Fish Abundance and Diversity During Low and High Flow Seasons of River Ravi, Punjab, Pakistan
Fish Abundance and Diversity During Low and High Flow Seasons of River Ravi, Punjab, Pakistan
Hafiz Muhammad Ashraf, Hafiz Abdullah Shakir* and Javed Iqbal Qazi
Institute of Zoology, University of the Punjab, Lahore, Pakistan.
ABSTRACT
The present study used multivariate analysis to determine the fish species diversity of the river Ravi, Punjab, Pakistan. Eight sampling sites were surveyed during the year of 2020 to ascertain fish diversity and abundance. Diversity indices computed by using Primer 7 Software for whole study compared seasonally. Cluster analysis (Euclidian distance) and poly component analysis performed by using Origin Software (2016). A total 877 (258 in low and 619 in high flow season) fish specimen was collected using a variety of fishnets and identified by using standard taxonomic keys based on morphometric characters. These fish specimens belong to 10 orders, 21 families, 37 genera and 50 species. The family Cyprinidae was containing 13 genera and 21 species. Eight species (Amblyceps mangois, Botia lohachta, Botia almorhae, Coptodon zillii, Ctenopharyngodon idella, Parambasis lala, Salmostoma phulo and Psilorhynchus nudithoracicus) were collected first time from river Ravi Pakistan. During high flow season, Cirrhinus mrigala and Captodon zilli showed highest relative abundance 13.89% and 13.73%, respectively. Highest frequency occurrence (FO) 87.5% was observed for Labeo rohita followed by two species Channa punctate and Cirrhinus mrigala (75%). Maximum Shannon-Weiner index 3.119 and 3.122, Margalef index 2.825 and 3.573, Evenness index 0.9728 and 0.9849 were recorded during low and high flow seasons, respectively. Taxonomic diversity (Delta) and total phylogenetic diversity (sPhi+) was found maximum during high flow season. Head Balloki upstream and Dhnad Balloki showed the highest diversity indices during low and high flow. The decline in number of fish species with less Shannon–Wiener index (<3.5) depicted the alarming situation of natural water body.
Article Information
Received 18 February 2023
Revised 25 April 2023
Accepted 16 May 2023
Available online 20 July 2023
(early access)
Published 16 July 2024
Authors’ Contribution
HAS and JIQ designed and supervised the study. HMA planned, surveyed, analyzed the data and prepared the manuscript. HAS and JIQ provided their expert guidelines and resources for study. HAS reviewed and finalized the manuscript.
Key words
Biodiversity, Abundance, Ravi Fish fauna, Ballok, Freshwater
DOI: https://dx.doi.org/10.17582/journal.pjz/20230218100231
* Corresponding author: [email protected]
0030-9923/2024/0005-2143 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Rivers are major parts of freshwater ecosystems. These water bodies are the determining factors in the growth of various civilizations in their vicinages (Benjamin et al., 1996). Freshwater fishes (almost 18,000 fish species) are one-fourth of all vertebrates on the world and deliver irreversible goods and services (Villéger et al., 2011; Allen and Pavelsky, 2018; Su et al., 2021; Laan, 2020). The decline in freshwater biodiversity due to habitat loss, overexploitation, discharge of untreated urban and industrial effluents in water bodies and changes in water flow is more when compared with terrestrial ecosystem (Dudgeon et al., 2006). About more than 14% of the
world’s freshwater fish are currently endangered or extinct (IUCN, 2021).
Rivers, streams, estuaries, man-made reservoirs and lakes, are abundant in Pakistan (FAO, 2003). Pakistan constitutes a transitional zone, which attributed the great influence and variation in fish fauna (Dudgeon et al., 2006). Pakistan has about 193 freshwater fish species (Rafique and Khan, 2012). These species were classified as Actinopterygii, Teleostei, 3 cohorts, 6 super orders, 13 orders, 30 families, and 86 genera (Costa and Schulz, 2010).
River Ravi is a small river of Indus water basin system with a total length of 750 km in Punjab province, Pakistan. This river rises from the glaciers in the mid-Himalayas of Himachal Pradesh, India, and flows northwestern through India and enters in Pathankot at Chaundh and forms a 37 km border between India and the state of Jammu and Kashmir before entering Pakistan via the villages of Tadyal and Kot Naina in Sialkot’s Shakargarh Tehsil, where it joins the Ujh river. After covering about 450 km, river Ravi joins River Chenab near Head Sidhnai at District Khanewal. The River has been highly affected by various anthropogenic activities like discharging of untreated industrial effluents and urban sewage that cause detrimental impacts on aquatic life especially on fish fauna (Shakir and Qazi, 2013; Shakir et al., 2013a, b).
Biodiversity is pivotal for outcomes of aquatic ecosystem such as water cleaning, nutrient cycling, livelihood, and food supply (Costanza et al., 1997). Therefore, maintaining biodiversity is important for a healthy environment and a good life (Helfrich et al., 2009). The study of fish biodiversity has widely been used to categorize habitats variability, diagnose temporal changes in the aquatic environment and helps in formulating the conservation and management plans (Dale and Beyeler, 2001; Lin and Caramaschi,, 2005; Costa and Schulz, 2010; Lakra et al., 2010).
Biodiversity refers to the variety of different types of life on Earth. It is the life support system and an important factor for the resilience of an ecosystem (Elmqvist et al., 2003; Rawat and Agarwal, 2015). The interaction between different species, as well as the physical and limnological properties of an aquatic ecosystem, may promote the composition and novel structure of the fish fauna (Agnisto et al., 2005). Depth, food availability, spawning sites, topography, water current, and water physicochemical properties all play a role in the diversity and distribution of fish in a habitat. Freshwater biodiversity provides a broad variety of valuable goods and services for human societies – some of which are irreplaceable (Covich et al., 2004a). The value of biodiversity includes its direct contribution to economic productivity (e.g. fisheries), its “insurance” value in the event of unforeseen events, its worth as a repository of genetic knowledge, and its value in supporting the provision of ecosystem services (e.g. cleaning water) (Pearce et al., 1998; Heal, 2000; Covich et al., 2004b). As a regular characteristic, precise statistical information on fish biodiversity, population dynamics, and recruitment patterns is essential in developing fish conservation and management plans for our natural fisheries resources (Holmlund and Hammer, 1999). Therefore, it is essential to study fish diversity continuously in various ecosystems across the region. The present study designed to estimate biodiversity and relative abundance of fish species during low (pre-monsoon) and high (post monsoon) flow seasons of river Ravi, Punjab, Pakistan.
MATERIALS AND METHODS
Sampling sites
Fish sampling was carried out at eight sites of river Ravi, Punjab, Pakistan. The sampling start from upstream site Ravi Syphon (31° 72΄ N and 74° 46΄ E) which is situated near village Ghazi Kakka, district Sheikhupura and approximately 20 Km upstream of New Ravi Bridge Shahdra. No point source of pollution at this site or above was identified after the entrance of river in Pakistan. This site was characterized with relatively good water quality due to least disturbance with respect to urban pollutants from Pakistan side. The downstream sampling site Shahdra (31° 60΄ N and 74° 28΄ E) is situated near New Ravi Bridge, Lahore. Three major pumping stations (North East, Shadbagh and Shahdra Gauging Station) are throwing untreated municipal sewage effluent of Lahore city into the Ravi between the sites Syphon and Shahdra. Dhand Nanodogar (31° 35΄N and 74° 06΄E) and Noul village (31° 35΄N and 74° 02΄E) sites are situated in district Sheikhupura. The water of river Ravi fill up the area of Dhand Nanodogar during monsoon flow and disconnect from mainstream of river during low flow season. There are four major pumping stations throwing untreated municipal effluent of Lahore city in to the river Ravi before these sampling sites (Dhand Nanodogar and Noul village). Site Dhand Balloki (31°25΄N and 73° 91΄E) situated 7 km upstream from Head Balloki. This is largest Dhand of Ravi River, which fill up due to overflow of river water during monsoon season and disconnect from mainstream of river during low flow season. Head Balloki is located about 75 km southwest of Lahore at river Ravi in District Kasur. There are two off-taking canals from Head Balloki namely Lower Bari Doab Canal (LBDC) and Balloki Sulemanki (B.S) Link Canal. Both canals irrigate a vast area in the southwest of the Punjab. Sampling was also done at Head Balloki about 500 m upstream (31° 25΄ N and 73° 91΄ E) and 500 m down steam (31° 22΄ N and 73° 89΄ E) situated in District Kasur. Head Sidhnai (30°.56΄N 72°.16΄ E) the last Headworks over Ravi River before its confluence into Chanab River about 15 km ahead was the last sampling points. It is situated about 2 km from Abdul Hakeem in District Khanewal (Fig. 1). The lifeline of Pakistan’s water resources is monsoon precipitation that falls in summer
from July to September (Naheed et al., 2013). During present study, each sampling sites were visited during low (pre-monsoon) and high (post-monsoon) flow season of rive Ravi twice for fish sampling.
Fish sampling
Fish specimens were collected using different nets (cast nets, gill nets, and hand nets) with the help of professional fishermen. Mesh size of nets ranged from 0.2–5 cm with length (10 m) and height (1.6 m) was used. Netting performed from 0900 h to 1600 h continuously on each sites. Wooden boats were used to mitigate sound disturbance for aquatic organisms. Fish specimens were kept in ice-box and transported to Fish Lab, Institute of the Zoology, University of the Punjab, Lahore immediately for further analyses. Each specimen of each site was tagged using specific code and separately packed in labeled (date, site, time, and locality) plastics jar in 95% ethanol. Each specimen then identified using local morphometric and meristic characters-based identification key (Mirza and Sharif, 1996; Mirza and Sandhu, 2007).
The relative abundance (RA) of fish species was calculated using the formula as given;
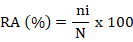
where, ni is number of individuals of a fish species, N = number of individuals of all fish species.
Occurrence frequency (OF) for all species calculated as following formula:
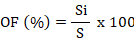
where, Si is number of sites in which a species found. S = total sampling sites.
Diversity indices
The diversity indices were calculated using following formulas.
Shannon–Wiener index calculated by;
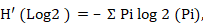
H′ is Shannon–Wiener index but for logs to the base two. Where’s pi is proportion of total sample represented by species i. Divide no. of individuals of species i by total number of samples.
Species richness was calculated by Margalef’s richness (d) as following;
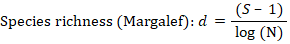
where S is number of species in a sample and N is number of specimens in the sample.
Evenness was calculated by using Pielou’s evenness formula as:

where’s H′ is Shannon Diversity index; S is number of Species.
Taxonomic diversity (Delta) and Total phylogenetic diversity (sPhi+) was also calculated by using Primer software.
Cluster, principal component and similarity percentile analyses
Clustering methods are widely used methods in identifying and recognizing similarity/dissimilarity patterns between sites. The cluster analysis was performed for all sampling sites with low and high flow seasons using Origin (Version 2016) statistical software (Clarke and Warwick, 2001).
The principal component analysis (PCA) is expressed by the following equation:
PCi = a1iV1 + a2iV2+……….. + aniVn
where PCi is the principal component i and ani is (n = 1….n) the loading (correlation coefficient) of the original variables Vn (Statheropoulos et al., 1998). PCA is a frequently used approach for reducing the number of variables in datasets and interpreting them in a meaningful way (Jolliffe and Cadima, 2016). The PCA was conducted using the statistical software Origin (2016).
Similarity percentile (SIMPER) analysis identified the species mainly responsible for the dissimilarity in abundance between sampling sites. It was determined by using PRIMER (Version 7) statistical software (Clarke and Warwick, 2001).
RESULTS
In present study, total 877 fish specimens were collected from eight sites of River Ravi; 258 during low and 619 high flow seasons. Each fish specimen was identified using morphometric and meristic characters. The sampled fishes belonged to 10 orders, 21 families, 37 genera and 50 species (Table II).
It was observed that the order Siluriformes showed more diversity with 8 (38.10 %) families, followed by Anabantiformes and Cypriniformes with 3 (14.29 %) for each, while order Cichliformes, Gobiiformes, Osteoglossiformes, Clupeiformes, Ovalentaria, Mugiliformes and Synbranchiformes were recorded with single (4.76%) family (Table III). The family Cyprinidae was the predominant representing 21 (42%) species followed by Bagridae with 4 (8%) species, Siluridae and Ambassidae with 3 (6%) each, Botiidae and Osphronemidae with 2 (4%), while other families observed with 1 (2%) species (Fig. 2).
Table I. List of fish spices collected during each season with their relative abundance (RA) and frequency of occurrence (FO).
|
Family/ Species |
Low flow season |
High flow season |
IUCN status |
||||
|
n |
RA % |
FO % |
n |
RA % |
FO % |
||
|
Osphronemidae |
|||||||
|
Trichogaster fasciata |
6 |
2.33 |
12.5 |
27 |
4.36 |
50 |
LC |
|
Trichogaster lalius |
7 |
2.71 |
25 |
9 |
1.45 |
12.5 |
LC |
|
Badidae |
|||||||
|
Badis badis |
1 |
0.39 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Channidae |
|||||||
|
Channa punctata |
15 |
5.81 |
75 |
12 |
1.94 |
37.5 |
LC |
|
Cichlidae |
|||||||
|
Coptodon zillii |
7 |
2.71 |
37.5 |
85 |
13.73 |
37.5 |
LC |
|
Botiidae |
|||||||
|
Botia almorhae |
0 |
0.00 |
0 |
7 |
1.13 |
12.5 |
LC |
|
Botia lohachata |
0 |
0.00 |
0 |
2 |
0.32 |
12.5 |
LC |
|
Cyprinidae |
|||||||
|
Amblypharyngodon mola |
23 |
8.91 |
25 |
80 |
12.92 |
12.5 |
LC |
|
Bangana dero |
6 |
2.33 |
25 |
1 |
0.16 |
12.5 |
LC |
|
Barilius modestus |
5 |
1.94 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Barilius vagra |
0 |
0.00 |
0 |
1 |
0.16 |
12.5 |
LC |
|
Chela cachius |
11 |
4.26 |
25 |
0 |
0.00 |
0 |
LC |
|
Cirrhinus mrigala |
28 |
10.85 |
62.5 |
2 |
0.32 |
12.5 |
LC |
|
Cirrhinus reba |
10 |
3.88 |
25 |
86 |
13.89 |
75 |
LC |
|
Ctenopharyngodon idella |
0 |
0.00 |
37.5 |
20 |
3.23 |
25 |
ND |
|
Cyprinus carpio |
4 |
1.55 |
0 |
10 |
1.62 |
12.5 |
VU |
|
Esomus danrica |
0 |
0.00 |
0 |
5 |
0.81 |
12.5 |
LC |
|
Hypophthalmichthys molitrix |
0 |
0.00 |
0 |
4 |
0.65 |
12.5 |
NT |
|
Labeo bata |
4 |
1.55 |
0 |
15 |
2.42 |
12.5 |
LC |
|
Labeo boggut |
0 |
0.00 |
0 |
31 |
5.01 |
12.5 |
LC |
|
Labeo calbasu |
4 |
1.55 |
25 |
4 |
0.65 |
37.5 |
LC |
|
Labeo catla |
2 |
0.78 |
25 |
1 |
0.16 |
12.5 |
LC |
|
Labeo dyocheilus |
2 |
0.78 |
12.5 |
1 |
0.16 |
12.5 |
LC |
|
Labeo rohita |
21 |
8.14 |
75 |
52 |
8.40 |
87.5 |
LC |
|
Osteobrama cotio |
33 |
12.79 |
50 |
48 |
7.75 |
37.5 |
LC |
|
Puntius chola |
1 |
0.39 |
12.5 |
5 |
0.81 |
12.5 |
LC |
|
Salmostoma bacaila |
11 |
4.26 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Table continues on next column.... |
|||||||
|
Family/ Species |
Low flow season |
High flow season |
IUCN status |
||||
|
n |
RA % |
FO % |
n |
RA % |
FO % |
||
|
Salmostoma phulo |
0 |
0.00 |
25 |
2 |
0.32 |
12.5 |
LC |
|
Psilorhynchidae |
|||||||
|
Psilorhynchus nudithoracicus |
2 |
0.78 |
12.5 |
0 |
0.00 |
|
ND |
|
Notopteridae |
|||||||
|
Chitala chitala |
1 |
0.39 |
12.5 |
1 |
0.16 |
12.5 |
NT |
|
Clupeidae |
|||||||
|
Gudusia chapra |
3 |
1.16 |
25 |
20 |
3.23 |
50 |
LC |
|
Ambassidae |
|||||||
|
Chanda nama |
18 |
6.98 |
25 |
7 |
1.13 |
25 |
LC |
|
Parambassis baculis |
0 |
0.00 |
0 |
21 |
3.39 |
12.5 |
LC |
|
Parambassis lala |
0 |
0.00 |
0 |
2 |
0.32 |
12.5 |
NT |
|
Gobiidae |
|||||||
|
Glossogobius guiris |
2 |
0.78 |
25 |
5 |
0.81 |
25 |
LC |
|
Mugilidae |
|||||||
|
Minimugil cascasia |
4 |
1.55 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Amblycipitidae |
|||||||
|
Amblyceps mangois |
4 |
1.55 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Bagridae |
|||||||
|
Mystus bleekeri |
3 |
1.16 |
12.5 |
4 |
0.65 |
25 |
LC |
|
Mystus cavasius |
1 |
0.39 |
12.5 |
11 |
1.78 |
25 |
LC |
|
Mystus vittatus |
0 |
0.00 |
0 |
7 |
1.13 |
12.5 |
LC |
|
Sperata seenghala |
3 |
1.16 |
25 |
0 |
0.00 |
0 |
LC |
|
Ritidae |
|||||||
|
Rita rita |
1 |
0.39 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Heteropneustidae |
|||||||
|
Heteropneustes fossilis Horabagridae |
0 |
0.00 |
0 |
5 |
0.81 |
12.5 |
LC |
|
Pachypterus atherinoides |
1 |
0.39 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Schilbeidae |
|||||||
|
Eutropiichthys vacha |
1 |
0.39 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Siluridae |
|||||||
|
Ompok bimaculatus |
1 |
0.39 |
12.5 |
0 |
0.00 |
0 |
NT |
|
Wallago attu Sisoridae |
9 |
3.49 |
50 |
21 |
3.39 |
62.5 |
VU |
|
Bagarius bagarius |
2 |
0.78 |
25 |
2 |
0.32 |
25 |
NT |
|
Glyptothorax telchitta |
1 |
0.39 |
12.5 |
0 |
0.00 |
0 |
LC |
|
Mastacembelidae |
|||||||
|
Macrognathus pancalus |
0 |
0.00 |
0 |
3 |
0.48 |
12.5 |
LC |
n, number of individuals; RA, realtive abundance; FO, frequency of occurrence.
Table II. Comparative account of various taxonomic studies of icthyofauna at River Ravi.
|
S. No |
Study year |
No of fish species |
References |
|
1 |
1943 |
49 |
Ahmed (1943) |
|
2 |
1970 |
65 |
Mirza (1970) |
|
3 |
2002 |
49 |
Ahmed and Mirza (2002) |
|
4 |
2001-2003 |
50 |
Khan et al. (2011) |
|
5 |
2011-2013 |
22 |
Rathore and Dutta 2015 |
|
6 |
2012-2013 |
19 |
Hussain et al. (2014) |
|
7 |
2014-15 |
38 |
Pervaiz et al. (2018) |
|
8 |
2020 |
50 |
Present study |
Table III. Number and percentage composition of families, genera and species of fishes under various orders.
|
Order |
Family n (%) |
Genus n (%) |
Species n (%) |
|
Anabantiformes |
3 (14.29) |
3 (8.11) |
4 (8) |
|
Cichliformes |
1 (4.76) |
1 (2.70) |
1 (2) |
|
Clupeiformes |
1 (4.76) |
1 (2.70) |
1 (2) |
|
Cypriniformes |
3 (14.29) |
15 (40.54) |
24 (48) |
|
Gobiiformes |
1 (4.76) |
1 (2.70) |
1 (2) |
|
Mugiliformes |
1 (4.76) |
1 (2.70) |
1 (2) |
|
Osteoglossiformes |
1 (4.76) |
1 (2.70) |
1 (2) |
|
Ovalentaria |
1 (4.76) |
2 (5.41) |
3 (6) |
|
Siluriformes |
8 (38.10) |
11 (29.73) |
13 (26) |
|
Synbranchiformes |
1 (4.76) |
1 (2.70) |
1 (2) |
The highest relative abundance (RA) were calculated as 13.89 and 12.79% for Cirrhinus mrigala and Osteobrama cotio during high and low flow seasons, respectively. While Barilius vagra, Labeo catla, Chitala chitala, Eutropiichthys vacha, Labeo dyocheilus, Bangana dero, Ompok bimaculatus, Pachypterus atherinoides and presented the lowest RA (0.16%) (Table I). Barilius vagra, Botia almorhae, Botia lohachata, Ctenopharyngodon idella, Esomus danricus, Heteropneustes fossilis, Hypophthalmichthys molitrix, Labeo boggut, Macrognathus pancalus, Mystus vittatus, Parambassis lala, Parambassis baculis and Salmostoma phulo missed in low flow season while Amblyceps mangois, Badis badis, Barilius modesticus, Chela cachius, Eutropiichthys vacha, Glyptothorax telchitta, Ompok bimaculatus, Pachypterus atherinoides, Rita rita, Salmostoma bacaila, Minimugil cascasia, Psilorhynchus nudithoracicus and Sperata seenghala missed in high flow season.
The highest frequency occurrence (FO) 87.5% was estimated for Labeo rohita in high flow season. While during low flow season highest FO 75% was recorded for two species (Labeo rohita and Channa punctata), followed by Cirrhinus mrigala (62.5%), Wallago attu (50%) and Osteobrama cotio (50%). Amblyceps mangois, Amblypharyngodon mola, Barilius modesticus, Barilius vagra, Botia almorhae, Botia lohachata, Labeo catla, Chitala chitala, Ctenopharyngodon idella, Esomus danricus, Eutropiichthys vacha, Heteropneustes fossilis, Labeo boggut, Labeo dyocheilus, Macrognathus pancalus, Mystus vittatus, Ompok bimaculatus, Parambassis baculis, Parambassis lala, Puntius chola, Pachypterus atherinoides, Rita Rita, Salmostoma bacaila and Minimugil cascasia had lower FO (12.5%).
Conservation status
The International Union for Conservation of Nature (IUCN) red list is well established, having a long history with the first red data books in the 1960s (Fitter and Fitter 1987). With respect to collected fish species in the present study, 4% are vulnerable (VU), 10% near threatened (NT), 4% not determined (ND) and 82 % least concern (LC) fish species according the Asia region red list by IUCN (2021) (Fig. 4). Moreover, fifteen-years (2007 to 2021) data of IUCN red list for fish species were at compared to assess the trend of annually increasing threatened species (Fig. 5).
Diversity indices
In the present study, the maximum value of shannon-wiener diversity calculated for Head Balloki downstream (3.119) and minimum for Head Sidhnai (1.835) during low flow season, while the maximum value for Head Balloki upstream (3.122) and minimum for Syphon site (0.65) during high flow season (Table IV).
The maximum species richness (d) recorded up to 2.825 for site Nanodogar Dhand and minimum 1.939 for site Dhand Balloki in low flow season. While maximum for site Dhand Balloki (3.573) and minimum for site Syphon (0.5581) were recorded during high flow season. The highest value of species richness (d) was found (3.573) during high flow season (Table IV).
Taxonomic diversity (Delta) found maximum for site Nanodogar Dhand (63.3) and minimum for site Head Sidhnai (34.86) during low flow season while maximum for site Head Balloki upstream (64.89) and minimum for Site Syphon (20.00) were noted during high flow season. Phylogenetic diversity (sPhi+) recorded maximum for the site Noul village (740) during low flow season and minimum for site Head Balloki upstream (480) while maximum for site Dhand Balloki (1040) and minimum for site Syphon (160) calculated during high flow season (Table IV).
HDB, Head Balloki; HDS, Head Sidhnai.
Cluster analysis
The dendrogram expressed that there was a prominent separation between samples collected from different sites. Two major clusters were arisen, corresponding to the low and high flow seasons, thus distinguished seasonally in the freshwater fish assemblages. The distance correlation range among sites was around 0.3833–1.059 in low flow season and maximum 0.3594–1.0098 distance in high flow season (Figs. ).
Poly component analysis (PCA)
The PCA was conducted for the most abundant fish species having RA% >2, using a correlation matrix and standardized variables. During low flow season Amblypharyngodon mola and Chanda nama showed highest abundance at site Dhand Nanodogar and Dhand Balloki, Cirrhinus mrigala, Captodon zilli, Labeo rohita and Wallago attu at site Head Balloki upstream, Shahdra and Noul villagea, while during high flow season. Cirrhinus mrigala, Labeo rohit and Wallago attu showed abundance at site Shahdra and Naoula and Cirrhinus reba, Trichogaster fasciata and Labeo bata at site Head Balloki downstream (Fig. 7)
SIMPER
During low flow seasons sites are divided into three groups (group a, Syphon; group b, Shahdra; Noul village, Head Balloki upstream and Head Sidhnai; group c, Dhand Nanodogar, Dhand Balloki and Head Balloki downstream). The average similarity levels of groups ‘b and c for the samples collected during low flow season were 45.89 % and 36.89 %, respectively while group (a) has less than two sites. The average dissimilarity between groups “a” and “b” was 92.67%; between “a” and “c” was 94.94% and between “b and c” was 79.74 %. During high flow season sites are divided into five groups (group a, Syphon; group b, Head Balloki downstream; group c, Dhand Nanodogar and Dhand Balloki; group d, Shahdra and Noul village and group e, Head Balloki upstream and Head Sidhnai). The average similarity levels of groups “c, d and e” were 40.35%, 65.55% and 26.20%, respectively while group (a and b) has less than two sites. The average dissimilarity (100, 91.77 and 76.31%) of group “a” with groups “c, d and e”; 91.75% between “a and b”; 89.28% (c and b), 85.59% (c and e), 85.23% (d and b), 78.00% (d and c), 76.31% (d and e) and 85.43% between (e and b) was recorded.
Discussion
In this study, we tried to estimate the seasonally (low and high flow season) abundance and diversity of fishes in river Ravi and recorded 50 species. In earlier surveys, Ahmed (1943) recorded 49 fish species, Mirza (1970) reported 65 and Ahmed and Mirza (2002) 49 fish species in river Ravi as discussed by Pervaiz et al. (2018). Khan et al. (2011) conducted an ichthyofaunal survey of the river Ravi and reported 50 species. Rathore and Dutta (2015) conducted the survey of the river Ujh (an important clean water tributary of the river Ravi, in Kathua district) and recorded the 5 orders, 10 families and 27 genera and 42 fish species. Hussain et al. (2014) conducted monthly surveys at floodplain situated on the River Ravi near Balloki Headworks from August 2012 to May 2013 and collected total 1703 fish samples which belongs to 7 orders, 8 families, 14 genera and 19 species. Hussain et al. (2015) reported 22 species and 9 families during their study (2011-2013) from river Ravi. Pervaiz et al. (2018) surveyed the river Ravi from the period of July, 2014 to June, 2015 and found 381 fish samples consists 38 species, 21 genera and 10 families. Numerically compared the fish species found from river Ravi found in previous studies with present study. Maximum 65 species was found in 1970 followed by 50 species in current study, while minimum 19 species reported during the study period of 2012 -2013.
In the present study, eight species (Amblyceps mangois, Badia badis, Botia almorhae, Captodon zilli, Glyptothorax telchitta, Hypophthalmichthys molitrix, Parambassis lala and Salmostoma phulo) were collected first time from river Ravi. These species were not reported from earlier studies conducted by (Ahmed, 1943; Mirza, 1970; Khan et al., 2011; Pervaiz et al., 2018). The 18 species Ailia punctata, Ailia coilia, Aspidoparia morar, Chanda ranga, Chanda baculis, Crossocheilus diplocheilus, Gagata cenia, Gara gotyla, Glyptothorax stocki, Glyptothorax punjabensis, Labeo dero, Macrognathus aculeatus, Monopterus cuchia. Nandus nandus, Nemacheilus sp., Sisor rhabdophorus and Tor putitora, were missing in the present study in comparison with earlier studies (Pervaiz et al., 2018). These differences may have arisen due to stray occurrences, sampling biases or the distribution of pollutants entering the river from various sources as well as other forms of human disturbances. When huge amounts of untreated urban and industrial effluents discharged in water bodies, it may cause rapid mortality of fish fauna (Austin, 1998). and Shakir et al. (2013) reported deteriorated water quality of river Ravi due to anthropogenic activities and industrial effluents. The reduction in water flow especially after the indus water basin treaty with India is also one of the cause of decline in fish diversity in river Ravi (Pervaiz et al., 2018)
Order Siluriformes was found dominant with 8(38.10%) families followed by anabantiformes and cypriniformes with 3 (14.29%) each. Similarly, Hussain et al. (2014) recorded that order Siluriformes was dominant with two families and dominant family Cyprinidae which was represented by six genera, followed by Notopteridae with two genera and Bagridae, Siluridae, Beloninidae, Channidae, Cichlidae and Mastacembelidae each represented with one genera. Rathore and Dutta (2015) expressed that the Siluriformes (4 families) was followed by Cypriniformes (3 families) while others Perciformes, Synbranchiformes and Beloniformes each constituted a single family. The faimily Cyprinidae found dominant with 21 species and 60.32% abundances, followed by Bagridae with 4 species, Ambassidae with 3 species, Botiidae, Mastacembelidae, Osphroneidae, Siluridae and Sisoridae each with 2 species, others with single species in present study. Family Cyprinidae showed dominance in the results of Pervaiz et al. (2018).
Cirrhinus mrigala and Osteobrama cotio have the maximum RA% (13.89 and 12.79) estimated during high and low flow season, respectively. Almost similar results were reported as highest RA (13.5%) for Labeo rohita followed by Wallago attu (13.2%) and Cirrhinus mrigala (12.6%). Khan et al. (2011) catched the more number of individuals Puntius sophore (439, RA=24.6%) followed by Oreochromis aureus Steindachner (169, RA = 9.5%) from river Ravi at Head Balloki. Latif et al. (2016) also calculated the highest (RA = 16.03%) for Puntius sophore from river Chenab. Akhi et al. (2020) was recorded a significantly higher (P < 0.05) number of fish individuals during the post-monsoon season followed by the pre-monsoon season, and the minimum number was reported during the monsoon season. Site based species diversity found highest at Dhand Balloki (54.05%) with population (32.96%) during high flow while during low flow season found highest species diversity (32.43%) at sampling site Dhand Nandogar and Noul village with population (20.16 and 18.99%, respectively) (Fig. 3). Labeo rohita showed highest frequency occurrence (87.5%) in high flow season while Channa punctata and Labeo rohita (75%) during low flow season. The findings of present study is in line with Hussain et al. (2016) in which Labeo rohita and Cirrhinus mrigala showed highest FO (100% for both) and RA (24.18 and 18.73 %) while Rita rita showed the lowest FO (20%) and RA (0.22%).
The IUCN provided a baseline information and a global context, to monitor the change in status of species and helpful for establishment of conservation priorities at the local level. In present study, the two VU fish species (Cyprinus carpio and Wallago attu), five NT species (Bagarius bagarius, Chitala chitala, Hypophthalmichthys molitrix, Ompok bimaculatus and Parambassis lala), 41 species are counted as LC were captured, while two species are ND (Table I, Fig. 5). Cyprinus carpio reported vulnerable speices in the IUCN status (IUCN, 2011, 2014, 2021). Significant decline of population of Wallago attu might be due to pollution and overharvesting (Rafique and Khan, 2012). The Wallago attu was declared as near threatened in 2014 and vulnerable in 2021 (IUCN, 2014, 2021). Some of the threatened species might become extinct in the near future (Albert et al., 2021). Fifteen years (2007 to 2021) data of IUCN red list for fish species showed the average annually increase in critically endangered (30), endangered (57) and vulnerable (47) fish species. The primary drivers of freshwater species reduction and ecosystem degradation are habitat loss and degradation, water withdrawal, overexploitation and pollution, and the introduction of non-native species (Ellison, 2004; Revenga and Kura, 2003).
The Shannon-Wiener diversity H′ (log 2) is a method used widely to compare diversity between seasons and different habitats. Comparison of both season showed the highest value of H′(log2) (3.122) during high flow season. Head Balloki up and down stream both sites showed the highest value of Shannon-Wiener diversity during high and low flow season, respectively. Hussain et al. (2014) studied commercial fish community of a floodplain lake situated on River Ravi and reported highest Shannon index (2.67581) in December and lowest (1.80945) in May. Hussain et al. (2015) assessed the relative diversity of the river Ravi’s commercially significant fish fauna and reported Shannon-wiener diversity index 2.749, 2.706 and 2.654 for the years 2011, 2012 and 2013, respectively. Purusothaman et al. (2016) studied the seasonal contribution of fish diversity from southeast coast of India and their findings showed that maximum H′(log2) (5.670) value in premonsoon and minimum (4.666) during monsoon. Ansari et al. (1995); and Sathianandan et al. (2012) also reported similar seasonal variations. Kindong et al. (2020) studied seasonal changes in fish diversity in the Yangtze River Estuary and recorded Shannon index values maximum (2.25) in December 2013. It was described that only in healthy and biodiversity rich areas have the Shannon value of more than 3.5 (Clarke and Warwick, 2001). In present study, the Shannon index was recorded lower than 3.5 indicted that all sampling sites are not healthy and biodiversity rich areas in both seasons. Shakir et al. (2013a, b) reported that the discharging of untreated urban sewage and industrial effluents cause detrimental effects on aquatic life especially on fish.
In the present study, the highest species richness index (d) was found (2.825 and 3.573) for the sites Dhand Nanodogar and Dhand Balloki during low and flow seasons, respectively. The species richness was comparatively higher during high flow season. Similarly, Hussain et al. (2015) reported species richness 3.515, 3.421 and 3.27 for the years 2011, 2012 and 2013, respectively. Hussain et al. (2016) calculated the maximum species richness for the month of February (3.4206) and minimum for the month of April (2.1722). The maximum species richness was reported for the month of December (2.25) (Kindong et al., 2020). Aziz et al. (2021) reported that fish species diversity indices declined remarkably due to anthropogenic activity. The highest species evenness index (J) up to 0.9728 and 0.9849 were calculated for site Head Balloki upstream during low and high flow seasons, respectively. Minimum species evenness index 0.6117 and 0.5892 was recorded during low and high seasons, respective for the site Head Sidhnai. Our results are in line with Hussain et al. (2016) reported maximum species evenness (0.95691) for the month of August and minimum (0.82351) for the month of May. Hussain et al. (2015)recorded species evenness 0.7442, 0.7131 and 0.7102 for the year 2011, 2012 and 2013, respectively Highest value of Delta and sPhi+ up to 64.89 and 1040 were recorded during high flow season.
Human impact is usually seen as the primary cause of the reduction in fish population, whether directly through habitat degradation or destruction or indirectly through global warming, eutrophication, pollution, groundwater exploitation, or the introduction of exotic species (Andermann et al., 2020; Dudgeon, 2020; Albert et al., 2021). The construction of dams on rivers inhibits the movement of fish, which may limit gene flow and create population difference (Meldgaard et al., 2003). Human populations have direct and indirect effects and lead to modify local species diversity (taxonomic and phylogenetic) (Leprieur et al., 2008; Villeger et al., 2011). Taxonomic and phylogenetic diversity determine how organisms affect functioning and stability of ecosystem and are thus important for conservation (Naeem et al., 2012; Craven et al., 2018; Brun et al., 2019; Pimiento et al., 2020). Low taxonomic values, i.e., reduced taxonomic/phylogenetic ‘breadth’ of assemblages for their number of species, can show significant environmental stress instigated by human impacts (Su et al., 2021) or fishing impacts (Mohamed et al., 2009), whereas higher values point toward normal environmental conditions with no major human impacts.
Seasonal comparison of the various diversity indices showed, the highest value of Shannon index (3.122), species richness (3.573), phylogenetic diversity (1040), evenness (0.9849), taxonomic diversity (64.89) during high flow season. Ansari et al. (1995) and Sathianandan et al. (2012) also reported similar seasonal variations. Akhi et al. (2020) studied the seasonal abundance and diversity indices of fish assemblage and concluded that the values of diversity indices (Shannon–Wiener diversity, Margalef’s richness, and Pielou’s evenness) varied from season to season.
The most extensively utilized approaches for finding and recognizing similarity/dissimilarity patterns between sites are clustering methods (Ripley, 1996). Cluster analysis proved useful in identifying natural groupings of samples, with samples within a group being more similar than samples from separate groups. It also identified species assemblages, which are groups of species that co-occur in a predictable pattern throughout months. Hierarchical cluster analysis, based on a pairwise distance matrix between all sampling sites was used to find patterns of fish diversity. The percent similarity matrix was used to analyze seasonal variations in the community and the association between species. In the present study, seasonal changes in the occurrence of species resulted in the creation of clusters. There was a clear distinction between samples taken in both seasons from different sites. Similarly, Hussain et al. (2014) performed the multivariate cluster analysis for the sampled fishes collected from floodplain of river Ravi. Ansari et al. (1995) observed similar groupings due to seasonal fluctuations on the west coast of India, and Purusothaman et al. (2016) reported similar groupings. The similarity trends by using Bray-Curtis cluster analysis were performed among the diversity indices and observed three groups of available fish species (Momi et al., 2021) and among different indigenous fish families based on the number of individuals belonging to each family from the Mahananda River (Galib et al., 2016).
The researchers were framed numerous techniques to determine the number of components to be extracted based on a subjective ground (Dancey and Reidy, 2007). The eigenvalue falls below 1 is a popular stopping criterion to determine the number of components is to be stopped (Jackson, 1993). The eigenvalues ≥1 were employed and extracted the three significant components for both low and high flow seasons data. The first three components have an Eigenvalue ≥ 1, the highest Eigenvalue (2.68 and 2.175) together explaining (33.34% and 27.2%) of the variance in the initial variables in low and high flow season, respectively.
SIMPER analysis indicated that the species mainly responsible for the dissimilarity in abundance between sampling sites. Similarity matrices were examined using Bray–Curtis similarity index (Bray and Curtis 1957). Similar method was reported by elsewhere (Purusothaman et al., 2016). Akhi et al. (2020) performed SIMPER analysis and reported 52.86% overall average dissimilarity among the seasons. Kindong et al. (2020) was performed SIMPER analysis for seasonal changes in fish diversity, density, biomass, and assemblage and reported 74.19% and 81.1% average dissimilarity among stations and months, respectively. Haque et al. (2022) studied seasonal analysis of food items and reported the average dissimilarity (30.02%) between summer and monsoon season, was with fish scales (19.71%) being the most prevalent food item making this difference.
Conclusions
In present study, total 50 fish species were recorded from river Ravi. The decline in number of fish species as compared to past surveys might be consequence of discharging of untreated urban swage and industrial effluents in river Ravi. The reduction of water flow in river Ravi especially after Indus treaty with India also cause detrimental effects on ichthyo-diversity. The present study depicted based on Shannon index (< 3.5) that the river Ravi, Pakistan is not a healthy and biodiversity rich habitat. To save the natural water bodies, the urgent focused strategies of concerned authorizes is suggested.
Acknowledgement
The authors would like to acknowledge the Higher Education Commission, Islamabad for support to complete this study.
Funding
The financial support of Higher Education Commission, Islamabad under National Research Programme for Universities vide letter No. 8201/Punjab/NRPU/R&D/HEC/2017 dated 27.03.2019
IRB approval
Not applicable.
Ethical statement
Not applicable.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Agnisto, A., Thomas, S. and Gomes, L., 2005. Conservation of the biodiversity of Brazil’s inland waters. Conser Biol., 19: 646-652. https://doi.org/10.1111/j.1523-1739.2005.00701.x
Ahmad, N., 1943. Fishes of Lahore. Bull. Dep. Zool. Panjab Univ., 1: 352-374.
Ahmed, Z. and Mirza, M.R., 2002. Fishes of river Ravi from Lahore to Head Balloki. Biologia, Pakistan, 48: 317-322.
Akhi, M.M., Jewel, M.A.S., Haque, M.A., Sarker, B.J., Khatun, M.S., Paul, A.K., Islam, M.S. and Das, S.K., 2020. Multivariate approaches to determine the relationship between fish assemblage structure and environmental variables in Karatoya River, Bangladesh. Comm. Ecol., 21: 171–181. https://doi.org/10.1007/s42974-020-00015-6
Albert, J.S., Destouni, G., Duke-Sylvester, S.M. Magurran, A.E., Oberdorff, T., Reis, R.E., Winemiller, K.O. and Ripple, W.J., 2021. Warning to humanity on the freshwater biodiversity crisis. Ambio, 50: 85–94. https://doi.org/10.1007/s13280-020-01318-8
Allen, G.H. and Pavelsky, T.M., 2018. Global extent of rivers and streams. Science, 361: 585–588. https://doi.org/10.1126/science.aat0636
Andermann, T., Faurby, S., Turvey, S.T., Antonelli, A. and Silvestro, D., 2020. The past and future human impact on mammalian diversity. Sci. Adv., 6: eabb2313. https://doi.org/10.1126/sciadv.abb2313
Ansari, Z.A., Chatterji, A., Ingole, B.S., Sreepada, R.A., Rivonkar, C.U. and Parulekar, A.H., 1995. Community structure and seasonal variation of an inshore demersal fish community at Goa, west coast of India. Estuarine, Coast. Shelf. Sci., 41: 593–610.
Austin, B., 1998. The effects of pollution on fish health. J. appl. Microbiol., 85(Suppl. 1): 234S-242S. https://doi.org/10.1111/j.1365-2672.1998.tb05303.x
Aziz, M.S.B., Hasan, N.A., Mondol, M.M.R., Alam, M.M. and Haque, M.M., 2021. Decline in fish species diversity due to climatic and anthropogenic factors in Hakaluki Haor, an ecologically critical wetland in northeast Bangladesh. Heliyon, 7: e05861. https://doi.org/10.1016/j.heliyon.2020.e05861
Benjamin, R., Chakrapani, B.K., Devashish, K., Nagarathna, A.V. and Ramachandra, T.V., 1996. Fish mortality in Bangalore Lakes, India. Electron. Green J., 1: 1-11. https://doi.org/10.5070/G31610252
Bray, J.R. and Curtis, J.C., 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr., 27: 325-349. https://doi.org/10.2307/1942268
Brun, P., Zimmermann, N.E., Graham, C.H., Lavergne, S., Pellissier, L., Münkemüller, T. and Thuiller, W., 2019. The productivity-biodiversity relationship varies across diversity dimensions. Nat. Commun., 10: 5691. https://doi.org/10.1038/s41467-019-13678-1
Clarke, K.R. and Warwick, R.M., 2001. Change in marine communities: An approach to statistical analysis and interpretation, second ed. PRIMER-E: Plymouth. Nat. Environ. Res. Council, UK, pp. 190.
Costa, P.F. and Schulz, U.H., 2010. The fish community as an indicator of biotic integrity of the streams in the Sinos River basin, Brazil. Braz. J. Biol., 70: 1195-1205. https://doi.org/10.1590/S1519-69842010000600009
Costanza, R., d’Arge, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., Limburg, K., Naeem, S., O’ Neill, R.V., Paruelo, J. and Raskin, R.G., Sutton, P. and Belt, M., 1997. The value of the world’s ecosystem services and natural capital. Nature, 387: 253-260. https://doi.org/10.1038/387253a0
Covich, A.P., Ewel, K.C., Hall, R.O., Giller, P.E., Goedkoop, W. and Merritt, D.M., 2004a. Ecosystem services provided by freshwater benthos. In: Sustaining biodiversity and ecosystem services in soil and sediments (ed. D.H. Wall). Island Press, Washington D.C., U.S.A., pp. 45–72.
Covich, A.P., Austen, M.C., Ba-Rlocher, F., Chauvet, E., Cardinale, B.J., Biles, C.L., Inchausti, P., Dangles, O., Solan, M., Gessner, M.O., Statzner, B. and Moss, B.R., 2004b. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. BioScience, 54: 767–775. https://doi.org/10.1641/0006-3568(2004)054[0767:TROBIT]2.0.CO;2
Craven, D., Eisenhauer, N., Pearse, W.D., Hautier, Y., Isbell, F., Roscher, C., Bahn, M., Beierkuhnlein, C., Bönisch, G., Buchmann, N., Byun, C., Catford, J.A., Cerabolini, B.E.L., Cornelissen, J.H.C., Craine, J.M., Luca, E.D., Ebeling, A., Griffin, J.N., Hector, A., Hines, J., Jentsch, A., Kattge, J., Kreyling, J., Lanta, V., Lemoine, N., Meyer, S.T., Minden, V., Onipchenko, V., Polley, H,W., Reich, P.B., Ruijven, J.V., Schamp, B., Smith, M.D., Soudzilovskaia, N.A., Tilman, D., Weigelt, A., Wilsey, B. and Manning, P., 2018. Multiple facets of biodiversity drive the diversity stability relationship. Nat. Ecol. Evol., 2: 1579-1587. https://doi.org/10.1038/s41559-018-0647-7
Dale, V.H. and Beyeler, S.C., 2001. Challenge in the development and use of ecological indicators. Ecol. Indicators, 1: 3-10. https://doi.org/10.1016/S1470-160X(01)00003-6
Dancey, C.P. and Reidy, J., 2007. Statistics without maths for psychology. Pearson education.
Dudgeon, D., 2020. Freshwater biodiversity: Status, threats and conservation. Cambridge University Press, Cambridge, 2020. https://doi.org/10.1017/9781139032759
Dudgeon, D., Arthington, A.H., Gessner, M.O., Kawabata, Z.I., Knowler, D.J., Leveque, Naiman, R.J., Prieur-Richard, A., Soto, D., Stiassny, M.L.J. and Sullivan, C.A., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev., 81: 163-182. https://doi.org/10.1017/S1464793105006950
EEA (European Environment Agency), 2020. The European environment state and outlook. Knowledge for transition to a sustainable Europe. Publications Office of the European Union, Luxembourg, 2019.
Ellison, A.M., 2004. Wetlands of Central America. Wetl. Ecol. Manage., 12: 3–55.
Elmqvist, T., Folke, C., Nyströme, M., Peterson, G., Bengtsson, J., Walker, B. and Norberg, J., 2003. Response diversity, ecosystem change and resilience. Front. Ecol. Environ., 1: 488-494. https://doi.org/10.1890/1540-9295(2003)001[0488:RDECAR]2.0.CO;2
FAO, 2003. Information of the fisheries management in the Islamic Republic of Pakistan. pp. 15.
Fitter, R. and Fitter, M., 1987. The road to extinction: problems of categorizing the status of taxa threatened with extinction. Proceedings of a Symposium Held by the Species Survival Commission, Madrid, 7 and 9 November 1984. IUCN.
Galib, S.M., Rashid, M.A., Chaki, N., Mohsin, A.B.M. and Joadder, M.A.R., 2016. Seasonal variation and community structure of fishes in the Mahananda River with special reference to conservation issues. J. Fish., 4: 325-334. https://doi.org/10.17017/j.fish.110
Haque, M.A., Paul, S., Jewel, M.A.S., Atique, U., Paul, A.K., Iqbal, S., Mahboob, S., Al-Ghanim, K.A., Al-Misned, F. and Ahmed, Z., 2022. Seasonal analysis of food items and feeding habits of endangered riverine catfish Rita rita (Hamilton, 1822). Brazil. J. Biol., 82: e237040. https://doi.org/10.1590/1519-6984.237040
Heal, G.M., 2000. Nature and the marketplace: Capturing the value of ecosystem services. Island Press, Washington D.C., U.S.A.
Helfrich, L.A., Neves, R.J. and Parkhurst, J.A., 2009. Sustaining america’s aquatic biodiversity. What is aquatic biodiversity; Why is it important? communications and marketing. College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University, Publication, pp. 420-520.
Holmlund, C.M. and Hammer, M., 1999. Ecosystem services generated by fish populations. Ecolog. Econ., 29: 253–268.
Hussain, A., Ashraf, M., Altaf, M., Khan, W.A., Akmal, M. and Qazi, J.I., 2015. Relative diversity and threats to commercially important fishes of the Ravi, Pakistan. Biologia, Pakistan, 61: 145–149.
Hussain, A., Sulehria, A.Q.K., Ejaz, M., Maqbool, A. and Mirza, M.R., 2014. Temporal variations in Commercial Fish Community of a Floodplain of the River Ravi, Pakistan. Biologia, Pakistan, 60 (1): 73–80.
Hussain, M.Z., Latif, A., Shahzadah, W.A., Hussain, S., Iqbal, R. and Ali, M., 2016. Diversity, abundance and seasonal variations of fish community in lentic water bodies of Indus River at Ghazi Ghat, Pakistan. Pakistan J. Zool., 48: 59-65.
IUCN, 2021. The IUCN red list of threatened species. Version 2021-1. https://www.iucnredlist.org. Downloaded on (13th Agugust 2021).
IUCN Red List version 2021-1: Table 8a Last Updated: 25 March 2021. https://www.iucnredlist.org. (Downloaded on 18th August 2021).
IUCN. 2011. IUCN protected area management categories. (http://www.iucn.org/about/work/programmes/pa/pa_products/ wcpa_categories/).
IUCN. 2014. The IUCN Red list of threatened species. Version 2014.1.International Union for Conservation of Nature. Accessed at http://www.iucnredlist.org, 30 March 2014.
Jackson, D.A., 1993. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology, 74: 2204–2214.
Jolliffe, I.T. and Cadima, J., 2016. Principal component analysis: a review and recent developments. Phil. Trans. R.S. math., phys. Engineer. Sci., 374: 20150202.
Kindong, R., Wu, J., Gao, C., Dai, L., Tian, S., Dai, X. and Chen, J., 2020. Seasonal changes in fish diversity, density, biomass, and assemblage alongside environmental variables in the Yangtze River Estuary. Environ. Sci. Pollut. Res., 27: 25461–25474.
Khan, A.M., Ali, Z., Shelly, S.Y., Ahmad, Z. and Mirza, M.R., 2011. Aliens, a catastrophe for native fresh water fish diversity in Pakistan. J. Anim. Pl. Sci., 21(2 Suppl.): 435-440.
Laan, R.V.D., 2020. Freshwater fish list 30th ed. Sept. 2020. Van der Laan, Almere, The Netherland. ISBN: ISSN: 2468-9157
Lakra, W., Sarkar, U., Kumar, R., Pondy, A., Dubey, V. and Gusain, O., 2010. Fish diversity, habitat ecology and their conservation and management issues of a tropical River in Ganga basin, India. The Environmentalist, pp. 1-14. https://doi.org/10.1007/s10669-010-9277-6
Latif, M., Siddiqui, S., Minhas, I.B. and Latif, S., 2016. Diversity and abundance of fish fauna at Head Marala, Chenab River, Punjab, Pakistan. Canad. J. Pure appl. Sci., 10: 3971–3979.
Leprieur, F., Beauchard, O., Blanchet, S., Oberdorff, T. and Brosse, S., 2008. Correction: Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol., 6: e322. https://doi.org/10.1371/journal.pbio.0060322
Lin, D.S.C. and Caramaschi, E.P., 2005. Responses of fish community to the flood pulse and siltation in a floodplain Lake of the Trombetas River, Brazil. Hydrobiologia, 545: 75-91. https://doi.org/10.1007/s10750-005-2186-x
Meldgaard, T., Nielsen, E.E. and Loeschcke, V., 2003. Fragmentation by weirs in a riverine system: A study of genetic variation in time and space among populations of European grayling (Thymallus thymallus) in a Danish River system. Conserv. Genet., 4: 735-747. https://doi.org/10.1023/B:COGE.0000006115.14106.de
Mirza, M.R., 1970. A contribution to the fishes of Lahore including revision of classification and addition of new records. Biologia, Pakistan, 16: 71-118.
Mirza, M.R. and Sandhu, I.A., 2007. Fishes of the Punjab, Pakistan. Polymer Publications, Pakistan.
Mirza, M.R. and Sharif, H.M., 1996. A key to the fishes of Punjab, Ilmi kitab khana, Lahore, Pakistan.
Mohamed, K.S., Sathianandan, T.V., Krishnakumar, P.K., Zacharia, P.U., Asokan, P.K., Abdurahiman, K.P., Durgekar, R.N., Shettigar, V., 2009. Biodiversity stressed fishing zones in Kerala and Karnataka and identification of marine protected areas. In: Marine ecosystems challenges and opportunities (ed. E. Vivekanandan). Book of Abstracts, Marine Biological Association of India, February 9–12.
Momi, M.M.A, Islam, M.S., Farhana, T., Iqbal, S. and Paul, A.K., 2021. How seasonal fish biodiversity is impacting local river fisheries and fishers socioeconomic condition: A case study in Bangladesh. J. Surv. Fish. Sci., 7: 79-103. https://doi.org/10.18331/SFS2021.7.2.7
Naeem, S., Duffy, J.E. and Zavaleta, E., 2012. The functions of biological diversity in an age of extinction. Science, 336: 1401–1406. https://doi.org/10.1126/science.1215855
Naheed, G., Kazmi, D.H. and Rasul, G., 2013. Seasonal variation of rainy days in Pakistan. Pak. J. Meteorol., 9: 9-13.
Pearce, G.R., Chaudry, R. and Ghulam, S., 1998. A simple methodology for water quality monitoring. HR Wallingford in collaboration with International Waterlogging and Salinity Research Institute, Lahore, pp. 1-29.
Pervaiz, K., Mirza, Z.S., Siddiqui, S., Naghma, K., Waheed, S.H. and Usman, K., 2018. Studies on the fish biodiversity of River Ravi in Punjab Pakistan. J. Entomol. Zool. Stud., 6: 1442-1448.
Pimiento, C., Leprieur, F., Silvestro, D., Lefcheck, J.S., Albouy, C., Rasher, D.B., Davis, M., Svenning, J.C., and Griffin, J.N., 2020. Functional diversity of marine megafauna in the Anthropocene. Sci. Adv., 6: eaay7650. https://doi.org/10.1126/sciadv.aay7650
Purusothaman, S., Jayaprabha, N. and Murugesan, P., 2016. Diversity and seasonal variation of fish assemblages associated with trawl catches from southeast coast of India. Region. stud. Marine Sci., 6: 29–36.
Rafique, M. and Khan, N.U.H., 2012. Distribution and status of significant freshwater fishes of Pakistan. Rec. Zool. Surv. Pak., 21: 90-95.
Rathore, V. and Dutta, S.P.S., 2015. Fish fauna of river Ujh, an important tributary of the river Ravi, District Kathua, Jammu. Environ. Conserv. J., 16: 81-86. https://doi.org/10.36953/ECJ.2015.161213
Rawat, U.S. and Agarwal, N.K., 2015. Biodiversity: Concept, threats and conservation. Environ. Conserv. J., 16: 19-28. https://doi.org/10.36953/ECJ.2015.16303
Revenga, C. and Kura, Y., 2003. Status and trends of biodiversity of inland water ecosystems. Technical Series no. 11. Montreal, Canada: Secretariat of the Convention on Biological Diversity.
Ripley, B.D., 1996. Pattern Recognition and Neural Networks. Cambridge University Press, pp. 403.
Sathianandan, T.V., Mohamed, K.S. and Vivekanandan, E., 2012. Species diversity in fished taxa along the southeast coast of India and the effect of the Asian Tsunami of 2004. Mar. Biodiv. 42: 179–187.
Shakir, H.A., Chaudhry, A.S. and Qazi, J.I., 2013a. Impact of anthropogenic activities on physico-chemical parameters of water and mineral uptake in Catla catla from river Ravi, Pakistan. Environ. Monit. Assess., 185: 2833-2842. https://doi.org/10.1007/s10661-012-2753-3
Shakir, H.A. and Qazi, J.I., 2013. Impact of industrial and municipal discharges on growth coefficient and condition factor of major carps from Lahore stretch of Ravi. J. Anim. Pl. Sci., 23: 167-173.
Shakir, H.A., Qazi, J.I. and Chaudhry, A.S., 2013b. Monitoring the impact of urban effluents on minerals contents of water and sediments of four sites of the river Ravi, Pakistan. Environ. Monit. Assess., 185: 9705-9715. https://doi.org/10.1007/s10661-013-3284-2
Statheropoulos, M., Vassiliadis, N. and Pappa, A., 1998. Principal component and canonical correlation analysis for examining air pollution and meteorological data. Atmosph. Environ.t, 32 :1087-1095.
Su, G., Logez, M., Xu, J., Tao, S., Villéger, S. and Brosse, S., 2021. Human impacts on global freshwater fish biodiversity. Science, 371: 835–838. https://doi.org/10.1126/science.abd3369
Villéger, S., Blanchet, S., Beauchard, O., Oberdorff, T. and Brosse, S., 2011. Homogenization patterns of the world’s freshwater fish faunas. PNAS U.S.A., 108: 18003-18008. https://doi.org/10.1073/pnas.1107614108
To share on other social networks, click on any share button. What are these?









