Histopathological Effect of Traces for Uranium on Cow Kidneys
Special Issue:
Emerging and Re-emerging Animal Health Challenges in Low and Middle-Income Countries
Histopathological Effect of Traces for Uranium on Cow Kidneys
Oula E. Hadi*, E.H. Altaee
Department of Pathology and Poultry Disease, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq.
Abstract | The pro-oxidant activity of uranium (U) is being evaluated in bovine kidneys, a tissue in which the toxic effects of this metal have been demonstrated. The study was conducted on several groups of grass-fed cows in different locations in Basra/Iraq. Histopathological examination of the kidneys revealed hemangiomatous transformation in cows with U in the kidney. Uranium levels were measured using a sodium iodide device, and samples were taken from the cows’ kidneys, as they are the organs most affected by uranium. It was noted that if low concentrations of uranium were taken, there would be inflammation in the tissues, and animal might die, leading to degeneration, necrosis, and inflammation. However, in high doses, it leads to the occurrence of various tumors in the kidneys or the occurrence of clear cell carcinoma, Sarcomatoid type of renal cell carcinoma. The results indicate that the levels of uranium found in grass lead to the depletion of the antioxidant defense system in cows and stimulate oxidative stress in the kidneys. Although at current uranium doses, restraint stressors have rarely shown additive adverse effects, their potential impact has not been underestimated.
Keywords | Histopathological effect of Uranium, Cell carcinoma, Necrosis, Inflammation
Received | September 21, 2024; Accepted | December 01, 2024; Published | December 09, 2024
*Correspondence | Oula E. Hadi, Department of Pathology and Poultry Disease, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq; Email: hsennaserh@yahoo.com
Citation | Hadi OE, Altaee EH (2024). Histopathological effect of traces for uranium on cow kidneys. J. Anim. Health Prod. 12(s1): 277-283.
DOI | https://dx.doi.org/10.17582/journal.jahp/2024/12.s1.277.283
ISSN (Online) | 2308-2801
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
The earth’s crust naturally contains uranium (Cuney, 2009). Its radioactivity and heavy metal toxicity make it one of the most severe pollution problems (Zou et al., 2011). Understanding the distribution and toxicity of uranium for humans and animals is crucial, especially in light of the rising amounts of uranium products being ingested or inhaled. Natural uranium comprises three isotopes including U234, U235, and U238. These isotopes differ in their radioactive properties but share similar chemical characteristics (ATSDR, 1999; Brugge et al., 2005).
One important method that uranium can contaminate animals with radionuclides is through their consumption of food, water, or soil (International Atomic Energy Agency, 2010). The known negative effects of uranium exposure from investigations and work-related exposures include chemically induced kidney toxicity, bladder damage, and lung cancer from exposure to radon, which is produced by radioactive decay (Chen et al., 2010).
Renal failure and injury to the proximal tubules may result from the location of the uranium accumulation in the tubules (Homma-Takeda et al., 2015). Moreover, numerous authors have shown that the liver (Souidi et al., 2005), the central nervous system (Lestaevel et al., 2005; Houpert et al., 2007), and the gastrointestinal tracts (Dublineau et al., 2007) are among the other biological targets of acute and chronic exposure to low uranium levels that have been shown to change.
Basrah Province is the main area in Iraq where uranium pollution is present. This study looked at the histopathological effects of uranium residues on cow kidney samples from the province of Basrah.
Cow kidney samples (n=100) were collected from the Basrah governorate, and these samples were gathered from agricultural fields in the same governorate where the animals are breeding there and produced.
To achieve an equilibrium state between the mother radionuclide and her daughters, the samples were cut, grilled, and exposed to the sun for a while before being dried in an oven at 70 °C for the entire night. They were then ground into a fine powder and kept in Marinelli beakers for a month. Finally, they were analyzed using a gamma spectroscopy detector NaI (TI) spectroscopy system with a crystal dimension of (3×3).
Calculations
Equation has been used to calculate the particular activity (Ahmed et al., 2013).
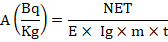
Where t is the measurement period (86400 sec), A is the sample’s specific activity, NET is the area under the peak, E is the detection efficiency, and Kg is the sample’s mass in kilograms.
The study was conducted in four different locations in the city of Basrah, where Uranium concentrations were studied in the four locations. It was found that the concentrations of site N were 40 samples, site E was 20 samples, site W was 20 samples, and site S was 20 samples, according to Table 1 shown.
Table 1: Uranium concentrations were studied in the four locations of Basrah?
|
No. |
Symbol |
Uranium concentration (Bk/Kg) |
|
1 |
N1 |
22.39±2.40 |
|
2 |
N2 |
22.90±2.37 |
|
3 |
N3 |
23.30±300 |
|
4 |
N4 |
23.30±3.01 |
|
5 |
N5 |
28.45±3.10 |
|
6 |
N6 |
28.50±3.09 |
|
7 |
N7 |
58.54±4.50 |
|
Table continued on next column...... |
||
|
No. |
Symbol |
Uranium concentration (Bk/Kg) |
|
8 |
N8 |
58.54±4.59 |
|
9 |
N9 |
66.80±2.03 |
|
10 |
N10 |
66.81±2.00 |
|
11 |
N11 |
85.90±0.37 |
|
12 |
N12 |
85.92±1.70 |
|
13 |
N13 |
85.90±0.73 |
|
14 |
N14 |
67.99±4.60 |
|
15 |
N15 |
67.99±5.00 |
|
16 |
N16 |
17.19±0.70 |
|
17 |
N17 |
17.19±0.68 |
|
18 |
N18 |
17.19±0.70 |
|
19 |
N19 |
17.19±0.70 |
|
20 |
N20 |
22.90±2.37 |
|
21 |
N21 |
22.93±2.40 |
|
22 |
N22 |
22.93±2.00 |
|
23 |
N23 |
22.90±2.40 |
|
24 |
N24 |
17.19±0.70 |
|
25 |
N25 |
17.19±0.70 |
|
26 |
N26 |
17.19±0.70 |
|
27 |
N27 |
17.22±0.70 |
|
28 |
N28 |
17.22±0.70 |
|
29 |
N29 |
17.22±0.70 |
|
30 |
N30 |
17.22±0.70 |
|
31 |
N31 |
17.22±0.68 |
|
32 |
N32 |
17.22±0.68 |
|
33 |
N33 |
17.22±0.68 |
|
34 |
N34 |
19.69±9.72 |
|
35 |
N35 |
19.70±5.68 |
|
36 |
N36 |
19.70±5.68 |
|
37 |
N37 |
19.70±5.68 |
|
38 |
N38 |
19.69±9.72 |
|
39 |
N39 |
19.17±9.00 |
|
40 |
N40 |
17.22±0.70 |
|
41 |
E1 |
0.0±0.030 |
|
42 |
E2 |
0.44±0.20 |
|
43 |
E3 |
2.72±O.070 |
|
44 |
E4 |
0.33±0.021 |
|
45 |
E5 |
1.15±0.50 |
|
46 |
E6 |
78.8±20.7 |
|
47 |
E7 |
0.36±0.24 |
|
48 |
E8 |
0.07±0.031 |
|
49 |
E9 |
0.85±0.47 |
|
50 |
E10 |
14.95±3.600 |
|
51 |
E11 |
0.07±0.030 |
|
52 |
E12 |
0.06±0.31 |
|
53 |
E13 |
0.16±0.80 |
|
Table continued on next page...... |
||
|
No. |
Symbol |
Uranium concentration (Bk/Kg) |
|
54 |
E14 |
0.61±038 |
|
55 |
E15 |
0.06±0.31 |
|
56 |
E16 |
0.06±0.31 |
|
57 |
E17 |
0.61±038 |
|
58 |
E18 |
0.61±038 |
|
59 |
E19 |
1.15±0.50 |
|
60 |
E20 |
1.15±0.50 |
|
61 |
W1 |
0.51±0.10 |
|
62 |
W2 |
2.19±0.10 |
|
63 |
W3 |
0.54±0.20 |
|
64 |
W4 |
2.93±0.14 |
|
65 |
W5 |
0.04±0.20 |
|
66 |
W6 |
5.74±0.21 |
|
67 |
W7 |
8.56±1.80 |
|
68 |
W8 |
8.43±0.33 |
|
69 |
W9 |
0.85±0.10 |
|
70 |
W10 |
10.03±2.50 |
|
71 |
W11 |
2.29±0.60 |
|
72 |
W12 |
6.71±1.70 |
|
73 |
W13 |
18.01±4.40 |
|
74 |
W14 |
64.81±17.00 |
|
75 |
W15 |
64.81±17.00 |
|
76 |
W16 |
64.81±17.00 |
|
77 |
W17 |
39.26±9.60 |
|
78 |
W18 |
39.26±9.60 |
|
79 |
W19 |
39.26±0.64 |
|
80 |
W20 |
39.26±0.64 |
|
81 |
S1 |
66.81±2.00 |
|
82 |
S2 |
39.26±0.64 |
|
83 |
S3 |
64.81±17.00 |
|
84 |
S4 |
60.81±17.01 |
|
85 |
S5 |
60.81±17.01 |
|
86 |
S6 |
64.81±17.00 |
|
87 |
S7 |
66.81±2.00 |
|
88 |
S8 |
85.92±1.70 |
|
89 |
S9 |
85.92±1.70 |
|
90 |
S10 |
33.92±1.22 |
|
91 |
S11 |
33.92±0.22 |
|
92 |
S12 |
21.15±0.50 |
|
93 |
S13 |
21.15±0.50 |
|
94 |
S14 |
21.15±0.55 |
|
95 |
S15 |
21.20±0.55 |
|
96 |
S16 |
22.20±0.50 |
|
97 |
S17 |
32.90±0.33 |
|
98 |
S18 |
32.90±0.33 |
|
99 |
S19 |
32.90±0.30 |
|
100 |
S20 |
30.22±0.22 |
Histopathological study
The tissue samples taken from the cow’s kidney were preserved in a 10% formaldehyde solution for fixation before being routinely processed with a histokine. Tissue slices were embedded in paraffin blocks, sectioned with a microtome, stained with hematoxylin and eosin, and examined under a light microscope to record the histological changes (Neamah et al., 2019).
As shown in Figure 1, cow’s kidney’s gross appearance reveals a proliferative, cortical, yellow orange to tan, well-circumscribed tumor that extends above the kidney’s capsule surface and resembles clear cell carcinoma.
Tumor mode cells in renal cancer with clear cell appearance are transparent. Tiny blood arteries that supply the tumor with copious amounts of blood frequently divide the tumor cell clusters. Chromophobe renal cell carcinoma is the opposite pathological disorder. It is a solid tumor of pale, granular cells with noticeable borders. Lastly, reticular cytoplasm perinuclear halos and wrinkled hyperchromatic nuclei are also observed in renal cell cancer sarcomatoid, which exhibits cellular atypia and a loss of typical epithelial higcellularity.
Numerous short- and long-term studies have demonstrated the toxicity of uranium to both people and animals (Mahdi et al., 2018). It is widely acknowledged that the kidney is the primary organ at risk for damage from heavy metal poisoning, with the proximal convoluted tubules bearing most of the damage. Our goal is to ascertain whether uranium-induced kidney damage progresses in terms of histopathology research. In the kidney of a cow with decreased uranium content, we saw vacuolation of the renal tubules, lobulated glomeruli infiltrations with intestinal fibrosis, and inflammatory cell infiltrations (Figure 2) compared to the histological chacteristics of the control (Figure 1).
Lesions showed signs of severe fibrosis and renal capsule invasion by mononuclear cells at higher concentrations. Many forms of renal cell cancers were discovered in areas with elevated uranium contents. The tumor in clear renal cell carcinoma comprises giant, spherical cells that join together to form enormous cell clusters. Many tumor cells had distinct cytoplasms or cell bodies, and the clusters of cells were frequently divided by microscopic blood veins that supplied the tumor with copious amounts of blood (Figure 3A). A solid tumor of granular pale cells with pronounced halos and wrinkled hyperchromatic nuclei was identified as chromophobe renal cell carcinoma. Sarcoma toid renal cell carcinoma also exhibited spindle cells, high cellularity, cellular atypia, and the absence of typical epithelial components.
Our research showed that uranium caused significant pathological alterations in cows kidneys. According to the research, exposure to uranium mainly affects the kidneys, bones, liver, lung, and central nervous system; nevertheless, the leading cause of death is renal insufficiency (Rozell et al., 2009; Al-Awadi and Alwan, 2014; Cheng et al., 2010). Following uranium injection, inhalation, or ingestion, inflammation is characterized as a harmful mechanism (Monleau et al., 2006; Weam et al., 2013; Orona and Tasat, 2012); nevertheless, there are few papers discussing its role in uranium nephrotoxicity (Zheng et al., 2015; I et al., 2018; Taulan et al., 2006). Despite the significant role, the inflammatory processes play a role in kidney injury (Ferenbach and Bonventre, 2015).
NF and KB activation was shown to elicit an inflammatory response in acutely exposed rats, and the transcriptome investigation (Taulan et al., 2006) observed the upregulation of three inflammatory genes, including osteopontin (opn), Pecam, and Gal-3, in mouse kidneys exposed to uranium. The inflammatory increase of intracellular and vascular cell adhesion molecules (ICAM) and VCAM generated by uranium (Bontemps et al., 2019) facilitates the recruitment of inflammatory cells to the kidney. The organ most vulnerable to uranium poisoning is the kidney, and it is widely known that uranium damages and malfunctions this organ, leading to either acute or chronic renal illness (UNSCEAR, 2016). Chronic oxidative stress, inflammation, DNA damage, and cell death are the leading causes of uranium-induced renal impairment (Guéguen et al., 2017; Haley et al., 1982; Diamond et al., 1989; Nada et al., 2011). Elevated urine glucose, protein, and electrolyte excretion indicate renal impairment (calcium, magnesium, sodium, potassium, and inorganic phosphate). Most likely due to decreased reabsorption from the proximal tubules or changed renal cell transport properties (Banday et al., 2008; Bontemps et al., 2019; Sánchez et al., 2001; Cappello and Macario, 2019).
Based on the exposure route and the molecule’s solubility, uranium has been classified as carcinogenic in several experimental investigations. One description of carcinogenesis is as a step event (Asic et al., 2017). The first step involves the direct and indirect mutation of kidney cells’ DNA (Yellowhair et al., 2018; Yazzie et al., 2003; Hamilton et al., 1997; Periyakaruppan et al., 2006; Wise et al., 2007), which turns them from healthy cells into possibly cancerous ones. After uranium exposure, DDR processes are presumably compromised, leading to an accumulation of nuclear DNA modification that facilitates their proliferation (Holmes et al., 2014; Saad et al., 2016; Adawiya et al., 2021).
Conclusions and Recommendations
The effect of uranium present in the content of herbs fed to cows in different areas of the city of Basra was studied, and the health and tissue effects on the kidneys of cows fed on these herbs were studied. Uranium levels were measured using a sodium iodide device, and samples were taken from the cows’ kidneys, as they are the organ most affected by uranium. If low concentrations of uranium are taken, there are cases of tissue inflammation, but in high doses they lead to the occurrence of various tumors in the kidneys. The study’s findings suggest that uranium levels in herbs cause cows’ antioxidant defense systems to weaken and increase oxidative stress in their kidneys.
Acknowledgments
The authors thank all the staff working in your journal for their continuous cooperation with us in presenting the manuscript in a good scientific form, The authors also thank all those who helped us in the laboratory work and the research sites from which the samples were taken.
Novelty Statement
Samples were taken from different locations using the latest methods and modern devices. The work was done in laboratories that have modern devices and technologies and keep pace with the modern era in analyzing site samples in the laboratory.
Author’s Contribution
The authors of the manuscript collected the data, reviewed it, studied the biostatistical analysis, and wrote the manuscript. The authors also declare that they have approved the manuscript and approved its content.
Funding
No funding to declare.
Data and materials
The respective authors will provide the datasets used in this study upon request.
Declarations
The College of Veterinary Medicine Clinic’s Ethical Committee permitted the study to proceed in Basra City. The authors declare no competing interests.
Conflict of interest
The authors have declared no conflict of interest.
Adawiya MA, Auday T, Al-Bayati, Khalid HM, Hazim LM (2021). Caculation of radiation nuclei concentrations in fertilized and unfertilized plants samples using gamma spectroscopy. Neuro Quantol., 19(2): 13-18. https://doi.org/10.14704/nq.2021.19.2.NQ21012
Ahmed FS, Mazin ME, Nada FT (2013). Determination of uranium concentration in urine of workers in an Iraqi phosphate mine and fertilizer plants. J. Radioanal. Nucl. Chem., pp. 187–193. https://doi.org/10.1007/s10967-013-2420-3
Al-Awadi A, Alwan JM (2014). The influence of whole sonicated pseudomonas aeruginosa antigens on experimental p. Aeruginosa arthritis in rabbits. Iraqi J. Vet. Med., 38(1): 1-10. https://doi.org/10.30539/iraqijvm.v38i1.246
Asic A, Kozaric A, Besic L, Mehinovic L, Hasic A, Kozaric M, Hukic M, Marjanovic D (2017). Chemical toxicity and radioactivity of depleted uranium: The evidence from in vivo and in vitro studies. Environ. Res., 156: 665–673. https://doi.org/10.1016/j.envres.2017.04.032
ATSDR (1999). Agency for toxic substances and disease registry. Toxicological profile for uranium (update) Atlanta, Georgia, USA: U.S. Department of Health and Human Services, Public Health Service.
Banday AA, Priyamvada S, Farooq N, Yusufi ANK, Khan F (2008). Effect of uranyl nitrate on enzymes of carbohydrate metabolism and brush border membrane in different kidney tissues. Food Chem. Toxicol., 46: 2080–2088. https://doi.org/10.1016/j.fct.2008.01.048
Bashair MS, Raad M, Al- Khafaji, Auday T, Al-Bayati (2016). Determination of radionuclides concentrations in soil around of Al-Tuwaitha nuclear research center in Iraq by using gamma spectroscopy analysis system. J. Chem. Biol. Phys. Sci., 6(3): 996-1005.
Bontemps A, Conquet L, Elie C, Magneron V, Gloaguen C, Kereselidze D, Tack K, Barbier OC, Guéguen Y (2019). In vivo comparison of the phenotypic aspects and molecular mechanisms of two nephrotoxic agents, sodium fluoride and uranyl nitrate. Int. J. Environ. Res. Publ. Health, 16: 1136. https://doi.org/10.3390/ijerph16071136
Brugge D, de Lemos JL, Oldmixon B (2005). Exposure pathway and health effects associated with chemical and radiological toxicity of natural Uranium: A review. Rev. Environ. Health, 20: 177–193. https://doi.org/10.1515/REVEH.2005.20.3.177
Cappello F, Macario AJL (2019). Depleted uranium induces human carcinogenesis involving the immune and chaperoning systems: Realities and working hypotheses. Med. Hypoth., 124: 26–30. https://doi.org/10.1016/j.mehy.2019.01.018
Chen J, Mereyhof DP, Tracy BL (2004). Model results of kidney burdens from uranium intakes. Health Phys., 86: 3-11. https://doi.org/10.1097/00004032-200401000-00003
Cheng KL, Hogan AC, Parvy DL, Markich SJ, Harford AJ, Van Dam RA (2010). Uranium toxicity and speciation during chronic exposure to the tropical freshwater fish, Mogurnda Mogurnda, Chemosphere, 79: 547–554. https://doi.org/10.1016/j.chemosphere.2010.02.017
Cuney M (2009). The extreme diversity of uranium deposits. Mineral. Depost., 44(1): 3-9. https://doi.org/10.1007/s00126-008-0223-1
Diamond GL, Morrow PE, Panner BJ, Gelein RM, Baggs RB (1989). Reversible uranyl fluoride nephrotoxicity in the Long Evans rat. Fundam. Appl. Toxicol., 13: 65–78. https://doi.org/10.1093/toxsci/13.1.65
Dublineau I, Grandcolas L, Grison S, Baudlin C, Paquet F, Voisin P, Aigueperse J, Gourmelon P (2007). Modifications of inflammatory pathways in Rat intestine following chronic ingestion of depleted uranium. Toxicol. Sci., 98(2): 458–468. https://doi.org/10.1093/toxsci/kfm132
Ferenbach DA, Bonventre JV (2015). Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol., 11: 264–276. https://doi.org/10.1038/nrneph.2015.3
Gilliand FD, Hunt WC, Pardilla M, Key CR (2000). Uranium mining and lung cancer among Navajo men New Mexico and Arizona, 1969-1993. J. Occup. Environ. Med., 41: 278–283. https://doi.org/10.1097/00043764-200003000-00008
Guéguen Y, Roy L, Hornhardt S, Badie C, Hall J, Baatout S, Pernot E, Tomasek L, Laurent O, Ebrahimian T, C Ibanez, S Grison, S Kabacik, D Laurier, M Gomolka. (2017). Biomarkers for Uranium risk assessment for the development of the CURE (Concerted Uranium Research in Europe) molecular epidemiological protocol. Radiat. Res., 187: 107–127. https://doi.org/10.1667/RR14505.1
Haley DP, Bulger RE, Dobyan DC (1982). The long-term effects of uranyl nitrate on the 35 structure and function of the rat kidney. Virchows Arch. B Cell Pathol. Incl. Mol., 41: 181–192. https://doi.org/10.1007/BF02890280
Hamilton MM, Ejnik JW, Carmichael AJ (1997). Uranium reactions with hydrogen peroxyde studied by EPR-spin trapping with DMPO. J. Chem. Soc. Perkin Trans., 12: 2491–2494. https://doi.org/10.1039/a702509b
Holmes AL, Joyce K, Xie H, Falank C, Hinz JM, Wise JP (2014). The impact of homologous recombination repair deficiency on depleted uranium clastogenicity in Chinese hamster ovary cells: XRCC3 protects cells from chromosome aberrations, but increases chromosome fragmentation. Mutat. Res. Mol. Mech. Mutagen., 762: 1–9. https://doi.org/10.1016/j.mrfmmm.2014.02.001
Homma-Takeda S, Kitahora K, Suzuki K, Blyth BJ, Suya N, Konishi T, Terada Y, Shimada Y (2015). Cellular localization of uranium in the renal proximal tubules during acute renal uranium toxicity. J. Appl. Toxicol., 35(12): 1594–1600. https://doi.org/10.1002/jat.3126
Houpert P, Frelon S, Monleau M, Bussy C, Chazel V, Paquet F (2007). Heterogenous accumulation of uranium in the brain of rats. Radiat. Prot. Dosimetry, 127(1-4): 86–89. https://doi.org/10.1093/rpd/ncm255
I J, Yuan Y, Zheng J, Hu N (2018). Hydrogen sulfide alleviates uranium-induced kidney cell apoptosis mediated by ER stress via 20S proteasome involving in Akt/GSK-3beta/Fyn-Nrf2 signaling. Free Radic. Res., 52: 1020–1029. https://doi.org/10.1080/10715762.2018.1514603
International Atomic Energy Agency (2010). Hand book of parameter values for the predication of radionuclide transfer in terrestrial and freshwater environments. Tech. Rep. Ser., 472: 194.
Lestaevel P, Bussy C, Paquet F (2005). Changes in sleep wake cycle after chronic exposure to uranium in rats. Neurotoxicol. Teratol., 27(6): 835–840. https://doi.org/10.1016/j.ntt.2005.07.005
Mahdi K, Auday TS, Najlaa RS, Ghuzlan SA (2018). Determination of radon concentrations in soil around al-Tuwaitha site using CR-39 detector. Eng. Technol. J., 36(2): 108-112. https://doi.org/10.30684/etj.36.2C.2
Monleau M, De Méo M, Paquet F, Chazel V, Duménil G, Donnadieu-Claraz M (2006). Genotoxic and inflammatory effects of depleted uranium particles inhaled by rats. Toxicol. Sci., 89: 287–295. https://doi.org/10.1093/toxsci/kfj010
Mulloy KB, James DS, Mohs K, Kornfeld M (2001). Lung cancer in a non smoking underground uranium miner. Environ. Health Perspect., 1009: 305–309. https://doi.org/10.1289/ehp.01109305
Nada FT, Rana MY, Nesreen B, Alrawi, Mazin ME (2011). Determination of uranium concentration in sheep organs for some Iraqi cities. Baghdad Sci. J., 8(2): 766-771. https://doi.org/10.21123/bsj.2011.8.3.766-771
Neamah G, Yousif E (2019). Determination of depleted uranium concentration and histopathological changes in local Iraqi fish and chickens. Iraqi J. Vet. Med., 43(2): 86-97. https://doi.org/10.30539/iraqijvm.v43i2.537
Neamah G, Yousif E, Anees AH (2019). Accumulation of chronic uranyl nitrate hexahydrate in kidneys and histopathology in adult albino rats. Onderst. J. Vet. Res., 23(11): 1095-1103.
Orona N, Tasat D (2012). Uranyl nitrate-exposed rat alveolar macrophages cell death: Influence of superoxide anion and TNF alpha mediators. Toxicol. Appl. Pharmacol., https://doi.org/10.1016/j.taap.2012.04.022
Periyakaruppan A, Kumar F, Sarkar S, Sharma CS, Ramesh GT (2006). Uranium induces oxidative stress in lung epithelial cells. Arch. Toxicol., 81: 389–395. https://doi.org/10.1007/s00204-006-0167-0
Rozell LE, Hahn FF, Lee RB, Parkhurst MA (2009). Assessing the renal toxicity of capstone depleted uranium oxides and other uranium compound. Health Phys., 96: 343–351. https://doi.org/10.1097/01.HP.0000338421.07312.ed
Saad MA, Taha YM, Muna AS, Asia H, Al-Mashhadani (2016). Assessment of radiological risk in the areas around surface disposal at Al-Tuwaitha site. Iraqi J. Phys., 14(30): 90- 97. https://doi.org/10.30723/ijp.v14i30.204
Sánchez DJ, Bellés M, Albina ML, Sirvent JJ, Domingo JL (2001). Nephrotoxicity of simultaneous exposure to mercury and uranium in comparison to individual effects of these metals in rats. Biol. Trace Elem. Res., 84: 139–154. https://doi.org/10.1385/BTER:84:1-3:139
Sangetha V, Rekha PDR, Dinesh U, Arun AB (2016). Biochemical and histopathological response of the Swiss albino micre treated with uranyl and its recovery, renal failure. Natl. Library Med., 38(5): 770–775. https://doi.org/10.3109/0886022X.2016.1160248
Souidi M, Gueguen Y, Linard C (2005). In vitro effects of chronic contamination with depleted uranium on CYP3A and associated nuclear receptors PXR and CAR in the rat. Toxicology, 214(1-2): 113–122. https://doi.org/10.1016/j.tox.2005.06.006
Taulan M, Paquet F, Argiles A, Demaille J, Romey MC (2006). Comprehensive analysis of the renal transcriptional response to acute uranyl nitrate exposure. BMC Genom., 7: 2. https://doi.org/10.1186/1471-2164-7-2
UNSCEAR (2016). Sources, effects and risks of ionizing radiation, united nations scientific committee on the effects of atomic radiation (UNSCEAR) 2016 Report; UNSCEARU: New York, NY, USA.
Weam S, Al-Hamadany, Khaid H, Mahdi A-U, Auday T, Subhi A-B, Hussein AS (2013). Detection of radiation pollution with depleted uranium in some Iraqi cattle with cancer and their field soils. Int. J. Adv. Res., 1(8): 7-11.
Wise SS, Thompson WD, Aboueissa AM, Mason MD, Sr JPW (2007). Particulate depleted uranium is cytotoxic and clastogenic to human lung cells. Chem. Res. Toxicol., 20: 815–820. https://doi.org/10.1021/tx700026r
Yazzie M, Gamble SL, Civitello AER, Stearns DM (2003). Uranyl acetate causes DNA single strand breaks in vitro in the presence of ascorbate (vitamin C). Chem. Res. Toxicol., 16: 524–530. https://doi.org/10.1021/tx025685q
Yellowhair M, Romanotto MR, Stearns DM, Lantz RC (2018). Uranyl acetate induced DNA single strand breaks and AP sites in Chinese hamster ovary cells. Toxicol. Appl. Pharmacol., 349: 29–38. https://doi.org/10.1016/j.taap.2018.04.022
Zheng J, Zhao T, Yuan Y, Hu N, Tang X (2015). Hydrogen sulfide (H2S) attenuates uranium- induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-kappaB pathways. Chem. Biol. Interact., 242: 353–362. https://doi.org/10.1016/j.cbi.2015.10.021
Zou W, Bai H, Zhao L, Li K, Han R (2011). Characterization and properties of zeolite as absorbent for removal of uranium (V1) from solution in fixed bed column. J. Radioanal. Nucl. Chem., 288(3): 779–788. https://doi.org/10.1007/s10967-011-1026-x
To share on other social networks, click on any share button. What are these?






