Iron Excess Impact on Pancreatic Beta Cell Structure and Fasting Blood Glucose Levels in Male Wistar Rats (Rattus norvegicus)
Research Article
Iron Excess Impact on Pancreatic Beta Cell Structure and Fasting Blood Glucose Levels in Male Wistar Rats (Rattus norvegicus)
Anisa Muthia Fakhira1, Madihah Madihah1, Susi Susanah2, Erick Khristian3, Ratu Safitri1*
1Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Department of Child Health, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 3Program Study of Medical Laboratory Technology, Faculty of Health and Science Technology Universitas Jenderal Achmad Yani, Cimahi, West Java, Indonesia.
Abstract | The body stores excess iron in several organs, including the pancreas, where it impairs its structure and function. The aims of this study is to ascertain the iron dosage that affects changes in pancreatic beta cell structure and fasting blood glucose levels in male Wistar rats (Rattus norvegicus). The study using 7 treatment groups with 4 replications. Iron dextran (ID) was administered intravenously in three 3-day intervals, with cumulative doses of 10, 20, 30, 40, 50, and 60 mg/kg BW, whether the control injected with physiological sodium chloride solution. The results of the study showed that both the necrosis score in pancreatic beta cells and fasting blood glucose levels demonstrated a dose-response pattern, with higher values corresponding to increasing dosages. The ID dose starting from 20 mg/kg BW showed a significant increase in pancreatic beta cell necrosis of 1.6 ± 0.55 and a fasting blood glucose level of 167.40±11.45 mg/dL, classifying it as a prediabetic state, that significantly different with control (p<0.05). Thus, ID as low as 20 mg/kg BW impaired the structure and function of pancreas of male wistar rats.
Keywords | Blood glucose, Iron excess, Pancreatic beta cells, Rat (Rattus norvegicus), Pancreas, Iron dextran
Received | November 20, 2023; Accepted | January 02, 2024; Published | February 03 2024
*Correspondence | Ratu Safitri, Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Bandung, West Java, Indonesia; Email: [email protected]
Citation | Fakhira AM, Madihah M, Susanah S, Khristian E, Safitri R (2024). Iron excess impact on pancreatic beta cell structure and fasting blood glucose levels in male Wistar rats (Rattus norvegicus). Adv. Anim. Vet. Sci., 12(3):399-404.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.3.399.404
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Iron is crucially involved in numerous vital cellular processes, such as facilitating the transport and exchange of oxygen and serving as the metallic component in a multitude of intracellular enzymes (Marku et al., 2021). However, in conditions of iron excess it will be dangerous for the body. Iron excess can result from both primary and secondary factors. Primary factors are attributed to genetic disorders, such as in hereditary hemochromatosis patients, where a genetic aberration impairs iron metabolism, leading to a persistent iron excess. Secondary factors are related to processes like transfusion, hemolysis, and excessive iron consumption (Gattermann et al., 2021; McDowell et al., 2022; Susanah et al., 2021). Patients with beta-thalassemia major, who require frequent blood transfusions to treat chronic anemia, are especially vulnerable to iron excess (Shah et al., 2019). Each unit of blood transfused introduces an additional 175–225 mg of iron into the body, whereas the body only excretes small amounts of iron every day, so patient who undergo transfusions are susceptible to experiencing iron excess (Humayun et al., 2021).
Excessive iron in the body activates Reactive Oxygen Species (ROS) via fenton reaction, which produces ferric iron, hydroxide, and the hydroxyl radical by oxidizing ferrous iron through a reaction with hydrogen peroxide (Backe et al., 2016). The hydroxyl radical will oxidize lipids, carbohydrates, proteins, DNA, and RNA. Therefore, when the production of ROS surpasses the ability of the cell to neutralize ROS, cellular ROS damage takes place that can cause damage and even cell death, one of which is pancreatic beta cells (Backe et al., 2016; Marku et al., 2021; Newsholme et al., 2019). Pancreatic beta cells have a relatively low quantity of antioxidant enzymes, including approximately 50% superoxide dismutase (SOD) and only 5% glutathione peroxidase (GPx) and catalase (CAT) compared to the liver, leading them to be more sensitive to ROS (Wang and Wang, 2017). Pancreatic beta cells are endocrine gland that produce insulin and regulate blood glucose levels in the body. When beta cells are damaged by oxidative stress, it results in insulin insufficiency, which causes high blood glucose levels, often known as hyperglycemia (Backe et al., 2016; Marku et al., 2021; Wang et al., 2019). Hyperglycemia is an early prognosis for diabetes mellitus, with the patients experiencing damage to around 50–90% of beta cells (Atkinson et al., 2020). Diabetes mellitus and impaired glucose tolerance have been reported in 30–60% of patients with hereditary hemochromatosis (Hatunic et al., 2010; Lockhart et al., 2023).
This study involves modeling iron excess in male Wistar rats and exploring its correlation with glucose metabolism. Several studies have been conducted regarding the impact of excess iron on glucose metabolism. According to Pramestiyani and Fadhilah (2017), reported that higher dosages of ferrous sulfate that given orally, were shown to elevate the blood glucose levels of rats. Delghingaro-Agusto et al. (2023), also reported rats given feed with a high iron content experienced an increase in impaired glucose tolerance by 17%.
Among the various studies, there has been no modeling of iron excess induced by iron dextran (ID) intravenously as an illustration of patients who frequently undergo blood transfusions, to determine its impact on pancreatic histopathology and fasting blood glucose levels. According to Maskoen et al. (2016) and Safitri et al. (2018), an iron excess condition may arise in rats administered with ID at a dosage of 60 mg/kg BW. The purpose of this study is to evaluate the possibility that ID, at dosages ranging from 10 to 60 mg/kg BW, damages pancreatic beta cells and raises fasting blood glucose levels in male rat.
MATERIALS AND METHOD
Animal treatment
This research was registered and approved by the Research Ethics Committee of Padjadjaran University with ethics approval number 605/UN6.KEP/EC/2021. Seven iron treatment groups were used in four replications of the experiment, which was carried out using a completely randomized design (CRD). The animal model for iron excess used in this study was male Wistar rats (Rattus norvegicus L.). Iron dextran (Sygma-Aldrich; MFCD00081553) is given intravenously at 3-days intervals with cumulative doses ranging from 10, 20, 30, 40, 50 and 60 mg/kg BW. In the control group, mice were treated with physiological NaCl. Dissection was performed every 3 days after each group reached the predetermined cumulative dose. Before dissection, rat were fasted for 8-10 hours. The rats were anesthetized using a ketamine-xylazine mixture (1:1) via intramuscular injection before the dissection.
Histological preparation of pancreatic beta cells with periodic acid schiff (PAS) staining
The pancreas, which has been fixed with Neutral Buffer Formalin (NBF) 10% for 24 hours, was then dehydrated with graded alcohol (70%, 85%, 95%, and absolute). The organ was subsequently infiltrated with xylene, followed by embedding in paraffin. The organ was then sliced at 5 μm using a microtome. The slides were air-dried, and deparaffinization was performed using xylene. The tissue stained with periodic acid schiff (PAS) (abcam; ab150680). The slides were treated with periodic acid for 5–10 minutes, followed by rinsing with distilled water. The slides were then stained with Schiff’s reagent, approximately 15–30 minutes, and washed with warm water. For counterstaining, hematoxylin was used for 5 minutes, then the slides were rinsed with tap water and cleared with xylene.
The observation of histopathological structures in pancreatic beta cells was conducted by analyzing the percentage of necrotized pancreatic beta cells in 50 cells from each of 5 fields at a magnification of 1000×. Histopathological observations were scored into categories as follows: score 0, indicating 0% necrosis of pancreatic beta cells; score 1, indicating 5–30% total necrosis of pancreatic beta cells; score 2, indicating 30–70% total necrosis of pancreatic beta cells; score 3, indicating >70% total necrosis of pancreatic beta cells (Setiadi et al., 2022).
Serum preparation and blood glucose level test
Blood was collected using a 5 ml syringe from the jugular vein of the rats. The blood was then placed in a clot activator and centrifuged at 3000 rpm for 15 minutes to separate the serum. The blood glucose levels were measured using serum with the Glucose GOD FS kit (DiaSys; 1 2500 99 10 021). A spectrophotometer was used to measure the absorbance at 540 nm, which was calculated using the following formula:
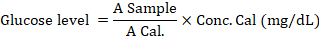
Statistical analysis
Pancreatic beta cell necrosis scores were analyzed using the Kruskal-Wallis and Mann-Whitney U tests, and fasting blood glucose levels were analyzed using one-way ANOVA and Duncan’s post hoc test with a confidence level of 95%. Data analysis was carried out with IBM@SPSS version 26.0 software.
RESULTS and Discussion
Considering the findings of the study, data were obtained, including scores of histopathological necrosis of pancreatic beta cells and fasting blood glucose levels in rats.
Histopathology of rat pancreatic beta cells post-induction of ID
Based on the results of histopathological observations (Figure 1), the Langerhans islet of the normal control group and the ID-treated group at a dose of 10 mg/kg BW showed densely packed cells, indicating a normal state (no damage observed). Empty spaces in the Langerhans islets were observed in rats induced with ID at a dose of 20–60 mg/kg BW, indicating cell necrosis, and the occurrence of larger empty spaces indicated a higher degree of necrosis (Herdiani et al., 2023).
The necrosis score did not differ significantly between the ID-treated at a dose of 10 mg/kg BW (1.2±0.45) and the normal control group (0.8±0.45) as shown in Figure 2. The group of rats that were given ID at doses of 20 mg/kg BW (1.6±0.55), showed a significant increase in necrosis scores compared to normal control rats (p<0.05). The necrosis score then increased significantly as the dose administered increased. The group of rats with ID doses of 30 and 40 mg/kg BW showed a significant increase in necrosis compared to normal control rat (p<0.05) to 1.8 ± 0.45. The 50 and 60 mg/kg BW ID dose group showed a score of 2.00±0.00 which was significantly different from the control and the 10 mg/kg BW group. These results indicate that iron in ID starting from a dose of 20–60 mg/kg BW, can increase beta cell necrosis as the dose increases.
Fasting blood glucose levels in rats post-induction of ID
The fasting blood glucose level in a normal control group (95.20±11.56 mg/dL) was categorized as a normal level. At an ID dose of 10 mg/kg BW (99.20±9.80 mg/dL), there was an increase in fasting blood glucose levels but not significantly different from the normal control group. A significant increase was observed starting at the ID of 20 mg/kg BW (167.40±11.45 mg/dL) as much as 75.84% compared to the normal control group (p<0,05), which is considered pre-diabetic (BPOM, 2021). The ID dose of 30–60 mg/kg BW showed no significant difference between each other, the highest increase in fasting blood glucose levels occurred with the administration of ID 60 mg/kg BW (197.2 ± 3.27 mg/dL), an increase of 107.14% compared to normal control rats (p<0.05) (Figure 3). The results of this study show that increasing iron from iron dextran can increase fasting blood glucose levels in rat, and the group of rat with prediabetes was started with an ID dose of 20 mg/kg BW.
The intravenous administration of ID to rat can cause iron buildup in their bodies (Musumeci et al., 2014). Excess iron in the body can activate ROS, leading to oxidative stress in the cells, resulting in cell damage and organ dysfunction (Newsholme et al., 2019). Necrosis of beta cells occurred in all treatments in this study as the number of IDs injected intravenously into rat increased. ID starting from 20 mg/kg BW showed a significant increase in pancreatic beta cell damage, as was illustrated in Figure 2. The highest necrosis score observed at doses of 50 and 60 mg/kg BW with a necrosis score of 2, which indicates moderate damage because more than 30% necrosis occurred (Setiadi et al., 2022). These findings are consistent with the study by Aldi et al. (2019), which showed that Fe tablets at a dose of 54 mg/kg BW caused damage to pancreatic beta cells with a damage score of 2.
Pancreatic beta cells need iron to aid insulin secretion (Hansen et al., 2012), however, its buildup is thought to be a biomarker of diabetes mortality and risk, and it plays a significant role in determining inflammation in the pancreatic islets (Marku et al., 2021). Under normal circumstances, body iron is bound by transferrin, but if transferrin capacity exceeds its limit, unbound iron circulates freely in the plasma as Non-Transferrin-Bound Iron (NTBI) (Backe et al., 2016; Susanah et al., 2021). Iron absorption in beta cells is mediated by endocytosis of the transferrin-transferrin receptor complex, and its release by the divalent metal ion transporter (DMT-1) from endosomes. As NTBI, it can be imported using the ZIP14 zinc transporter being toxic as ion-free. After that, Fe2+ is dispersed easily to be stored, bound to ferritin, or used by surrounding proteins such lipocalin and PCBP (Hansen et al., 2012; Marku et al., 2021). Excessive iron increases the production of ROS through the Fenton reaction (Gattermann et al., 2021). This catalyzes the conversion of single electrons in LOOH into LO-, which is one form of free radical (Ursini and Maiorino, 2020), causing oxidative stress in organs (Taher and Saliba, 2017). Pancreatic beta cells are sensitive to ROS because they have less antioxidants, approximately 50% SOD and 5% GPx and CAT compared to antioxidant enzymes in the liver (Wang and Wang, 2017). As a result, beta cells are more susceptible to ferroptosis, there is an increase in oxidative levels within the cell, resulting in lipid peroxidation and the accumulation of ROS that damage cell membranes. This leads to cellular membrane failure and ultimately necrosis or cell death (Miao et al., 2023).
Damage to pancreatic beta cells causes disruption of insulin production, so that when the body lacks insulin, blood glucose levels rise, resulting in hyperglycemia (Wang et al., 2022). In this study, starting at an ID dose of 20 mg/kg BW showed a significant increase in fasting blood glucose levels compared to normal control rat, and also showed pre-diabetic conditions (BPOM, 2021). The more ID doses given, the higher the fasting blood glucose levels. This is in line with Pramestiyani and Fadhilah (2017) research showed that blood glucose levels in mice increased along with increasing doses of iron sulfate as an inducer of iron overload, with the average blood glucose levels in controls 30 and 60 mg/kg BW were 150.67, 175.33, and 191.50 mg/dL, respectively.
The oxidative stress caused by excess iron, also a major factor in the pathogenesis of hyperinsulinemia and insulin resistance, because it can inhibit the internalization and action of insulin (Tangvarasittichai, 2015). Certain studies suggest that ROS activate stress kinases, which can then directly block insulin signaling pathway, particularly in H9c2 cardiomyoblasts (Al-Lahham et al., 2016; Sung et al., 2019). A study on healthy men also found a higher body iron stores may contribute to impaired insulin sensitivity through increased oxidative stress (Syrovatka et al., 2009).
Hyperglycemia is one of the signs of Diabetes Mellitus (DM), which can be caused by disturbances in insulin secretion, insulin function, or both. Chronic hyperglycemia tends to increase the formation of free radicals through glucose metabolism pathways such as increased activation of protein kinase C (PKC), production of AGEs, glucose autooxidation, methylglyoxal formation metabolism, and oxidative phosphorylation (Bhatti et al., 2022). These free radicals can trigger oxidative stress for normal cellular homeostasis and the body’s defense against infection. Excessive production of free radicals can also disrupt normal protective mechanisms and cause diabetes complications (Yaribergi et al., 2019). The research findings strongly support the association between iron excess and the development of type 2 diabetes. Liu et al. (2020) reported that, type 2 diabetes was associated with higher serum ferritin levels, which indicates the body has excess iron. Gao et al. (2022) also reported that women who experience excess iron have a higher risk of diabetes than men, the presence of liver injury (alanine transaminase) and abnormalities of lipid metabolism (triglycerides, apolipoprotein B) contribute to the association between iron overload and diabetes.
CONCLUSIONS and Recommendations
The conclusion of this study is that the administration of ID, starting from a dose of 20 mg/kg BW, increases the number of necrotic pancreatic beta cells and blood glucose levels in rats, and this damage becomes more severe with increasing doses administered.
ACKNOWLEDGEMENTS
Thank you for Indonesia Endowment Funds and Education for funding this research in Academic Leadership Supervised and LPDP by Prof. Dr. Ratu Safitri, M.S. 2023 for Commercialization Sappan Wood as Adjuvant Chelation Therapy for Thalassemia.
Novelty Statement
This study aims to ascertain the iron dosage that affect changes in pancreatic beta cell structure and fasting blood glucose levels in male Wistar rats (Rattus norvegicus). To the best of our knowledge, there has been no modeling of iron excess induced by ID intravenously, to determine its impact on pancreatic histopathology and fasting blood glucose levels.
Author’s Contribution
All authors contributed equally to the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Aldi A, Kalsum U, Fatmawati F (2019). The effect of high doses of iron (Fe) supplementation on the condition of pancreatic beta cells in white rats (Rattus norvegicus) wistar strain pregnant (In Indonesian language). J. Issues Midwif., 3(1): 20–25.
Al-Lahham R, Deford JH, Papaconstantinou J (2016). Mitochondrial-generated ROS down regulates insulin signaling via activation of the p38MAPK stress response pathway. Mol. Cell. Endocrinol., 419: 1–11. https://doi.org/10.1016/j.mce.2015.09.013
Atkinson M, Campbell-Thompson M, Kusmartseva I, Kaestner K (2020). Organisation of the human pancreas in health and in diabetes. Diabetologia, 63(10): 1966–1973. https://doi.org/10.1007/s00125-020-05203-7
Backe MB, Moen IW, Ellervik C, Hansen JB, Mandrup-Poulsen T (2016). Iron regulation of pancreatic beta-cell functions and oxidative stress. Ann. Rev. Nutri., 36, 241–273. https://doi.org/10.1146/annurev-nutr-071715-050939
Bhatti J, Sehrawat A, Mishra J, Sidhu I, Navik U, Khullar N, Reddy P (2022). Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med., 184: 114–134. https://doi.org/10.1016/j.freeradbiomed.2022.03.019
BPOM (2021). Guidelines for preclinical pharmacodynamic testing of traditional medicines (In Indonesian language). (Internet). Available from: https://jdih.pom.go.id/download/product/1282/18/2021
Delghingaro-Augusto V, Hosaka A, Estaphan S, Richardson A, Dahlstrom JE, Nolan CJ (2023). High dietary iron in Western diet-fed male rats causes pancreatic islet injury and acute pancreatitis. J. Nutr., 153(3): 723–732. https://doi.org/10.1016/j.tjnut.2023.01.009
Gao H, Yang J, Pan W, Yang M (2022). Iron overload and the risk of diabetes in the general population: Results of the Chinese health and nutrition survey cohort study. Diabetes Metab. J., 46(2): 307–318. https://doi.org/10.4093/dmj.2020.0287
Gattermann N, Muckenthaler MU, Kulozik AE, Metzgeroth G, Hastka J (2021). The evaluation of iron deficiency and iron overload. Deutsches Ärzteblatt Int., 118(49): 847–856. https://doi.org/10.3238/arztebl.m2021.0290
Hansen JB, Tonnesen MF, Madsen AN, Hagedorn PH, Friberg J, Grunnet LG, Heller RS, Nielsen AØ, Størling J, Baeyens L, Anker-Kitai L, Qvortrup K, Bouwens L, Efrat S, Aalund M, Andrews NC, Billestrup N, Karlsen AE, Holst B, Mandrup-Poulsen T (2012). Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic β cell fate in response to cytokines. Cell Metab., 16(4): 449–461. https://doi.org/10.1016/j.cmet.2012.09.001
Hatunic M, Finucane FM, Brennan AM, Norris S, Pacini G, Nolan JJ (2010). Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism, 59(3): 380–384. https://doi.org/10.1016/j.metabol.2009.08.006
Herdiani N, Wirjatmadi B, Kuntoro EAW (2023). Effect of mangosteen peel extract on increasing the number of endocrine cells in pancreas Langerhans islands and decreasing tumor necrosis factor alfa in streptozotocin-induced male rats. J. Pharma. Negative Results, 14(3): 3539–3549.
Humayun S, Farooq G, Farid N, Asmat S, Mehwish A, Ali M (2021). Haematological and biochemical effects of transfusion of stored blood in transfusion-dependent thalassemia patients. Pak. J. Physiol., 17(3): 14–18.
Liu J, Li Q, Yang Y, Ma L (2020). Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J. Diabetes Investig., 11(4): 946–955. https://doi.org/10.1111/jdi.13216
Lockhart M, Salehmohamed MR, Kumar D, Cummiskey AG, Seong KC, Sreenan S, McDermott J (2023). Screening for hereditary hemochromatosis in newly referred diabetes mellitus. Am. J. Med. Open, 10: 100046. https://doi.org/10.1016/j.ajmo.2023.100046
Marku A, Galli A, Marciani P, Dule N, Perego C, Castagna M (2021). Iron metabolism in pancreatic beta-cell function and dysfunction. Cells, 10(11): 2841. https://doi.org/10.3390/cells10112841
Maskoen AM, Safitri R, Milanda T, Reinarti L, Fauziah PN (2016). Iron chelation ability of granule Sappan wood (Caesalpinia sappan L.) extract on iron-overloaded. Int. J. Pharm. Tech. Res., 9(5): 299–305.
McDowell LA, Kudaravalli P, Sticco K (2022). Iron overload (Internet). Treasure Island: StatPearls Publishing; (cited 2023 Oct 13). Available from: https://www.ncbi.nlm.nih.gov/books/NBK526131/
Miao R, Fang X, Zhang Y, Wei J, Zhang Y, Tian J (2023). Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: Mechanisms and therapeutic opportunities. Cell Death Dis., 14(3): 1–7. https://doi.org/10.1038/s41419-023-05708-0
Musumeci M, Maccari S, Stati T, Sestili P (2014). Iron excretion in iron dextran-overloaded mice. Blood Transf., 12: 485–490.
Newsholme P, Keane KN, Carlessi R, Cruzat V (2019). Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: Importance to cell metabolism, function, and dysfunction. Am. J. Physiol. Cell Physiol., 317(3): C420–C433. https://doi.org/10.1152/ajpcell.00141.2019
Pramestiyani M, Fadhilah S (2017). Effect of giving various doses of ferrous sulphate on blood glucose levels in pregnant rats (Rattus norvegicus) (In Indonesian language). J. Ilmu Kebidanan., 3(2): 140–144.
Safitri R, Maskoen AM, Syamsunarno M, Ghozali M, Panigoro R (2018). Iron chelating activity of Caesalpinia sappan L. extract on iron status in iron overload rats (Rattus norvegicus L.). AIP Conf. Proc., 2018: 1–6. https://doi.org/10.1063/1.5050146
Setiadi E, Peniati E, Susanti RSR (2022). The effect of aloe vera skin extract on blood sugar levels and histopathological features of the pancreas of rats induced by alloxan (In Indonesian language). Life Sci., 9(2): 171–185.
Shah FT, Sayani F, Trompeter S, Drasar E, Piga A (2019). Challenges of blood transfusions in β-thalassemia. Blood Rev., 37: 1–13. https://doi.org/10.1016/j.blre.2019.100588
Sung HK, Song E, Jahng JWS, Pantopoulos K, Sweeney G (2019). Iron induces insulin resistance in cardiomyocytes via regulation of oxidative stress. Sci. Rep., 9(1): 4668. https://doi.org/10.1038/s41598-019-41111-6
Susanah S, Rakhmilla LE, Ghozali M, Trisaputra JO, Moestopo O, Sribudiani Y (2021). Iron status in newly diagnosed β-thalassemia major: High rate of iron status due to erythropoiesis drive. Biomed. Res. Int., 2021: 1–7. https://doi.org/10.1155/2021/5560319
Syrovatka P, Kraml P, Potockova J, Fialova L, Vejrazka M, Crkovska J, Andel M (2009). Relationship between increased body iron stores, oxidative stress and insulin resistance in healthy men. Ann. Nutr. Metab., 54(4): 268–274. https://doi.org/10.1159/000229507
Taher AT, Saliba AN (2017). Iron overload in thalassemia: Different organs at different rates. Hematol. Am. Soc. Hematol. Educ. Program., 2017(1): 265–271. https://doi.org/10.1182/asheducation-2017.1.265
Tangvarasittichai S (2015). Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes, 6(3): 456. https://doi.org/10.4239/wjd.v6.i3.456
Ursini F, Maiorino M (2020). Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med., 152(3): 175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
Wang HY, Li QM, Yu NJ, Chen WD, Zha XQ, Wu DL, Luo JP (2019). Dendrobium huoshanense polysaccharide regulates hepatic glucose homeostasis and pancreatic β-cell function in type 2 diabetic mice. Carbohyd. Poly., 211(1): 39–48. https://doi.org/10.1016/j.carbpol.2019.01.101
Wang J, Wang H (2017). Oxidative stress in pancreatic beta cell regeneration. Himdawi: Oxid. Med. Cell. Long., 2017(1): 1–9. https://doi.org/10.1155/2017/1930261
Wang Z, Fang S, Ding S, Tan Q, Zhang X (2022). Research progress on relationship between iron overload and lower limb arterial disease in type 2 diabetes mellitus. Diabetes, Metab. Synd. Obes. Targets Ther., 2022(15): 2259–2264. https://doi.org/10.2147/DMSO.S366729
Yaribeygi H, Atkin SL, Sahebkar A (2019). A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol., 234(2): 1300–1312. https://doi.org/10.1002/jcp.27164
To share on other social networks, click on any share button. What are these?





