |
11(R) |
8(R) |
6(R) |
2(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS442 |
3(R) |
2(R) |
5(R) |
3(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS113 |
10(R) |
11(R) |
10(R) |
9(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS151 |
11(R) |
5(R) |
10(R) |
1(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
|
BS376 |
12(R) |
12(R) |
9(R) |
6(R) |
0(R) |
0(R) |
0(R) |
24(S) |
0(R) |
Key: R: Resistant; I: Intermediate; S: Sensitive; ATM: aztreonam; CPL: chloramphenicol; GTN: gentamicin; TTE: tetracycline; SXT: sulphamethoxazole/ trimethoprim; AMC: amoxicillin/ clavulanic acid; MEM: meropenem; CIP: ciprofloxacin; CRO: ceftriaxone.
Table 2: Lytic spectra of two bacteriophage isolates determined on eight Pseudomonas host strains from the abattoir and residential wastewaters.
|
P. aeruginosa strains |
Lysis by bacteriophages |
|
|
δ274 |
Ψ102 |
|
|
ABA5 |
++ |
+ |
|
ABA1 |
+ |
+ |
|
RES141 |
++ |
++ |
|
RES144 |
++ |
- |
|
ABA31 |
++ |
++ |
|
ABA41 |
++ |
- |
|
ABA42 |
+ |
+++ |
|
ABA61 |
+ |
++ |
Key: + number of plaques.
Conclusions and Recommendations
The phages isolated from residential waste waters were specific to the MDR Pseudomonas aeruginosa. The re-emergence of phages as therapeutic tools has attracted and increased the expectations of public health workers in ameliorating the menace of antimicrobial resistance. Further in vitro and in vivo studies will be done to establish the protection of the isolated phages against Pseudomonas sp.
Novelty Statement
The manuscript is original and has not been published in any other journal.
Author’s Contribution
Stephen Emencheta and Damian Agbo did the work under the supervision of Ebele Onuigbo, Chinelo Eze and Anthony Attama.
Conflict of interest
The authors have declared no conflict of interest.
References
Afunwa, R., J. Ezeanyika, E. Afunwa and A. Udeh. 2020. Multiple antibiotic resistance index of gram-negative bacteria from bird droppings in two commercial poultries in Enugu State, Nigeria. Open J. Med. Microbiol., https://doi.org/10.4236/ojmm.2020.104015
Attama, A.A., I.S. Agbo, I.E. Eke, E.B. Onuigbo and J.C. Ogbonna. 2017. Bacteriophage: Clinical applications. In: Mendez-Vilas, A (ed), Antimicrobial research: Novel bioknowledge and educational programs, Ist ed. pp. 260-269.
Brzozowska, E., A. Lesniewski, and J. Niedziolka-Jonsson. 2018. Interactions of bacteriophage T4 adhesin with selected lipopolysaccharides studied using atomic force microscopy. Sci. Rep., 8: 10935. https://doi.org/10.1038/s41598-018-29383-w
Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing. 30th ed., CLSI Supplement M100-S12, Wayne, PA.
Diggle, S., and M. Whiteley. 2020. Microbe profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology, 166(1): 30-33. https://doi.org/10.1099/mic.0.000860
Domingo-Calap, P., and J. Delgado-Martinez. 2018. Bacteriophages: Protagonists of a post-antibiotic era. Antibiotics, 2018: 1-16. https://doi.org/10.3390/antibiotics7030066
El-Haddad, L., C.P. Harb, M.A. Gebara, M.A. Stibich and R.F.A. Chemaly. 2019. Systematic and critical review of bacteriophage therapy againstmultidrug-resistant ESKAPE organisms in humans. Clin. Infect. Dis., 69: 167-178. https://doi.org/10.1093/cid/ciy947
Gupta, R., and Prasad, Y., 2011. P-27/HP endolysin as antibacterial agent for antibiotic resistant Staphylococcus aureus of human infections. Curr. Microbiol., 63: 39-45. https://doi.org/10.1007/s00284-011-9939-8
Hirsch, E.B., and V.H. Tam. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Exp. Rev. Pharmacoecon. Outcomes Res., 1094: 441-451. https://doi.org/10.1586/erp.10.49
Holger, D., R. Kebriaei, T. Morrisette, K. Lev, J. Alexander and M. Rybak. 2021. Clinical pharmacology of bacteriophage therapy: A focus on Multidrug-Resistant Pseudomonas aeruginosa infections. Antibiotics, 10(5): 1-21. https://doi.org/10.3390/antibiotics10050556
Krumperman, P.H., 1983. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol., 46: 165-170. https://doi.org/10.1128/aem.46.1.165-170.1983
Kulikov, E.E., K. Alla, and A.V.L. Golomidova. 2019. High-throughput LPS profiling as a tool for revealing of bacteriophage infection strategies. Sci. Rep., 9: 2958. https://doi.org/10.1038/s41598-019-39590-8
Mogasale, V., P. Saldanha and V. Mogasale. 2021. A descriptive analysis of antimicrobial resistance patterns of WHO priority pathogens isolated in children from a tertiary care hospital in India. Sci. Rep., 11: 5116. https://doi.org/10.1038/s41598-021-84293-8
Moradali, M.F., S. Ghods and B.H.A. Rehm. 2017. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival and Persistence. Front. Cell. Infect. Microbiol., https://doi.org/10.3389/fcimb.2017.00039
Murray, A.K., 2020. The novel coronavirus COVID-19 outbreak: Global implications for antimicrobial resistance. Front. Microbiol., 11: 1020. https://doi.org/10.3389/fmicb.2020.01020
Patil, A., R. Banerji, P. Kanojiya, S. Koratkar and S. Saroj. 2021. Bacteriophages for ESKAPE: Role in pathogenicity and measures of control. Exp. Rev. Anti-Infect. Ther., 19: 845-865. https://doi.org/10.1080/14787210.2021.1858800
Petrovic, F.A., and R.C. Lin. 2020. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Natl. Microbiol., 5: 465-472. https://doi.org/10.1038/s41564-019-0634-z
Poole, K., 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol., 3: 255-264.
Qin, J., Wu, N., Bao, J., Shi, X., Ou, H., Ye, S., Zhao, W., Wei, Z., Cai, J., Li, L., Guo, M., Weng, J., Lu, H., Tan, D., Zhang, J., Huang, Q., Zhu, Z., Shi, Y., Hu, C., Guo, X., Zhu, T. 2020. Heterogeneous Klebsiella pneumonia co-infections complicate personalized bacteriophage therapy. Front. Cell. Infect. Microbiol., 10: 608402. https://doi.org/10.3389/fcimb.2020.608402
Rahman, S., A. Bharatha, M. Haque, and M.G. Hilaire. 2020. Review: Antimicrobial stewardship: Fighting antimicrobial resistance and antimicrobial stewardship: Fighting antimicrobial resistance and protecting global public health, 2020. Infectious Drug Research.
Sharma, S., S. Datta, and S.K. Dwivedi. 2021. Isolation and Characterization of a lytic bacteriophage against Pseudomonas aeruginosa. Sci. Rep., 11: 193093. https://doi.org/10.1038/s41598-021-98457-z
Witzenrath, M., B. Schmeck, J.M. Doehn, T. Tschemig, J. Zahlten, J.M. Loeffler, M. Zemlin, H. Müller, B. Gutbier, H. Schütte, S. Hippenstiel, V.A. Fischetti, N. Suttorp, and S. Rosseau. 2009. Systemic use of the endolysins Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit. Care Med., 37: 642-649. https://doi.org/10.1097/CCM.0b013e31819586a6
World Health Organisation, 2020. Antimicrobial resistance. Available at: https://ahpsr.who.int/publications/action-plan-on-antimicrobial-resistance
World Health Organization, 2017. Publishes list of Bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
Zhang, Y., Y. Li, J. Zeng, Y. Chang, S. Han, J. Zhao, Y. Fan, Z. Xiong, X. Zou, C. Wang, B. Li, H. Li, J. Han, X. Liu, Y. Xia, B. Lu, and B. Cao. 2020. Risk factors for mortality of in-patients with Pseudomonas aeruginosa bacteremia in China: Impact of Resistance profile in the mortality. Infect. Drug Resist., 13: 4115-4123. https://doi.org/10.2147/IDR.S268744
To share on other social networks, click on any share button. What are these?





