Evalution of Bionematicides and Chemical Nematicides in Controlling Meloidogyne incognita on Grape, with Estimating Ethoprophos and Fenamiphos Residues and Chlorophyll Degree
Evalution of Bionematicides and Chemical Nematicides in Controlling Meloidogyne incognita on Grape, with Estimating Ethoprophos and Fenamiphos Residues and Chlorophyll Degree
Mohamed Adam1*, Hassan Sobhy2, Mohamed Abouzid3, Dalia Elhafny4 and El-Desouki Ibrahim5
1Department of Zoology and Nematology, Faculty of Agriculture, Cairo University, Egypt; 2Animal Resources, Faculty of African Post Graduate Studies, Cairo University, Egypt; 3Green Egypt Copmany Agriculture, Egypt; 4Pesticides Residues and Environmental Pollution Deptartment, Central Agricultural Pesticides Laboratory, Agricultural Research Center, Giza, Egypt; 5Department of Economic Entomology and Pesticides, Faculty of Agriculture, Cairo, Egypt.
Abstract | The potential of bionematicides: Taglis 90% w/w (Tagetes sp. 80% andAlgaesea 10%) and bioniconemal (Pacilomyceslilacinus 2× 106cfu/ gm) was assessed to control the root- knot nematode Meloidogyne incognita on grape, in comparison with chemical nematicides: Nemacab (ethoprophos 20% Ec), Dento (fenamiphos 40% Ec) and Vaydate (oxamyl 24% Ec) during two successive seasons (2016 and 2017) under field conditions. Drenching of soil with a suspension of all tested products significantly reduced the nematodes populations in soil compared with the untreated control in both trails. Among chemical and bio-nematicides, there was no significant differences in their control potential towards M. incognita. The best bionematicide was Taglis causing over 85% reduction while the best chemical nematicide was Nemacab with over 94% reduction in nematode populations after two months from their application in 2016. The same trend in the control potential for each treatment was also obtained in 2017 experiment. The residues of ethoprophos and fenamiphos in leaves and fruits were not detected during the whole experiment. The amounts of ethoprophos and fenamiphos decreased from zero to 5 days from (4.88 to 3.61 ppm) and (15.25 to 7.31 ppm) with loss of 26.02% and 47.93 %, respectively. For all tested products, no impacts on Chlorophyll degree in leaves were detected when applied on the soil. From the result of this research, it is recommended that both bionematicides could be used for nematode control in different agricultural systems especially, sustainable and organic farming, while the three chemical nematicides could be used in conventional agriculture without any negative impacts on plant and soil.
Received | April 11, 2023; Accepted | May 09, 2023; Published | May 30, 2023
*Correspondence | Mohamed Adam, Department of Zoology and Nematology, Faculty of Agriculture, Cairo University, Egypt; Email: mohamed-adam2007@agr.cu.edu.eg
Citation | Adam, M., Sobhy, H., Abouzid, M., Elhafny, D. and Ibrahim, E-D., 2023. Evalution of bionematicides and chemical nematicides in controlling Meloidogyne incognita on grape, with estimating ethoprophos and fenamiphos residues and chlorophyll degree. Pakistan Journal of Nematology, 41(1): 45-55.
DOI | https://dx.doi.org/10.17582/journal.pjn/2023/41.1.45.55
Keywords | Grape, Nematode, Bionematicides, Control, Residues, Clorophyll
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Grape (Vitis vinifera L.) is one of the most important fruit crops consumed in the world as fresh and processed products, such as jam, juice, jelly, grape seed extract, raisins, vinegar and grape seed oil (Ye et al., 2018). In 2019, approximately 6.9 million hectares throughout the world are devoted to grape production, with total production up to 75 million of tons (Unusan, 2020). In Egypt, grapevine is one of the most important and favorable fruit crops, and became the second fruit crop after citrus, where the planted area reached up to 188543 feddan producing 1378815 tons (El-Hady et al., 2015). Recently, plant-parasitic nematodes (PPN) pose a serious problem facing grapevines producers especially in tropical and sub-tropical countries (Briar et al., 2016). Root-knot nematodes (RKN, Meloidogyne spp.) is considered to be the most prevalent and economically important genus found in grape farms in the world. In Egypt, root-knot nematode (RKN) is the most common nematode occurring in both conventional and organic grape farms (Adam et al., 2014). It caused a severe problem especially in sandy soil, where, the environmental conditions are favorable for the development of these soil-dwelling parasites. RKN induce giant cell formation, resulting in root galls, causing deficiency of the uptake of water and nutrients (Williamson and Hussey, 1996). In addition to that, it plays role in transmission of grapevine fan leaf virus and is a specific vector of yellow vein virus. Consequently, poor growth and reduced root resistance to other soil pathogens, leading to plant death and a decline in quality and yield of the crop (Jacquet et al., 2005). The global estimated losses caused by nematode damage ranges from 12.5% to 20.0% of vine growth and productivity.
Chemical nematicides is still the most effective and reliable method of controlling PPN, however, their negative impacts on the environment and human health led to restrictions on their use (Nyczepir and Thomas, 2009). In addition, nematicides often do not provide long-term suppression of the pathogen. Whilst, other options e.g. cultural control and host plant resistance are often not practical or unsatisfactory, making their management more difficult in recent years (Anwar and McKenry, 2007; Tian et al., 2007). Natural nematicides based on bioagents and/or phytochemicals are alternatives for nematode control, especially in modern agricultural production systems that encourage concerns regarding food safety and environmental production (El-Saadony et al., 2021). However, only few commercial products are available, and these are frequently of limited efficacy when applied to field soils.
Due to the limited availability of remedies for them, management of PPN puts the sustainable success of grape farms at risk. The combination of both conventional and new control strategies has become a feasible concept of integrated pest management IPM. Numerous commercial products are available in the Egyptian market. These products are diverse in their chemical and biological activities and in their impacts on plant and soil. In the present work, two bio-nematicides and three chemical nematicides belonging to different groups were selected to assess their potential against the root-knot nematode M. incognita on grape. The objectives of this study were (i) to evaluate the efficacy of bio-nematicides in comparison with chemical nematicides in controlling M. incognita on grape in the field, and (ii) to estimate the impacts of these products on plant and soil.
Materials and Methods
Nematicides
Five commercial products namely Nemacab, Dento, Vaydate, Taglis and Bioniconemal were tested on grapevines in open field to evaluate their efficacy against RKN. These nematicides were applied in growing season as soil drench after dissolving in water following the recommended doses. The evaluation included six treatments (Table 1) plus check treatment.
Field experiments
Field experiments were conducted at the Noamany farm located in Al-Beheira governorate, 70 km Cairo-Alexandria desert road. This farm had ten-year old Fleam seedless vines heavily infected by RKN. Two field experiments were carried out in May–July 2016 and 2017 to evaluate the potential of five products to control RKN on grapes. The vines were grown in lines spaced 3m apart and the distance between each vine was 2m. The experiment consisted of six treatments and untreated control; seven treatments× four replicates × six vines/replicate in completely random block design. Before application of nematicides, soil samples were taken from each treatment to determine initial population. Each sample consisted of about 1.5 kg
Table 1: Trade name, active ingredients, chemical group and doses of the Nematicides used.
|
Trade name |
Active ingredients |
Chemical group |
Dosage / L water |
|
Vaydate |
Oxamyl 24% Ec |
Carbamate |
2 ml |
|
Nemacab |
Ethoprophos 20% Ec |
Organophosphorus |
2 ml |
|
Dento |
Fenamiphos 40% Ec |
Organophosphorus |
2 ml |
|
Taglis |
Tagets 80%, Algaesea 10% W/W |
Natural |
4 ml |
|
Bioniconemal I |
Pacilomyces lilacinus (2×106cfu/ gm) WP |
Natural |
2 gm |
|
Bioniconemal II |
Pacilomyces lilacinus (2×106cfu/ gm) WP |
Natural |
4 gm |
composed of 12 soil cores taken from the top 20 cm. Nematodes were extracted from a 250g subsample of well-mixed soil from each replicate by using Cobb’s sieving and decanting technique followed by a modified Baermann technique (Hooper et al., 2005) and counted under a stereoscopic microscope. All nematicides were applied at the recommended dosage as soil drench after dilution in tap water (Table 1), with 2 L of a nematicide suspension per tree and the control only received an equivalent amount of tap water. The evaluation of the experiment was determined after one and two months from nematicides application by estimating nematode population in all replicates of each treatment. Soil samples were collected and analyzed as described above. Numbers of J2 per 250g soil were calculated. Percentage nematode reduction in soil was determined according to Henderson and Tilton formula (Puntener, 1981) as follows:
Nematode reduction %= [1-(PTA/PTB×PCB/PCA)]×100
PTA = population in treatment after application, PTB = population in the treatment before application, PCB = population in the control before application, PCA = population in the control after application.
Residues of nematicides
Chemicals and reagents: Certified reference standards of ethoprophos (Figure 1) and fenamiphos (Figure 2) were obtained from Dr. Ehrenstorfer (Augsburg, Germany). Stock standard solutions (1000 μg/ml) were prepared individually in acetonitrile. All standard solutions were stored in the dark at 4ºC. Acetonitrile (HPLC grade) and acetic acid were purchased from Merck. Anhydrous magnesium sulfate and sodium chloride, purchased from Merck, were activated by heating at 250 ºC for 4 h in the oven before being used and kept in desiccators. Bulk primary secondary amine (PSA) sorbent (Bondesil-PSA, 40μm) and GCB were purchased from Supelco (Supelco, Bellefonte, USA). Sodium acetate was purchased from Aldrich.
Pesticide technical formulation of (Nemacab 20% EC), was supplied by Suez Canal Company, Egypt and was used at application rate of 2ml/L. (Dento 40% EC) was supplied by Shoura Company, Egypt and was used at application rate, 2 ml. /L.
Sample processing
For sampling; fruits, leaves and soil were collected at 0 (2 hours), 5, 10, 15 and 30 days after pesticides application. Sampling was performed randomly by collecting 2 kg of leaves, fruits and soil representative from each untreated and treated area at the previously mentioned intervals to study the dissipation of the pesticides. Field samples were transported in ice boxes to the laboratory and homogenized by ultra-thorax homogenizer except for soil samples. The homogenate and soil samples were stored at -5oC until further steps.
Standard preparation
Stock solutions: 1000 ug/ml reference standard solution of each pesticide was prepared in acetonitrile. Intermediate solutions: mixture standards of 100 ug/ml of each pesticide was prepared by diluting stock solution in acetonitrile. Calibration solutions: Calibration mixtures of concentration levels (0.05, 0.5, 1, 2, 4 and 8 µg/ml) for ethoprophos and (0.05, 0.5, 1.25, 2.5, 5, 10 and 20 ug/ml) for fenamiphos were prepared in acetonitrile.
Extraction and clean up
The samples were prepared with the QuEChERS method according to Anastassiades (Anastassiades et al., 2003). Ten grams of homogenized grapes and grapes leaves was weighed into a 50 ml PTFE centrifuge tube, 10 mL of acetonitrile was added, the tube was vigorously hand shaken for 1 min, 4 g of anhydrous MgSO4 plus 1 g of sodium chloride were added, the tube was hand shaken for 1 min, and the mixture was centrifuged at ≤ 4000 rpm for 5 min. Acetonitrile 1.0 mL was transferred into centrifuge tube for cleanup. An aliquot of 1.0 mL was transferred into the DSPE tubes containing 25 mg PSA and 150 mg MgSO4 for grapes samples and for grape leaves 10 mg GCB were added. The tubes were well capped and vortexed for 1 min., then centrifuged for 5 min at ≤4000 rpm. The combined eluate was filtered through a 0.22-µm nylon syringe filter into an auto sampler vial for GC injection.
Three grams of soil samples were weighted into 50 ml PTFE centrifuge tube and 7 ml of water were added, then the tube was vigorously hand shaken for 30 min. to hydrate the soil samples. Ten mL of acetonitrile with 1% acetic acid were added. NaOAc 1g and 4 g of anhydrous MgSO4 were added. The mixture was vortexed again for 1 min and centrifuged at ≤4000 rpm for 5 min. The combined eluate was filtered through a 0.22-µm nylon syringe filter into an auto sampler vial for GC injection (Yu et al., 2016).
Instrument condition
Determination of ethoprophos using GC-FPD: The gas chromatograph (HP6890) equipped with a flame photometric detector (FPD) with a phosphorus filter was used. A 30 m x 0.32 mm capillary column coated with a 0.25 μm thick film of 14% cyanopropil siloxane (PAS-1701) from Hewlett and Packard was used in combination with the following oven temperature program:
Initial temperature 190 ºC for 10 min. The carrier gas (N2) flow rate was 4 ml/min., splitless injection of a 2μl volume was carried out at 240 ºC. Hydrogen and air were used at flow rate 75 and 100 ml/min., respectively. Detector temperature were 250 ºC. The retention time was 1.33 min.
Determination of fenamiphos using GC-μECD
The gas chromatograph (HP6890) equipped with an auto-sampler (HP7673), a micro electron-capture detector was used. A 30 m x 0.32 mm capillary column coated with a 0.25 μm thick film of 5% phenylmethylpolysiloxane (HP-5) from Hewlett and Packard was used in combination with the following oven temperature program.
Initial temperature 220 ºC for 2 min., 5 ºC / min. up to 260 ºC and held for 1 min. The carrier gas (N2) flow rate was 4 ml/min., splitless injection of a 1μl volume was carried out. Thedetector and injector temperatures were 300 ºC and 280 ºC, respectively with retention time of 7.14 min.
Method validation
The linearity of the method was calculated from the results, directly proportional to the concentration of tested pesticide in solvent. Linearity was assessed by the correlation coefficient (R2) which resulted from the six points calibration curve at levels (0.05, 0.5, 1, 2, 4 and 8 µg/ml prepared in acetonitrile) for ethoprophos and the seven- points (0.05, 0.5, 1.25, 2.5, 5, 10 and 20 µg/ml) for fenamiphos. Matrix-matched calibration was used to compensate for the matrix effects. The matrix effectwasdefined as the influence of one or more co-extracted components from the sample on the measurement of tested pesticide concentration. The presence of these effects is demonstrated by comparing the response produced from the tested pesticide in a pure solvent solution with the samples, which were extracted and then spiked with tested pesticide in the same solvent at the same concentration levels.
Matrix effects (% ME) were calculated using the equation:
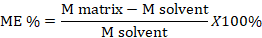
Where; ME: the matrix effect; M matrix: Slope of calibration curve in matrix; M solvent: Slope of calibration curve in the pure solvent. The %ME could be negative or positive and would be classified in three categories: no matrix effect (between -20% and 20%), medium matrix effect (between -50% and -20%) or (between 20% and 50%) and strong matrix effect (between -50% or above 50%).
The trueness, mean of recovery study was carried out on untreated grapes and soil sample by fortifying five replicates of the samples with tested pesticides standards at three levels ranging from 0.1 to 1 mg/kg by spiking of blank samples with standard solution.
Trueness was calculated using the following equation:
% R = (X/µ) × 100
% R: recovery percentage; X: experimental concentration of ethoprophos and fenamiphos mg/kg; µ: calculated concentration of ethoprophos and fenamiphosmg/kg.
The repeatability precision (RSDr) involved repeat of recovery levels (0.1 to 1 mg/kg), five replicates for each level per day on three different days.
RSDr = (SD / M) × 100
Where; SD: standard deviation of the replicates; M: the mean value of the recovery.
LOD for ethoprophos and fenamiphos was calculated as the minimum level at which the analyte can be reliably detected and LOQ is set by determining the analyte at different detectable concentrations based on SANTE (Sante/12682, 2019).
Estimation of chlorophyll degree
The Chlorophyll degree was estimated to determine whether these tested nematicides have any negative effects on plant leaves. The Chlorophyll degree was measured using a SPAD-502 handheld chlorophyll meter (SPAD-502plus, Knica Minolta, Osaka, Japan) (Zhang et al., 2022). After one and two months from nematicides application, five trees of each treatment were randomly selected as replicates to measure the Chlorophyll degree in their leaves. Readings were taken from mature leaves opposite the first bunch of the new shoot and the average values in the same treatment were calculated (Brunetto et al., 2012).
Statistical analysis
Field experiments data are presented as the means value ± standard deviation of 4 replicates. Statistical analyses were performed by IBM SPSS Statistics version 16. Data were subjected to an analysis of variance (One-way AVOVA). Differences between means were reported as significant if P < 0.05 using Tukey’s test. Five replicates were done for each sample. Microsoft Excel Program was used to calculate and analyze mean values and standard deviations. The other calculations were done using the above mentioned equations.
Results and Discussion
Effectiveness of bio-nematicides compared with nematicides in controlling M. inognitaon in grapes
The effect of bio-nematicides in comparison with chemical nematicides in reducing the population density of M. inognita on grapes was assessed in two trails in 2016 and 2017 (Figures 3 and 4). Before nematicide application, no significant differences in the average of initial population of RKN J2 levels among all treatments and the untreated control was detected in both trails. After application, all treatments significantly reduced the nematode population densities on grapes compared to the untreated check in both samples taken within the two months (P ≤ 0.05, Figures 1 and 2). All treatments revealed persistently suppressive effect on nematode counts along the evaluation period of two months. Both bio-nematicides; Taglis and Bioniconemal II gave a high decrease in the total number of J2 in soil samples reached over 84% and 81%, respectively, in comparison with Nemacab that resulted in the highest reduction reached over 90% after two months from their application in 2016. The other two nematcides; Vaydet and Dento caused a reduction in the number of J2 in soil samples reached over 87%, after 2 months from the application time in 2016. Among all treatments, no significant differences in their effectiveness in decreasing the nematode population density was observed, expect that the control potential obtained by Bioniconemal I was significantly lower than those obtained by Nemacab and/or Vaydet. The lowest reduction in nematode population was achieved by Bioniconemal I (one dose), which did not significantly differ from Bioniconemal II (duplicated dose). The same trend in the control potential for each treatment was also obtained in 2017 trail.
Our results showed that both commercial products P. lilacinus and Tagetes spp. were effective in managing M. incognita in soil and plant roots. These results agree with previous studies (Kiewnick and Sikora, 2003; Krueger et al., 2007; Karakas and Bolukbasi, 2019; Al-Hazmi et al., 2017).
P. lilacinus strain 251(PL251) was produced by solid state fermentation and water dispersible granule (WDG) formulation led to an excessive use of this biological nematicide in an integrated approach to control PPN (Kiewnick and Sikora, 2003). P. lilacinus strain UP1 formulated on rice substrate in powder form caused a significant reduction in number of nematodes on tomato in comparison with the commercial nematicide Nemacur (Oclarit and Cumagun, 2009). In this study Bioniconemal II (4 g/ L water) used as soil drench on grapevines gave over 81% reduction in the number of J2 in the soil compared with the untreated control. Pre-planting soil treatment by PL251 reduced the final nematode population in the tomato roots by up to 71% compared to the inoculated control (Kiewnick and Sikora, 2006). Our results showed that the nematode population density decreased clearly as the concentration of P. lilacinus (2× 106cfu/g product) applied in soil increased, where Bioniconemal II (4 g/ L water) was better than Bioniconemal I (2 g/ L water). The optimum doses of PL251were 1 × 106 CFU/g soil and 6 × 106 CFU/g soil for suppressing M. incognita and Radopholussimilis, respectively (Kiewnick and Sikora, 2006; Mendoza et al., 2007). Also, the EC50 values for the commercially formulated product ranged between 0.097 g and 0.08 g/500 cm3 soil, equivalent to 1.29 × 106 and 9.88 × 105 CFU/g soil for gall index and final population per root, respectively (Kiewnick and Sikora, 2006). The highest reduction in gall number was achieved with 7.92 x 106 spores per ml of P. lilacinus strain UP1 (Oclarit and Cumagun, 2009). Marigold (Tagetes spp.) has been known to suppress multiple genera of PPN especially M. incognita (Marahatta, 2012; Mervat et al., 2012). In this study, Tagiles (80% tagetsand 10% algaesea) caused 84% reduction in nematode population on grapes. This is due to production of a number of toxic bioactive compounds which included alpha terthienyl that is thought to be the main allelopathic compound responsible for nematode suppression (Gommers and Bakker, 1988; Marahatta et al., 2012). Nemagold is a commercial liquid product extracted from T. erecta and used for nematode control (Marahatta et al., 2012). For chemical nematicides, the three compounds effectively controlled RKN population associated with grapes when applied as soil drench with (4ml/grapevine). Nemacab (ethoprophos) that was the best one, has been previously used for controlling RNK in grapevines causing high reduction reachd up to 99%in soil with recommended rate 10-12 L ha incorporated in a drip irrigation of two hours (German et al., 2019). Application of oxamyl at the recommended dose (5 ml/ grapevine) resulted in high reduction of RKN reproduction on grapes in Egypt (Abdel-Sattar et al., 2020). Fenamiphos applied on grapes at 0.04% (0.1% v/v) reduced the nematode population over 80% compared with the untreated control (Aballay and Sepúlveda, 2004). These compounds are systemic nematicides that can inhibit feeding, temporarily inactivate, repel or kill PPN in the plant rhizosphere (Al-Azzeh and Abu-Gharbieh, 2004).
Residues of nematicides
Method validation: Evaluation of calibration curve linearity of ethoprophos and fenamiphos were done based on injections of standard solutions prepared in pure acetonitrile in series at (8, 4, 2, 1, 0.5, and 0.05 ug/g) for ethoprophos and (20, 10, 5, 2.5, 1.25, 0.5 and 0.05 ug/g) for fenamiphos for GC injection. Standard calibration curve was constructed by plotting analyte concentrations against peak areas. The correlation coefficient were (R2= 0.98 and R2= 0.99).
Regarding matrix effect, the relative responses were -9.02 and -19.44 for grapes and soil for ethoprophos, respectively and were -7.56 and –15.79 for grapes and soil for fenamiphos, respectively. It can be concluded that the matrix did not significantly suppress or enhance the response of the instrument. These results showed that there was no interfering endogenous peak and good performance of analysis was also achieved, indicating that this method meets the criteria of the European Union. Hence, this method can be used for routine residue analysis of ethoprofos and fenamiphos in grapes and soil matrices.
The trueness, mean recovery was carried out on untreated samples that were spiked with ethoprophos and fenamiphos at three levels (0.1-1ug/g) for grape and soil samples in five replicates. The method trueness and precision parameters in terms of average recovery and relative standard deviation were calculated and measured according to the European Union guidelines (Sante, 2019). The percentage recovery of ethoprophos and fenamiphos from the fortified grapes and soil samples is presented in Tables 2 and 3. Data showed that the obtained mean recoveries for grape and soil, respectively ranged from 95.21 to 100.58% and 79.51 to 85.27% with relative standard deviation (RSDs) ranged from 2.97 to 3.58 and 3.82 to 4.99 for ethoprophos. While the obtained mean recoveries for fenamiphos from grape and soil, respectively ranged from 90.55 to 98.24% and 75.12 to 89.36% with relative standard deviation (RSDs) ranged from 1.45 to 3.20 and 4.66 to 7.13. According to (Sante, 2019), the obtained mean recoveries were within the acceptable range (70-120%). Data indicated that the recovery from the grape samples was slightly higher than that from the soil. So, the value indicating that the method was sensitive and able to detect and quantify the analyte at low levels, and it is suitable for the determination of tested pesticide residue in grapes and soil.
The repeatability precision (RSDr) involved repeat of recovery levels (0.1 to 1 mg/kg), five replicates for each level per day on three different days. The (RSDr) value ranged from 5.82-8.44% and 6.47-8.79 for ethoprophos in grape and soil and 3.54-7.68% and 5.73-9.22 for fenamiphos in grape and soil. According to (Sante, 2019) the obtained (RSDr) values were within the acceptable range ≤20%.
Table 2: Recovery percentages of ethoprophos in grape and soil.
|
Fortification level ug/g n=5 |
Grape |
Soil |
|||
|
% Recovery ±RSD |
% RSDr |
% Recovery ±RSD |
% RSDr |
||
|
0.1 |
95.21±2.97 |
8.44 |
79.51±3.82 |
6.47 |
|
|
0.5 |
97.34±3.01 |
5.82 |
82.19±3.96 |
6.91 |
|
|
1 |
100.58±3.58 |
6.57 |
85.27±4.99 |
8.79 |
|
Table 3: Recovery percentages of fenamiphos in grape and soil.
|
Fortification level ug/g n=5 |
Grape |
Soil |
|||
|
% Recovery ±RSD |
% RSDr |
% Recovery ±RSD |
% RSDr |
||
|
0.1 |
90.55±1.45 |
3.54 |
75.12±4.99 |
5.73 |
|
|
0.5 |
95.05±2.22 |
4.39 |
80.86±4.66 |
7.57 |
|
|
1 |
98.24±3.20 |
7.68 |
89.36±7.13 |
9.22 |
|
SANTE 2019. Guidance SANTE/12682/2019-guidance document on analytical quality control and method validation procedures for pesticide.
The detection and quantification limit for ethoprofos and fenamifos were (0.05 and 0.1 ug/g, respectively, indicating good analytical precision.
Table 4: Dissipation of ethoprophos nematicide in soil, leaves and fruits of grape.
|
Time after application (days) |
Soil residues (µg/g) |
Leaves |
Fruits |
||
|
Ppm |
Loss% |
Persistence |
|||
|
Initial |
4.88±0.62 |
- |
100 |
N.D |
N.D |
|
5 |
3.61±0.17 |
26.02 |
73.97 |
N.D |
N.D |
|
10 |
1.8±0.28 |
63.11 |
36.88 |
N.D |
N.D |
|
15 |
0.19±0.03 |
96.1 |
3.89 |
N.D |
N.D |
|
30 |
0.06±0.02 |
98.77 |
1.23 |
N.D |
N.D |
Table 5: Dissipation of fenamiphos nematicide in leaves, fruits grape and soils.
|
Time after application |
Soil residues (µg/g) |
Leaves |
Fruits |
||
|
(days) |
PPM |
Loss% |
Persistence |
||
|
Initial |
15.25±0.80 |
- |
100 |
N.D |
N.D |
|
5 |
7.31±1.00 |
47.93 |
52.07 |
N.D |
N.D |
|
10 |
6.76±0.86 |
44.32 |
55.68 |
N.D |
N.D |
|
15 |
3.64±0.38 |
23.68 |
76.32 |
N.D |
N.D |
|
30 |
0.54±0.08 |
3.54 |
96.46 |
N.D |
N.D |
Determination of Ethoprophos and Fenamiphos nematicides in soil, leaves and grapes fruits
The initial deposits and the residual behavior of ethoprophos and fenamiphos in soil, leaves and grape fruits are presented in Tables 4, 5 and Figure 5. It was noticed that the initial amount of both nematicides was low. Although the same dose for both nematicides was used (2ml/L water), the initial deposit of fenamiphos and ethoprophos was 15.25 and 4.88 ppm, respectively. The initial deposit of fenamiphos in soil was higher than that obtained from ethoprophos, this is due to the active ingredients fenamiphos and ethoprophos were 40% and 20% in the commercial formulation, respectively. During the experimental period, the degradation of residual amounts of fenamiphos was faster than that, which occurred in the residual amounts of ethoprophos. This agrees with Caceres who found that fenamiphos was degraded faster in the alkaline soil than in the neutral and acidic soils, indicating that the farm soil used in this study is alkaline soil type as the majority of Egyptian soils (Cáceres et al., 2011).
Table 6: Decomposition rate (K) and half–life (RL50) of ethoprophos in soil.
|
Regression equation |
Y= 189.7205x + 11.9743 |
|
Regression coefficient |
0.9839 |
|
K |
0.11 |
|
RL50 (days) |
9.61 |
After five days from the application, the amount of ethoprophos continually degraded until it reached to 3.61 ppm with 26.02% loss. The calculated half-life value of ethoprophos was 9.61days in soil (Table 6). The reduction of the ethoprophos nematicide residues may be due to the nematicide dissipation leading to volatilization (Boesten et al., 1993). Ethoprophos was more persistent in conventional treatments.
The amounts of fenamiphos decreased sharply in soil from 15.25 to 7.31 ppm after zero to 5 days from application with a very high loss of 47.93%, meaning that the amount dropped to near half value after 5 days from the initial deposits. The rapid degradation of fenamiphos continued to reach 0.54 ppm with 96.46 loss after 30 days from the initial deposit. The calculated half- life value of fenamiphos was 4.80 in the soil (Table 7). The residual amounts of ethoprohos and Fenamiphos in grape leaves and fruits after 30 days from application was not detected.
Table 7: Decomposition rate (K) and half–life (RL50) of fenamiphos in soil.
|
Regression equation |
Y= 189.7205x + 11.9743 |
|
Regression coefficient |
0.9975 |
|
K |
0.07 |
|
RL50 (days) |
4.80 |
Sante, 2019. Guidance SANTE/12682/2019-guidance document on analytical quality control and method validation procedures for pesticide.
Table 8: Clorophyll degree estimate in grape leaf after application with nematicides.
|
Treatment |
Rate/ L water |
Degree of clorophyll in grape leaf after application |
Means |
|
|
1 month |
2 months |
|||
|
Vaydet |
2m/l |
30.49±1.50 a |
56.32±3.86 a |
43.40±2.68 |
|
Nemacab |
2m/l |
28.77±1.15 a |
57.15±8.50 a |
42.96±4.82 |
|
Dento |
2m/l |
30.02±2.02 a |
58.47±1.66 a |
44.24±1.84 |
|
Taglis |
4m/l |
31.07±0.69 a |
56.70±2.90 a |
43.88±1.79 |
|
Bioniconemal 1 |
2gm/l |
30.47±2.28 a |
57.57±2.44 a |
44.02±2.36 |
|
Bioniconemal II |
4gm/l |
28.72±1.22 a |
57.00±1.70 a |
42.86±1.46 |
|
Control |
- |
30.22±1.05 a |
59.20±4.09 a |
44.71±2.57 |
Means followed by the same letter within each column are not significantly different (P ≤ 0.05 )according to Tukey’s test (n = 5).
These findings agree with Yuan (Yuan et al., 2021) who studied the dissipation of ethoprophos nematicide in soil, and its proclivity for translocation to spinach. He found that the initial levels of ethoprophos residue in the soils after 30 and 60 days from pesticide treatment were 2.74 and 0.21 mg/kg, respectively. Also, the half-live of ethoprophos in soils was determined to be 7.6 days (Yuan et al., 2021).
Estimation of chlorophyll degree
Chlorophyll degree was estimated in grape leaves after one and two months from the application of nematicides (Table 8). Results showed that no significant differences were recorded among the plants regarding the Chlorophyll SPAD index and the control, indicating that all treatments didn’t have any negative effects on the Chlorophyll degree. These results agree with different works on estimating the Chlorophyll degree in cucumber, sugar beet and tomato (Enan et al., 2016; El-Sayed et al., 2018; El-Eslamboly et al., 2019).
Conclusions and Recommendations
From our results it can be concluded that both bio-nematicides were effective to control RKN associated with grapes, which could be used as safe alternatives to the three chemical nematicides used in this study. Therefore, both formulations could be promising products for controlling PPN associated with crops grown in both modern and conventional agricultural systems. Whilst, the three chemical products could be used for nematode control in traditional grape farms without any negative effects on the plant and soil.
Acknowledgments
The Faculty of African post graduate studies, Cairo University, Egypt, The Faculty of Agriculture, Cairo University, Egypt, The Central Agriculture pesticide laboratory Agriculture research center and The Noamany farm located at 70 km Cairo-Alexandria desert road supported this work.
Novelty Statement
The current study showed that bionematicides are alternative ways to chemical nematicides, which could be used for nematode control in both traditional and modern agricultural systems without any negative impacts on the environment health.
Author’s Contribution
MA and EI designed, wrote and implemented this manuscript.
HS, MA and EI supervised the work and reviewed the manuscript.
MA, EI and MA carried out the field experiments.
MA examined them in the laboratory.
DE, EI and MA executed Chlorophyll and residuals analysis.
MA carried out statistical analysis.
All authors read and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest
References
Aballay, E. and Sepúlveda, R., 2004. Insunza, V. Evaluation of five nematode-antagonistic plants used as green manure to control Xiphinema index Thorne et allen on Vitis vinifera L. Nematropica, 34: 45-51.
Abdel-Sattar, M., Haikal, A.M. and Hammad, S.E., 2020. Meloidogyne incognita population control and nutritional status and productivity of Thompson seedless grapevines managed with different treatments. PLoS One, 15(10): e0239993-e0239993. https://doi.org/10.1371/journal.pone.0239993
Adam, M., Heuer, H., Ramadan, E.M., Hussein, M.A. and Hallmann, J., 2014. Occurrence of plant-parasitic nematodes in organic farming in Egypt. Int. Nematol., 23: 82-89.
Al-Azzeh, T.K. and Abu-Gharbieh, W.I., 2004. Effect of oxamyl and fenamiphos on egg hatching, motility and root penetration of Tylenchulus semipenetrans. Nematol. Mediterr., 32: 19-23.
Al-Hazmi, A.S., Dawabah, A.A., Al-Nadhari, S.N. and Al-Yahya, F.A., 2017. Comparative efficacy of different approaches to managing Meloidogyne incognita on green bean. Saudi J. Biol. Sci., 24: 149-154. https://doi.org/10.1016/j.sjbs.2016.05.013
Anastassiades, M., Lehotay, S.J., Štajnbahar, D. and Schenck, F.J., 2003. Fast and easy multiresidues method employing acetonitrile extraction/ partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J. AOAC Int., 86: 412-431. https://doi.org/10.1093/jaoac/86.2.412
Anwar, S.A. and McKenry, M.V., 2007. Variability in reproduction of four populations of Meloidogyne incognita on six cultivars of cotton. J. Nematol., 39: 105-110.
Boesten, J.J.T.I., Van Der Pas, L.J.T. and Smelt, J.H., 1993. Field test of the PESTLA model for ethoprophos on a sandy soil. In integrated soil and sediment research: A basis for proper protection, pp. 241-245. https://doi.org/10.1007/978-94-011-2008-1_50
Briar, S.S., Wichman, D. and Reddy, G.V.P., 2016. Plant-parasitic nematode problems in organic agriculture, In: Organic farming for sustainable agriculture. D. Nandwani, ed. (Cham: Springer International Publishing), pp. 107-122. https://doi.org/10.1007/978-3-319-26803-3_5
Brunetto, G., Trentin, G., Ceretta, C.A., Girotto, E., Lorensini, F.V., Miotto, A., Moser, G.R.Z., Melo, G.W.B.d., 2012. Use of the SPAD-502 in estimating nitrogen content in leaves and grape yield in grapevines in soils with different texture. Am. J. Plant Sci., 3: 1546-1561. https://doi.org/10.4236/ajps.2012.311187
Cáceres, T.P., Megharaj, M. and Naidu, R., 2011. Toxicity and transformation of insecticide fenamiphos to the earthworm Eiseniafetida. Ecotoxicology, 20: 20-28. https://doi.org/10.1007/s10646-010-0552-6
El-Eslamboly, A.A.S.A., Abd El-Wanis, M.M. and Amin, A.W., 2019. Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt. J. Biol. Pest Contr., 2(27): 9-18. https://doi.org/10.1186/s41938-019-0122-z
El-Hady, E.S., Mahgoob, E.A., Desouky, I.M., Shaltout, A.D. and Haggag, L.F., 2015. Effect of root-knot nematode on the growth and yield of some grapevine cultivars grafted onto nematode resistant rootstocks. Middle East J. Agric. Res., 5: 1091-1097.
El-Saadony, M.T., Abuljadayel, D.A., Shafi, M.E., Albaqami, N.M., Desoky, E.M., El-Tahan, A.M., Mesiha, P.K.,Elnahal, A.S.M., Almakas, A., Taha, A.E., Abd El-Mageed, T.A., Hassanin, A.A., Elrys, A.S. and Saad, A.M., 2021. Control of foliar phytoparasitic nematodes through sustainable natural materials: Current progress and challenges. Saudi J. Biol. Sci., 28: 7314-7326. https://doi.org/10.1016/j.sjbs.2021.08.035
El-Sayed, A.B., Shehata, S.A., Taha, S.S., Hamouda, H.A., Abdelgawad, K.F. and Youssef, D., 2018. Algae extract overcoming the adverse effects of saline stress in hydroponic grown tomato plants. J. Food Agric. Environ., 16: 92-99.
Enan, S.A.A., El-saad, A.M. and El-sayed, A.B., 2016. Impact of foliar feeding with alga extract and boron on yield and quality of sugar beet grown in sandy soil. Egypt J. Agron., 38: 319-336. https://doi.org/10.21608/agro.2016.622
German, E., Mauricio, T., Eduardo, S. and Araya, M., 2019. Chemical control of Meloidogyne spp. in grapevines (Vitis vinifera). J. Appl. Biosci., 136: 3896. https://doi.org/10.4314/jab.v136i1.6
Gommers, F.J. and Bakker, J., 1988. Physiological diseases induced by plant responses or products. In: (G.O. Poinar and H.B. Jansson Eds.), Diseases of nematodes. pp. 3-22. https://doi.org/10.1201/9781351071475-1
Hooper, D.J., Hallmann, J. and Subbotin, S., 2005. Methods for extracting and processing detection of plant and soil nematodes. In: (eds. Luc, R.A.S., J. Bridge), Plant parasitic nematodes in subtropical and tropical agriculture. CABI Publishing, Wallingford, UK, pp. 53-86. https://doi.org/10.1079/9780851997278.0053
Jacquet, M., Bongiovanni, M., Martinez, M., Verschave, P., Wajnberg, E. and Castagnone-Sereno, P., 2005. Variation in resistance to the root-knot nematode Meloidogyne incognita in tomato genotypes bearing the Mi gene. Plant Pathol., 54: 93-99. https://doi.org/10.1111/j.1365-3059.2005.01143.x
Karakas, M. and Bolukbasi, E., 2019. A review using marigolds (Tagetes spp.) as an alternative to chemical nematicides for nematode management. Int. J. Adv. Eng. Manage. Sci., 5: 556-560. https://doi.org/10.22161/ijaems.59.3
Kiewnick, S., Sikora, R.A., 2003. Efficacy of Paecilomyces lilacinus (strain 251) for the control of root-knot nematodes. Commun.Agric. Appl. Biol. Sci., 68:123-128.
Kiewnick, S., Sikora, R.A., 2006. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol. Contr., 38: 179-187. https://doi.org/10.1016/j.biocontrol.2005.12.006
Krueger, R., Dover, K.E., McSorley, R. and Wang, K.H., 2007. Marigolds (Tagetes spp.) for nematode management. Entomology and Nematology Department, Florida Cooperative Extension Service. University of Hawaii. HI, 96822. https://doi.org/10.32473/edis-ng045-2007
Marahatta, S.P., Wang, K.H., Sipes, B.S. and Hooks, C.R., 2012. Effects of Tagetes patula on active and inactive stages of root-Knot nematodes. J. Nematol., 44: 26-30.
Mendoza, A., Sikora, R. and Kiewnick, S., 2007. Influence of Paecilomyces lilacinus strain 251 on the biological control of the burrowing nematode Radopholus similis in banana. Nematropica, pp. 203-214.
Mervat, A.A., Shawky, S.M. and Shaker, G.S., 2012. Comparative efficacy of some bioagents, plant oil and plant aqueous extracts in controlling Meloidogyne incognita on growth and yield of grapevines. Ann. Agric. Sci., 57: 7-18. https://doi.org/10.1016/j.aoas.2012.03.009
Nyczepir, A.P. and Thomas, S.H., 2009. Current and future management strategies in intensive crop production systems. Root-knot nematodes, pp. 412. https://doi.org/10.1079/9781845934927.0412
Oclarit, E. and Cumagun, C., 2009. Evaluation of efficacy of Paecilomyces lilacinus as biological control agent of Meloidogyne incognita attacking tomato. J. Plant Prot. Res., 49(4): 337-340. https://doi.org/10.2478/v10045-009-0053-x
Puntener, W., 1981. Manual for field trials in plant protection. Basle, Switzerland: Agric. Division, Ciba Geigy, pp. 205.
Sante 12682, 2019. Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2019-12682.pdf (accessed on 25 October 2022).
Tian, B., Yang, J. and Zhang, K.Q., 2007. Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol., 61: 197-213. https://doi.org/10.1111/j.1574-6941.2007.00349.x
Unusan, N., 2020. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods, 67: 103861. https://doi.org/10.1016/j.jff.2020.103861
Williamson, V.M. and Hussey, R.S., 1996. Nematode pathogenesis and resistance in plants. Plant Cell, 8: 1735-1745. https://doi.org/10.1105/tpc.8.10.1735
Wood, C.W., Reeves, D.W. and Himelrick, D.G., 1992. Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status and crop yield a review. Proc. Agron. Soc. N. Z., 23: 1-9.
Ye, J., Wu, J., Qin, F., Sun, S., Dalmia, A., Reuter, W. and Kero, F., 2018. Analysis of 213 pesticide residues in grapes by LC-MS/MS with time-managed MRM. Food and beverage. Appl. Noteb., 3(31): 1-7.
Yu, Y., Liu, X., He, Z., Wang, L., Luo, M., Peng, Y. and Zhou, Q., 2016. Development of a multi-residue method for 58 pesticides in soil using QuEChERS and gas chromatography-tandem mass spectrometry. Anal. Methods, 8: 2463-2470. https://doi.org/10.1039/C6AY00337K
Yuan, X., Lee, J., Han, H., Ju, B., Park, E., Shin, Y. and Kim, J.H., 2021.Translocation of residual ethoprophos and tricyclazole from soil to spinach. Appl. Biol. Chem., 64: 1-10. https://doi.org/10.1186/s13765-021-00619-0
Zhang R., Yang, P., Liu S., Wang, C. and Liu J., 2022. Evaluation of the methods for estimating leaf Chlorophyll content with SPAD Chlorophyll meters. Remote Sens., 14(20): 5144. https://doi.org/10.3390/rs14205144
To share on other social networks, click on any share button. What are these?





