In vitro Antihelminthic Activities of Artocarpus heterophyllus (Jackfruit) and Artocarpus camansi (Breadnut) Leaf Extracts on the Model Nematode, Caenorhabditis elegans
In vitro Antihelminthic Activities of Artocarpus heterophyllus (Jackfruit) and Artocarpus camansi (Breadnut) Leaf Extracts on the Model Nematode, Caenorhabditis elegans
Estrelle Anne Tacbas1, Neil Pep Dave Sumaya2 and Nanette Hope Sumaya1,3*
1Department of Biological Sciences, College of Science and Mathematics, Mindanao State University-Iligan Institute of Technology, A. Bonifacio Ave., Tibanga, Iligan City, Philippines; 2Division of Plant Pathology, University of Southern Mindanao, Kabacan, Cotabato, Philippines; 3FBL-Nematology Research Group, Premier Research Institute of Science and Mathematics, Mindanao State University-Iligan Institute of Technology, A. Bonifacio Ave., Tibanga, Iligan City, Philippines.
Abstract | Caenorhabditis elegans can represent a model organism for herbal medication against parasitic nematodes in determining the anthelmintic potential for the following concentrations of Artocarpus heterophyllus and Artocarpus camansi, 10000 ppm, 7500 ppm, 5000 ppm. The phytochemical results revealed the presence of alkaloids, flavonoids, saponins, steroids, and tannins in both plant crude extracts. Different developmental stages of C. elegans (i.e., 1st to 4th larval stages (L1-L4), young adult (YA) and adult nematodes) were used for in vitro anthelmintic assay, mortality, development of life stages, and reproduction of the nematode. Both plant extracts caused high mortality in the L4 stage for their LC50 and LC90 values. Notably, the crude extracts of both plants delayed the development of L4 for almost 48 hours. Thus, the results suggest that the extracts of A. heterophyllus and A. camansi can be a potential alternative for anthelmintic treatment or with further research, can be utilized as a natural source and active ingredient for a bio-based antihelmintic pharmaceutical drug.
Received | July 20, 2024; Accepted | August 07, 2024; Published | September 14, 2024
*Correspondence | Nanette Hope Sumaya, Department of Biological Sciences, College of Science and Mathematics, Mindanao State University-Iligan Institute of Technology, A. Bonifacio Ave., Tibanga, Iligan City, Philippines; Email: [email protected]
Citation | Tacbas, E.A., Sumaya, N.P.D. and Sumaya, N.H., 2024. In vitro antihelminthic activities of Artocarpus heterophyllus (Jackfruit) and Artocarpus camansi (Breadnut) leaf extracts on the model nematode, Caenorhabditis elegans. Pakistan Journal of Nematology, 42(2): 136-145.
DOI | https://dx.doi.org/10.17582/journal.pjn/2024/42.2.136.145
Keywords | Antihelmintic, Moraceae, Parasitic nematodes, Phytoconstituents, Plant extracts
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Parasitic nematodes continue to place a considerable burden on human health, the productivity of developing countries, and the livestock industry. These nematodes threaten the health of the livestock and cause a major financial and socioeconomic burden to modern society (Vadnal et al., 2017; Zanzani et al., 2014). The parasitic gastrointestinal nematodes, such as Ascaris lumbricoides, Trichuris trichuria, Necator americanus, and Ancylostoma duodenale are a group of gastrointestinal soil-transmitted helminths that pose a significant public health concern in the Philippines (Bethony et al., 2006). If infected, children can suffer from profound physical deficits, including anemia and malnutrition, stunted growth, reduced fitness, and cognitive delays (Soares et al., 2015).
Despite this, only a few anthelmintic drugs are presently available on the market. These include benzimidazoles, macrocyclic lactones (ivermectin), imidazothiazoles (levamisole), and cyclic octadepsipeptides (emodepside), most of which were introduced decades ago (Wink, 2012). However, the extensive and sole reliance on anthelmintics with their indiscriminate use is under serious threat due to the rapid and widespread emergence of anthelmintic-resistant strains of parasites worldwide including in the Philippines (Rupa and Portugaliza, 2016). Moreover, some of these drugs are unaffordable, inaccessible, or inadequately available to the resource-poor farmers of developing countries. Thus, this paved the way for traditional herbal remedies to be used as an alternative source of anthelmintic.
It has been well evidenced that traditional medicines including plants and plant-derived preparations hold great promise as a source of easily available effective anthelmintic treatments, including anthelmintic agents (Aremu et al., 2012). The genus Artocarpus of the Moraceae family is used traditionally in many types of diseases, including the treatment of various parasites (Rotylenchulus reniformis, Meloidogyne incognita, etc.) with its nematicidal effect. Artocarpus heterophyllus (Jackfruit) and Artocarpus camansi (Breadnut) are the species belonging to this genus which are known to possess phytochemicals that may potentially be used as an anthelmintic, and it is also known for its high nutritional value (Hari et al., 2014).
The free-living nematode Caenorhabditis elegans may offer a convenient alternative model system to search for medicinal plants with anthelmintic activity (Burns et al., 2015). C. elegans is a small, free-living soil nematode (roundworm) that lives in many parts of the world and survives by feeding on microbes, primarily bacteria (Palikaras and Tavernakis, 2013). This nematode completes a reproductive life cycle in 2.5-3 days at 25 °C (or in 3.5 days at 20 °C), progressing from fertilized embryos which take 14 hours to complete, then hatch to L1 stage which proceeds through four larval stages (L1–L4) for the next 50 hours to become egg-laying adults. According to Katiki et al. (2011), this nematode satisfies many of the criteria needed for an in vitro test of anthelmintics because it is cheap, readily available, and easy to work with.
The mode of action of anthelmintics can be evaluated in vitro through nematode mortality, development, and reproduction. This study aims to investigate the anthelmintic activity of A. heterophyllus (jackfruit) and A. camansi (breadnut) against the model nematode, C. elegans, screen the phytochemical components of A. heterophyllus and A. camansi leaves extract, assess the mortality of the C. elegans after 24 hours of exposure to different concentrations of the extracts, observe the development and reproduction of the surviving nematodes after exposure to varying concentrations of the extracts, and compare the anthelmintic efficacy of A. heterophyllus and A. camansi with the commercially available anthelmintic drug. With further research, it may be utilized as an alternative for a new environmentally safe and sustainable medicine for anthelminthic treatments or can be used as a natural source and active ingredient for a pharmaceutical drug.
Materials and Methods
Plant collection and preparation
Mature leaves of Artocarpus heterophyllus and Artocarpus camansi were collected from the rural area of Midsalip, Zamboanga del Sur (8.027792, 123.318313). The area where the leaves were collected is far from the public road, the soil is dry, slightly rocky and the trees are fully exposed to the sunlight. After collection, the leaves were washed with tap water to remove dust that was on the leaves. Then, the leaves were air dried at room temperature (25oC-27 oC) under the shade of light with sufficient air circulation around the leaves. When the leaves dried, they were ground to a coarse powder by using a blender.
Extraction and phytochemical screening
The powdered leaves were sent to the Chemistry Department of Mindanao State University, Iligan Institute of Technology (MSU-IIT), and were extracted and analyzed for the presence of phytochemicals viz alkaloids, anthraquinones, flavonoids, glycoside, steroids, and tannins using the standardized methods.
Preparation of Escherichia coli
Standard methods (Riley et al., 2017) were used to grow a liquid culture of E. coli OP50 strain obtained from e-nema GmBH, Schwentinental Germany. A small volume of existing bacterial culture was inoculated into a 20-milliliter test tube of fresh LB broth and was incubated for 12 hours. The bacterium was used as a food source for the nematode C. elegans.
Bacterial cell count of Escherichia coli
Following the protocol of Abcam (2019), a hemacytometer was used to provide for a more quantified and standardized distribution of bacterial cells in each plate. About 10 microliter of cultured broth was taken from the 20 milliliters test tube and transferred to an Eppendorf tube. The broth in the Eppendorf tube was then diluted using a dilution factor of 5, and a drop of the diluted broth was then placed on the side of the coverslip. Live cells of E. coli OP50 were counted on the large squares (16) of the hemacytometer. After counting the live cells, the formula below was used to obtain the target density of 105 bacterial cells. This target density of 105 will be used to seed the nematodes on the anthelmintic assay later on.
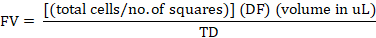
FV= Final volume (uL); No. of squares= 16; DF= dilution factor (5); Volume in uL= 104 (constant); TD= Target density (106).
Preparation of nematode Caenorhabditis elegans
Standard methods of Katiki et al. (2011) were followed with some modifications. A monoxenic strain of N2 (wild type) of C. elegans acquired from the Aging Physiology and Molecular Evolution Laboratory of Ghent University, Belgium was sliced and transferred to a nematode agar (NA) medium composed of 400 milliliter of distilled water and 11.2 grams of agar. The medium was cooked and then supplemented with 10 milliliters of buffer (KH2PO4) and 75 microliters of cholesterol. When the medium solidified, it was then seeded with 500 microliters of E. coli and was incubated for 12 hours. After 12 hours, a sliced strain of N2 C. elegans was transferred and raised to the nematode agar medium and incubated for 7 days at 25oC.
In vitro anthelmintic assay
The following procedure followed is according to the study of Santhi et al. (2017) with some modifications. About 500 microliters of Escherichia coli with a target density of 105 were grown on the nematode agar medium supplemented with different concentrations (5000, 7500, and 10000 ppm) of the two plant extracts (A. heterophyllus and A. camansi) in a 5-centimeter diameter Petri dishes. Treatment of Albendazole drug was used as a positive control and phosphate-buffered saline (PBS) as a negative control. Following 12 hours of incubation, each plate was inoculated with 10 individuals of L4 stage nematode. L4 stage larvae can be distinguished by the presence of a small white half-circle patch in the worm midsection which will eventually develop to vulva in the adult stage. Each treatment (plant extract at a given concentration) consisted of 5 replicates (wells) with 3 trials, multiplied by 2 (plants) so there were 40 plates per trial. The incubated nematodes were examined for 3 consecutive days (72 hours). Assessment of the mortality of the nematodes was checked on day 1 (24 hours after inoculation of nematodes), and observation of the development and reproduction rates of the nematodes were checked on days 2-3 (48–72 hours).
Statistical analysis
For the effect of plant extracts on anthelmintic assays, the data (numerical data - number of each nematode stage) were transformed into percentages) was analyzed using a repeated-measures analysis of two-way variance ANOVA, and a post-hoc Tukey’s test to confirm the differences that occurred between groups. Probit analysis and LC50 were also used to determine the lethal concentration required to kill 50% of the population.
Results and Discussion
Phytochemical screening of crude extract
The results of the phytochemical screening showed that both A. heterophyllus and A. camansi extracts contain alkaloids, flavonoids, saponins, steroids, and tannin compounds (Table 1). Despite similarities in the presence of the compounds, it was evident that A. heterophyllus contains a higher amount of saponins and tannins than that of A. camansi.
Table 1: Phytochemical screening of the ethanolic leaf extracts of Artocarpus heterophyllus and Artocarpus camansi.
|
Plant |
Alkaloids |
Anthraquinones |
Glycosides |
Flavonoids |
Saponins |
Steroids |
Tannins |
|
Artocarpus heterophyllus |
(+) |
(-) |
(-) |
(+++) |
(+++) |
(+++) |
(+++) |
|
Artocarpus camansi |
(+) |
(-) |
(-) |
(+++) |
(+) |
(+++) |
(++) |
Legend: (+) present, (-) absent.
Leaf extracts of both A. heterophyllus and A. camansi have been characterized before (Durga et al., 2022; Adan et al., 2020; Rabeta et al., 2016) and phytochemical constituents identified were more or less similar to this study. The presence of such phytoconstituents in both plant extracts, especially flavonoids and tannins, is significant since they are known effective against parasitic nematodes (Speigler et al., 2017). To maximize the potential of plant extracts, proper extraction procedures could be the key. This is evident in a recent report showcasing that the extraction method could affect the content and bioefficacy of compounds. For example, Indrianingsih et al. (2024) highlighted that jackfruit leaves extracted with the use of the ultrasonication method yielded better results in terms of antioxidant and antibacterial activity, and total phenolic content than the traditional maceration technique. This could serve as a basis for further studies utilizing jackfruit and breadnut leaf extracts to maximize their potential.
LC50 and LC90 of A. heterophyllus and A. camansi
Lethal concentration (LC) is a very important parameter in determining the efficacy of plant extracts. Based on the in vitro assay, the most lethal concentration that can kill 50% of the population of nematodes is 3500-3800 ppm for A. heterophyllus and 4000 ppm for A. camansi (Figure 1). Based on the figure, it can easily be observed that A. heterophyllus is much more toxic than A. camansi but according to the statistical analysis, there was no significant difference between the two (P>0.860).
A similar trend was observed in LC90 where 11500 ppm of A. heterophyllus was required to kill 90% of the nematodes whereas a higher concentration of 14700 ppm for A. camansi was needed (Figure 2). This also indicated that A. heterophyllus was numerically more toxic than A. camansi, but similar to the LC50, there was no significant difference between the two (P>0.096).
Although the results were statistically comparable for the two plant extracts tested, the potency of jackfruit extract could be attributed to the fact that it has a higher level of tannin present compared to breadnut. Tannins bind in nematode cuticle and interfere with the cuticle structure ultimately leading to increased cuticle rigidity (Greiffer et al., 2022). So far, this is the first study that showed the lethal concentration values of jackfruit and breadnut leaf extracts against the model nematode, C. elegans.
Effect of A. heterophyllus and A. camansi on C. elegans mortality
The exposure of L4 to the different concentrations of the extract shows a significant effect on the mortality of C. elegans in any concentration (P<0.0001) (Figure 3). At 10000, 7500, and 5000 ppm, A. heterophyllus exhibited 85-87%, 71-73%, and 59-62% mortality, respectively. The different concentrations of the extract killed more than 50% of the population and it is concentration dependent, starting from the most lethal group; Group A (10000 ppm and Albendazole), group B (10000 ppm and 7500 ppm), group C (7500 ppm and 5000 ppm) and group D (PBS). Groups A, B, and C showed a significant difference to the (-) control group D (PBS) having the lowest mortality. On the other hand, group A, Albendazole and 10000 ppm have no significant difference with each other (P = 0.0993) indicating that the concentration of 10000 ppm of A. heterophyllus is as effective as that of the anthelmintic drug Albendazole.
Meanwhile, different concentrations of A. camansi also showed a significant effect on the mortality of L4 of C. elegans (P<0.0001) (Figure 4). At 10000 ppm and 7500 ppm, A. camansi exhibited 81-82% and 66-70% of mortality, respectively. While at 5000 ppm exhibited 55% and no more than 60%. Nevertheless, each concentration killed 50% of the population. The mortality rate is also concentration dependent starting from the most lethal group; group A (Albendazole and 10000 ppm), group B (10000 ppm and 7500 ppm), group C (7500 ppm and 5000 ppm), and the (-) control group D (PBS). Groups A, B, and C showed a significant difference to the (-) control group D (PBS) having the lowest mortality. On the other hand, like A. heterophyllus, Albendazole, and 10000 ppm have no significant difference with each other (P=0.2074) indicating that the concentration of 10000 ppm of A. camansi is as effective as that of the anthelmintic drug albendazole.
Direct anthelmintic effects of tannin-rich plants have already been reported to have negative effects on the mortality and development of gastrointestinal nematodes (Novobilsky et al., 2011) and a study (Katiki et al., 2013) showed up to 80% mortality of C. elegans treated with a tannin-rich plant. A. heterophyllus is a tannin-rich plant (Table 1) which also showed nematicidal activity against various nematodes (Prakash et al., 2009). A. camansi (++) is also a tannin-rich plant next to A. heterophyllus (+++) (Table 1). Like A. heterophyllus, exhibits a significant nematicidal effect. In line with the study of Katiki et al. (2013), plants with the most amount of tannin are much more effective in anthelmintic activity than those who have less, which correlates to the results (Figures 3 and 4) showing that A. heterophyllus is much more effective than A. camansi.
Effect of A. heterophyllus and A. camansi on the development and reproduction of C. elegans
The extracts of A. heterophyllus on day 1 (24 hours) (Figure 5A), delayed the development of L4 stage larva to young adult (P<0.0001). At concentrations of 10000 ppm, 7500 ppm, and 5000 ppm, 1, 3, and 4 nematodes, respectively stayed in their L4 stage for at least 24 hours. Whereas (-) control treatment (PBS) showed a higher development rate with 5 young adults and 2 fully developed adults, on the other hand, the (+) control treatment (albendazole) showed the least number of survivors. The survival of nematodes within the different concentrations
showed significant differences with each other, starting from the most number of survivors; group A (-) control (PBS), group B (7500 ppm and 5000 ppm), group C (10000 ppm and 7500 ppm) and group D (+) control (Albendazole) having the least number of survivors. Developmental resumption of L4 in young adults is shown on day 2 (Figure 5B) (P<0.0001). This time, the L4 larvae have proceeded to develop into young adults, and reproduction of larvae (L1-L4) has started. At concentrations 10000 ppm, 7500 ppm and 5000 ppm only 1, 3 and 4 larvae have developed further to young adults, respectively. It can be observed that L1 larvae (P= 0.0005) have a greater number of individuals among the larval stages, with the means of 7.2778 in 5000 ppm; 3.6667 in 7500 ppm, and 0.9667 in 10000 ppm, followed by L2 (P= 0.0004) then L3 (P= 0.0006). The lowest concentration (5000 ppm) was able to produce more L1-L4 stages than the highest concentration (10000 ppm) with the least number of L1-L4 larvae which indicates that it is concentration-dependent. The (-) control treatment still showed a higher development rate with now fully developed adults.
On day 3, it has more offspring and all the life stages are present from L1 to fully developed adult (Figure 5C). It can be observed that L1 (P<0.0001), has a greater number of individuals followed by L2 (P<0.0001), L3 (P<0.0001), and L4 (P<0.0001) having the least number of individuals present per concentration.
The extracts of A. camansi on day 1 (24 hours) (Figure 6A), delayed the development of L4 stage larva to young adult (P<0.0001). At concentrations of 10000 ppm, 7500 ppm, and 5000 ppm, only 2, 4, and 5 nematodes respectively stayed in their L4 stage for at least 24 hours. Whereas (-) control treatment (PBS) showed a higher development rate with 7 young adults, on the other hand, the (+) control treatment (Albendazole) showed the least number of survivors. The survival of nematodes within the different concentrations showed significant differences with each other, starting from the most number of survivors; group A (-) control (PBS), group B (7500 ppm and 5000 ppm), group C (10000 ppm and 7500 ppm) and group D (+) control (Albendazole) having the least number of survivors.
On day 2 (Figure 6B), the developmental resumption of L4 in young adults is shown (P<0.0001). The L4 larvae have proceeded to develop into young adults, still, it was delayed because it took almost 48 hours for
the L4 to develop into a young adult, and reproduction of larvae (L1-L4) has started. At concentrations 10000 ppm, 7500 ppm and 5000 ppm only 2, 3 and 5 L4 larvae have developed further to young adults, respectively. It can be observed that L1 larvae (P= 0.0003) have a greater number of individuals among the larval stages, with the means of 6.5278 in 5000 ppm; 4.7500 in 7500 ppm; and 1.0833 in 10000 ppm, followed by L2 (P= 0.0001) then L3 (P= 0.0001). The lowest concentration (5000 ppm) was able to produce more L1-L4 stages than the highest concentration (10000 ppm) with the least number of L1-L4 larvae, which indicates that it is concentration-dependent. The (-) control treatment still showed a higher development rate with now fully developed adults.
On day 3 (Figure 6C), there were more offspring and all the life stages were present from L1 to fully developed adult. It can be observed that L1 (P<0.0001), has a greater number of individuals followed by L2 (P<0.0001), L3 (P<0.0001), and L4 (P<0.0002) having the least number of individuals present per concentration.
According to the study of Palikaras and Tavernakis (2013). The life cycle of C. elegans completes only for 3 days at 25oC. When the egg hatches into L1 larvae, it takes 12 hours before it proceeds to L2 stage, from L2 stage it takes 8 hours to develop in the L3 stage, and from the L3 stage comes another 8 hours to become L4 larvae, from L4 larvae it takes 10 hours to become a young adult and another 8 hours to become a fully developed adult. On day 1, the development of L4 to young adults has been delayed for 24 hours which is supposed to be 10 hours only, here the larvae are still dormant to its L4 stage. Resumption of the development of L4 to young adults happened on day 2, still, it was delayed, because it took almost 48 hours in all for the L4 to proceed to young adults. On day 3, the young adult continued to develop into a fully developed adult and all larval stages are now present. The development was delayed or inhibited from its normal life cycle due to a trade-off. This may be attributed to the energy spent or underlying mechanisms (gene expression levels) that are needed to protect itself from foreign substances (as a defense mechanism) rather than to grow and develop. This may be in line with the study of Azaizeh et al. (2013), that tannin-rich plants impair the exsheathment or molting of L3-L4 larvae thus, inhibiting its growth and restricting the nematode from feeding. Previous research has also indicated that dietary restriction in C. elegans slows nematode development as well as increases its lifespan, although whether these two effects are linked is considered controversial (Bull et al., 2007). Furthermore, it is shown that A. heterophyllus extract slows, but does not prevent nematode development and reproduction. The development of the L4 larvae stages is the same as in the results of A. heterophyllus, on days 1, 2, and 3, where it delayed the development of the L4 stage instead of their usual 10 hours’ development time (Palikaras and Tavernakis, 2013). Thus, the observation shows that A. camansi extract also slows, but does not prevent nematode development and reproduction.
In comparison to the (+) control (Albendazole), in terms of mortality, A. heterophyllus and A. camansi would have the potential to be a source for antihelmintic medicine at high concentrations (10000 ppm) or more. But in terms of preventing the development and reproduction of the nematodes, it just slows its development and reproduction and does not completely inhibit unlike that of Albendazole which kills and also inhibits the nematode development and reproduction.
Conclusions and Recommendations
The study showed that A. heterophyllus and A. camansi have both phytochemical compounds such as alkaloids, flavonoids, saponins, steroids, and tannins but each plant had varying quantities. Both are tannin-rich plants A. heterophyllus (+++) and A. camansi (++) which could be responsible for most of the nematicidal activity. The LC50 and LC90 of both plants show that they are toxic and have positive anthelmintic activity in all concentrations for it killed 50% of the population even with the lowest concentration (5000 ppm). The remaining surviving nematodes continued to develop and reproduce but the extracts of different concentrations delayed the development of L4 larvae to young adults for about 48 hours. Despite the delay in the development and reproduction of the nematodes, A. heterophyllus and A. camansi can potentially become an anthelmintic medicine at higher concentrations.
The experimental setup presented here can give the foundation for further studies of the morphological effect on C. elegans treated with A. heterophyllus and A. camansi. Moreover, it could also serve as the basis for the development of a bio-based antihelmintic drug as an alternative to synthetic antihelmintic drugs.
Acknowledgement
The authors thank Prof. Dr. Bart Braeckman of the Laboratory for Aging Physiology and Molecular Evolution at the Faculty of Sciences, Ghent University for the C. elegans provision.
Novelty Statement
This study was first to assess the in vitro antihelminthic potential of the two crude extracts of A. heterophyllus and A. camansi leaves using the model nematode, C. elegans.
Author’s Contribution
Estrelle Anne Tacbas: Conceptualization, Methodology, Investigation, Data Curation, Resources, Formal Analysis, Writing-Original draft
Neil Pep Dave Sumaya: Conceptualization, Methodology, Data Curation, Formal Analysis, Resources, Supervision, Writing- Review and Editing
Nanette Hope Sumaya: Conceptualization, Methodology, Data Curation, Formal Analysis, Writing- Review and Editing
Conflict of interest
The authors have declared no conflict of interest.
References
Abcam, 2019. Counting cells using a hemacytometer. Retrieved from https://www.abcam.com/protocols/counting-cells-using-a-haemocytometer
Adan, A.A., Ojwang, R.A., Muge, E.K., Mwanza, B.K. and Nyaboga, E.N., 2020. Phytochemical composition and essential mineral profile, antioxidant and antimicrobial potential of unutilized parts of jackfruit. Fd. Res., 4(4): 1125-1134. https://doi.org/10.26656/fr.2017.4(4).326
Aremu, A.O., Finnie, J.F. and Van Staden, J., 2012. The potential of South African medicinal plants used as anthelmintics their efficacy, safety concerns, and reappraisal of current screening methods, South African, J. Bot., 82: 134e150. https://doi.org/10.1016/j.sajb.2012.05.007
Azaizeh, H., Halahleh, F., Abbas, N., Markovics, A., Mukalda, H., Ungar, E.D. and Landau, S.Y., 2013. Polyphenols from Pistacia lentiscus and Phillyrea latifolia impair the exsheathment of gastro-intestinal nematode larvae. Vet. Physiol., 191: 44-50. https://doi.org/10.1016/j.vetpar.2012.08.016
Bethony, J., Brooker, S., Albonico, M., Geiger, S.M., Loukas, A., Diemert, D. and Hotez, P.J., 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet, 367(9521): 1521–1532. https://doi.org/10.1016/S0140-6736(06)68653-4
Bull, K., Cook, A., Hopper, N.A., Harder, A., Holden-Dye, L. and Walker, R.J., 2007. Effect of the novel anthelmintic emodepside on the locomotion, egg-laying behavior, and development of Caenorhabditis elegans. Int. J. Parasitol., 37: 627–636. https://doi.org/10.1016/j.ijpara.2006.10.013
Burns, A.R., Luciani, G.M., Musso, G., Bagg, R., Yeo, M., Zhang, Y. and Roy, P.J., 2015. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun., 6(1). https://doi.org/10.1038/ncomms8485
Durga, R., Kanimozhi, G., Senthilkumar, G. and Panneerselvam, A., 2022. Evaluation of phytochemical compounds from jackfruit leaves (Artocarpus heterophyllus, Lam.) and its GCMS Analysis. J. Sci. Trans. Environ. Technol., 16(1): 5-11.
Greiffer, L., Liebau, E., Herrmann, F.C. and Spiegler, V., 2022. Condensed tannins act as anthelmintics by increasing the rigidity of the nematode cuticle. Sci. Rep., 12(1): 18850. https://doi.org/10.1038/s41598-022-23566-2
Hari, A., Revikumar, K.G. and Divya, D., 2014. Artocarpus: A review of its phytochemistry and pharmacology. J. Pharma Search, 9(1): 7. Retrieved from http://nationalcollegeofpharmacy.yolasite.com/resources/2014-01-02.pdf.
Indrianingsih, A.W., Styaningrum, P., Windarsih, A., Suryani, R., Noviana, E. and Itoh, K., 2024. The effect of extraction method on biological activity and phytochemical content of Artocarpus heterophyllus (jackfruit) leaves extract concurrent with its principal component analysis. Process Biochem., 143: 135-147. https://doi.org/10.1016/j.procbio.2024.04.034
Katiki, L.M., Ferreira, J.F.S., Gonzalez, J.M., Zajac, A.M., Lindsay, D.S., Chagas, A.C.S. and Amarante, A.F.T., 2013. Anthelmintic effect of plant extracts containing condensed and hydrolysable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol., 192(1-3): 218–227. https://doi.org/10.1016/j.vetpar.2012.09.030
Katiki, L.M., Ferreira, J.F.S., Zajac, A.M., Masler, C., Lindsay, D.S., Chagas, A.C.S. and Amarante, A.F.T., 2011. Caenorhabditis elegans as a model to screen plant extracts and compounds as natural anthelmintics for veterinary use. Vet. Parasitol., 182(2-4): 264–268. https://doi.org/10.1016/j.vetpar.2011.05.020
Novobilský, A., Mueller-Harvey, I. and Thamsborg, S.M., 2011. Condensed tannins act against cattle nematodes. Vet. Parasitol., 182(2-4): 213–220. https://doi.org/10.1016/j.vetpar.2011.06.003
Palikaras, K. and Tavernarakis, N., 2013. Caenorhabditis elegans (Nematode). Found. Res. Technol., 1: 251-256. Retrieved from http://www.tavernarakislab.gr/publications/EoG2e.pdf
Prakash, O., Kumar, R., Gupta, R. and Mishra, A., 2009. Artocarpus heterophyllus (Jackfruit): An overview. Phcog. Rev., 3(6): 353-358. Retrieved from https://www.researchgate.net/publication/279761143_Artocarpus_heterophyllus_Jackfruit_An_overview
Rabeta, M.S. and Nor Syafiqah, M.J., 2016. Proximate composition, mineral and total phenolic contents, and scavenging activity of breadnut fruit (Artocarpus camansi). J. Trop. Agric. Food Sci., 44(1): 1-7.
Riley, S.P., Woodman, M.E., Holt, J. and Stevenson, B., 2017. Culture of Escherichia coli and related bacteria. Curr. Protoc. Essent. Lab. Tech., 15: 4.2.1–4.2.30. https://doi.org/10.1002/cpet.17
Rupa, A.P.M. and Portugaliza, H.P., 2016. Prevalence and risk factors associated with gastrointestinal nematode infection in goats raised in Baybay City, Leyte, Philippines. Vet World, 9(7): 728–734. https://doi.org/10.14202/vetworld.2016.728-734
Santhi, V.S., Salame, L., Dvash, L., Muklada, H., Azaizeh, H., Mreny, R. and Glazer, I., 2017. Ethanolic extracts of Inula viscosa, Salix alba, and Quercus calliprinos, negatively affect the development of the entomopathogenic nematode, Heterorhabditis bacteriophora. A model to compare gastro-intestinal nematodes developmental effect. J. Invert. Pathol., 145: 39–44. https://doi.org/10.1016/j.jip.2017.03.005
Soares Magalhães, R.J., Salamat, M.S., Leonardo, L., Gray, D.J., Carabin, H., Halton, K. and Clements, A.C.A., 2015. Mapping the risk of soil-transmitted helminthic infections in the Philippines. PLoS Negl. Trop. Dis., 9(9): e0003915. https://doi.org/10.1371/journal.pntd.0003915
Spiegler, V., Liebau, E. and Hensel, A., 2017. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Natl. Prod. Rep., 34(6): 627-643. https://doi.org/10.1039/C6NP00126B
Vadnal, J., Ratnappan, R., Keaney, M., Kenney, E., Eleftherianos, I., O’Halloran, D. and Hawdon, J.M., 2017. Identification of candidate infection genes from the model entomopathogenic nematode Heterorhabditis bacteriophora. BMC Genom., 18(1). https://doi.org/10.1186/s12864-016-3468-6
Wink, M., 2012. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules, 17(11): 12771–12791. https://doi.org/10.3390/molecules171112771
Zanzani, S., Gazzonis, A., Di Cerbo, A., Varady, M. and Manfredi, M., 2014. Gastrointestinal nematodes of dairy goats, anthelmintic resistance and practices of parasite control in Northern Italy. BMC Vet. Res., 10(1): 114. https://doi.org/10.1186/1746-6148-10-114
To share on other social networks, click on any share button. What are these?




