Evaluation of Anti-Sperm Antibodies (ASAs) in the Serum and Seminal Plasma of Dromedary Male Camels with Infertility History
Research Article
Evaluation of Anti-Sperm Antibodies (ASAs) in the Serum and Seminal Plasma of Dromedary Male Camels with Infertility History
Saleh M. Albarrak1*, Fahad S. Aldahmashi1,2, Abdulrhman A. Alrubayan1,2, Abdel Kader A. Zaki1,3
1Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia; 2Ministry of Environment, Water and Agriculture, Qassim region, Saudi Arabia; 3Department of Physiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Abstract | Anti-sperm antibodies (ASAs) have been shown to contribute to male infertility in multiple species but never been examined in male camels. We have developed and evaluated an ELISA assay to specifically detect and quantify ASAs in the serum and seminal plasma of 29 infertile dromedary male camels, which were of two different breeds (Waddah & Majaheem) and three different ages (> 3 to ≤5, ≥5 to ≤7, and >7 years). ASAs were detected in ≥ 80% of the examined animals with considerable individual variation within each group. The serum and seminal plasma ASAs indexes (%) were significantly elevated in the >7 years group compared to the >3 to ≤5 and ≥5 to ≤7 years groups (P ≤ 0.05), and in the ≥5 to ≤7 years group compared to the >3 to ≤5 years group (P ≤ 0.05). Serum and seminal plasma ASAs indexes were significantly higher in the Waddah breed compared to the Majaheem breed (P ≤ 0.05). The sperm motility and viability were significantly higher in the >7 years group compared to the > 3 to ≤5 years and ≥5 to ≤7 years groups (P ≤ 0.05 and P ≤ 0.01, respectively). Significant differences were also observed between the examined two breeds concerning sperm viability and motility with sperm viability being higher in the Majaheem breed (P ≤ 0.05) and sperm motility in the Waddah breed (P ≤ 0.05). Our data demonstrated the presence of ASAs in sera and seminal plasma of infertile dromedary male camels. Our results suggested that age and breed influenced serum and seminal plasma levels of ASAs in male camels. Data presented in the current study highlight the potential role of ASAs in camel infertility; however, more work is needed to determine ASAs’ contribution to reproductive challenges in camels.
Keywords | Anti-sperm antibodies, Dromedary Camels, Seminal plasma, Serum, ELISA
Received | September 06, 2022; Accepted | September 28, 2022; Published | October 20, 2022
*Correspondence | Saleh M Albarrak, Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia; Email: salbarrak@qu.edu.sa; salbarrak7@hotmail.com
Citation | Albarrak SM, Aldahmashi FS, Alrubayan AA, Zaki AKA (2022). Evaluation of anti-sperm antibodies (ASAs) in the serum and seminal plasma of dromedary male camels with infertility history. Adv. Anim. Vet. Sci. 10(11): 2447-2456.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.11.2447.2456
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Experts, researchers, veterinarians, and farmers have been tackling the issue of camels’ low reproductive efficiency for decades (Faye, 2017). Despite the availability of cutting-edge technologies, research on camel infertility remains limited. One of the gaps and difficulties in enhancing camel reproduction is the inadequate understanding of camel infertility. Not all causes of infertility have been identified in camels as in cattle (Bello and Bodinga, 2020). Therefore, identifying the causes preventing reproduction and finding solutions is critical. Unfortunately, specialist clinical monitoring was not extensive as camel producers have provided the majority of the data gathered on the subject of camel fertility. Impotantia is a term used to describe several male camel infertility conditions, including weak or absent sexual drive, weak capacity to have penetration, and the inability to childbearing. Transient and chronic infertility is associated with orchitis and epididymitis.
Studies on male camels’ infertility, particularly its immunological origin, are lacking. Anti-sperm antibodies (ASAs) have been reported to associate with male infertility in human and multiple veterinary species including cattle, horses, dogs and deer (Gupta et al., 2018; Kawase and Jimbo, 2018, Lu et al., 2019, Ferrer et al., 2020, Esmailnejad et al., 2020; Shibahara et al., 2022). Sperm antigenicity was initially discovered in guinea pigs inoculated with bull sperm in 1899 (Landsteiner, 1899). Since then, a number of publications have verified that sperm can cause immunological responses and ASAs (Parsonson et al., 1971, Wright et al., 1980; Kim et al., 1999). ASAs are likely to cause immunological sterility via traversing and entering the site of egg fertilization preventing contact between the sperm and the egg (Restrepo et al., 2013). Studies have shown that ASAs cause direct damage to the sperm, affecting their counts and motility (Archana et al., 2019). The development of ASAs is likely caused by traumatic disturbance or developmental problems with the blood-testicular barrier that separates the sperm antigens from the immune system. Generation of ASAs mainly occurs in the epididymis, and ASAs have been detected in both blood serum and seminal fluids of infertile men, dogs, bulls, and stallions (McLachlan, 2002, Cuia et al., 2015, Lu et al., 2019; Ferrer et al., 2020). Because ASAs have been detected in 5 - 15% of infertile males, it is thought that ASAs may contribute to male infertility in humans (Lu et al., 2019). According to Cuia et al. (2015), ASAs resulted in immunological male infertility in dogs, although the exact mechanism was unknown. The results of the study demonstrated that ASAs had a detrimental impact on sperm activity, quantity, and motility. Ferrer et al. (2020) detected ASAs in serum and seminal plasma of bulls with seminal vesicle inflammation and suggested that ASAs may be connected to lower pregnancy outcomes.
To the best of our knowledge, studies investigating the presence of ASAs in male camels are lacking. The detection of ASAs using ELISA technology is of clinical importance with the ELISA technique has been proven sensitive and accurate in detecting ASAs (Kawase and Jimbo, 2018). Therefore, in the current study, we aimed to develope and evaluated an in-house ELISA assay designed to specifically detect ASAs in either blood serum or seminal and seminal plasma obtained from twenty-nine male camels with a history of fertility problems.
MATERIALS AND METHODS
Animals
Camel semen, seminal plasma, and blood serum samples were obtained from 29 camel males examined or treated at the Qassim University Veterinary Hospital, KSA. Samples were collected from the Majaheem and the Waddah breeds during the breeding season (November and December of 2021). Animals involved in the current study were sexually mature and had a history of fertility problems. They weighed 423 to 573 kg, with body condition scores ranging from 3.5 to 4.5 (scale: 1–5). The animals were divided according to their ages into three groups, the first group aged >3 to ≤5 years, the second group was >5 to ≤7 years, and the third group aged more than seven years old.
Samples Collection
Male camels are prepared for sampling firstly by fasting from 24-36 hours before sample collection. The sedation process was carried out by intravenous injection using a xylazine drug (xylazine 20 mg/ml) at a dose of 1.1 ml/100 kg body weight /IV as referred by Khalil et al. (2019). Blood samples were drawn directly from the jugular vein and centrifuged at 3000 rpm for 10 minutes for serum separation. The serum samples were labeled and saved at -20 C until analyzing the ASAs by ELISA. The semen samples were collected using an electro-ejaculator according to the specifications of the manufacturer (ElectroJac 5 Neogen, Lexington, KY, USA). The electric pulsation-cycling program was done automatically as; in the automatic mode, the circuit delivers a series of 32 cycles. Each cycle lasts 2 seconds, followed by a 2-second pause. Immediately after camel sedation, the penis was cleaned with saline, and a falcon graduated tube was placed to collect the semen sample at the time of ejaculation. The falcon tube was immediately closed to avoid contamination. The semen samples were centrifuged at 3000 rpm for 10 mins for separation of the seminal plasma for measuring the existence of ASAs by ELISA assay.
Semen Samples Analysis
The collected semen samples (ranged volume from 3-7 ml) were incubated at 37 °C for 10-20 mins for liquefaction time measurement of each semen sample. After liquefaction, a drop from each semen sample was examined under the microscope for evaluating mass sperm motility. The semen samples were diluted with sodium citrate 2.9% (drop by drop) for the individual sperm motility examination. Semen films stained with Eosin and Nigrosin were prepared for calculating the live/dead ratio as well as sperm abnormality (Wani et al., 2008). The semen sample volume was calculated from the graduated collection tube. The method described by Kershaw-Young et al. (2013) was used to measure the viscosity of the semen sample. Briefly, a 100 ml pipette was used to extract 50 ml of semen sample. After releasing 25 ml onto a glass slide, the pipette was quickly lifted. The viscosity standard was determined by measuring the length at which semen thread breaks. The viscosity of semen samples were assessed before incubation (at 0 mins) and after incubation for 5, 10, 15, 30, 60, 120, and 240 mins in a water bath at 37 ℃. The formula (Viscosity at Given Time/Initial Viscosity) X100 was used to determine the viscosity percentage.
Individual motility of the camel sperms was assessed manually. Ten µL of the semen sample was spread on a warm grease-free glass clean slide, and a cover-slip was placed over it. Examination took place under × 400 magnification using a light microscope. Each motile sperm whether oscillatory or progressive was considered motile and used to generate a value for the total motility, as described previously (Kershaw-Young and Maxwell, 2011). Viability (%) was assessed using the conventional method Eosin-Nigrosin staining technique. Briefly, one drop of the semen sample was mixed with two drops of the Eosin-Nigrosin dye, smeared on a clean grease-free glass slide, and then dried. Slides were examined under ×400 light microscopy, and spermatozoa were classified as live if they remained unstained with Eosin and dead if they got stained with the dye. A procedure for evaluating the integrity of the acrosome in ethanol-fixed alpaca sperm was used (Morton et al., 2010). Following dilution, all observations of live, dead, and acrosome integrity were recorded immediately. Sperm concentration/ml was calculated using a Neubauer hemocytometer slide after 1:100 dilutions in formal citrate, sperm counts were expressed as in 106/ml.
Development Of Camel Asas-Specific Elisa
Indirect enzyme-linked immunosorbent assay (ELISA), which is a modified version of the Standard indirect solid-phase ELISA, was used to measure the amounts of immunoreactive ASAs (Esmailnejad et al, 2020).
Preparation Of Sperm Proteins As Antigen For Elisa
For preparing sperm antigens, testicles were obtained from a slaughterhouse in a cooled container. Semen of the testis was isolated as previously described by Chiu et al. (2004). The seminiferous tubules were taken out and smashed in a PBS-filled Petri dish (pH 7.4). After allowing the sperm to separate from the tubules, they were aspirated with a Pasteur pipette. Each aliquot of sperm was resuspended with1 ml PBS (pH 7.4) and centrifuged at 1500 g for 15 mins at 4°C. The remaining fluid was considered testicular seminal plasma. Collected semen droplet was washed three times. The sperm samples were sonicated to break up the tissue and free the proteins. The sonicates was centrifuged at 8500 g for 90 mins at 4°C. The total protein concentration in the supernatant was determined using a colorimetric method (Biuret reagent) according to Doumas et al. (1981).
Preparation Of Asas In Rabbits
Three male rabbits were immunized with sperm to produce a pooled hyperimmune serum. Three ml of blood were collected from each rabbit before immunization and preserved at –20ºC as the negative control serum. An equal volume of Freund’s complete adjuvant (Difco, Detroit, MI, USA) was added to the sperm and emulsified. Each animal was administrated intra-peritoneally with 0.5 ml of the mixture containing 400 mg sperm protein. Each animal received boosting doses at the 2nd, 4th, and 6th weeks post the initial inoculation. Blood samples were obtained by cardiac puncture 10 days after the fourth immunization. Sera were separated, pooled, placed in aliquots, and frozen at -20ºC. The pooled hyperimmune serum was used for potency titration and as a positive control in the ELISA assay.
Titration Of The Detection Antibodies
Two ELISA microtiter – plates (Nunc, Roskilde, Denmark) were coated with double-fold serial dilutions of sheep anti-rabbit IgG (product no. A-5279, Sigma co., St. Louis, U.S.A) and anti-camel-IgG (ALPHA DIAGNOSTICS INTERNATIONAL) conjugated with Horse Radish Peroxidase (HRP) starting with a 1/10 concentration. This was done by transferring 25 ul of HRP-conjugated Ab from a well into the next well that contains 25 ul of blocking buffer (PH 7.2), followed by adding 25 ul of the substrate solution to each well. Then, the plates were placed in the incubator at 37ْC for 30 minutes. The best dilutions were the highest concentration which gave a strong positive signal. The best dilutions were 1:4000 and 1:3000 for sheep anti-rabbit IgG and anti-camel-IgG-conjugates, respectively.
Determination Of Checkerboard Titration
Checkerboard titration was performed to find the optimum dilution of the antigen and tested antisera and to know the potency of prepared sperm protein antisera in the rabbits. Serial dilutions of the tested antigen were used in coating an ELISA plate at concentrations of 1000, 500, 250, 125, 62.5, 31.25 ng, and a negative control antigen versus different dilutions of tested anti-sera diluted, as 1:5,1:10,1:20,1:40, and 1:80. The serum dilution plus the lowest concentration of antigen gave a clear differentiation under known dilutions of the conjugated antibodies and the substrate and was considered an optimum condition to be used in further tests. The potency of the sperm antisera prepared in rabbits against 500 ng sperm protein was constructed. The results of the potency of the sperm antisera prepared in rabbits are shown in B Figure 1B. The optimum dilution of the antisera was 1:80.
Standard Log-Dose Response Curve To Determine The Optimum Concentration Of The Antigen (Sperm Proteins)
Serial dilutions of the sperm-derived proteins (antigen) were placed in the plate (dilution/row) in the following order 350, 175, 87.5, 34.75, 21.87 ng. The negative control antigen was done similarly. The plate was then incubated for 1 hour at 37°C with shaking and washed 3 times with the washing buffer (Phosphate buffer saline pH 7.2 containing 0.05% Tween – 20). Each well received 100 µl of the rabbit antisera (1:80 in PBS) followed by incubation for 1 hour at 37°C with shaking. After the incubation, the plate was washed 3 times using the washing buffer. 100 µl of the diluted HRP-labeled sheep anti-rabbit IgG (1:4000 dilution in PBS, PH 7.2) was added to each well and incubated for 1 hour at 37°C in a shaking water bath. After incubation, the plate was washed 3 times with the washing buffer, and 100 µl of the substrate buffer containing TMB was added to each well. The plate was incubated in the dark for 7 mins at room temperature. The reaction was stopped by adding 50 µl of the stopping solution to each well. The signal of the enzyme-mediated reaction was measured at a wavelength of 492 nm using a microplate ELISA reader. A standard curve was constructed by plotting the optical density against the log dose of the antigen (Figure 1-B).
Elisa Cut-Off Values And Antibodies Index (%)
Cut-off values discriminate positive results from background readings. It has been the goal of several standard procedures to determine the best cut-offs (Lopez-Raton et al., 2014). Typically, and particularly with homemade ELISAs, cut-off values are calculated using known negative sera that are mixed in with the unknown (examined) samples on the titer plates. A cut-off value can be determined using the following general formula: Cut-off = a*X + f * SD, where X is the mean, SD is the standard deviation of independent negative control readings, and a and f are two multipliers. We used a = 1 with f = 3 (i.e., cut-off = mean + 3 times the standard deviation) (Classen et al., 1987). The titer of serially diluted sera samples was determined by comparing with the optical density (OD) of three well-known negative controls (mature adults female camels that were clinically normal in all regards) and three known positive controls from immunized rabbits.
The percentage of antibodies was calculated for each serum and seminal plasma samples by comparison with positive and negative control values. Additionally, the sensitivity% and specificity % were calculated according to Tabouret et al. (2001) and Bauer et al. (2002).
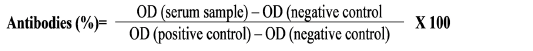
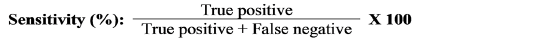
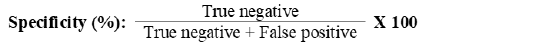
The Elisa Procedure
The ELISA microplate wells were coated each with 100 µl of the camel sperm proteins (125ug) diluted in carbonate bicarbonate coating buffer (0.159% sodium carbonate and 0.293% sodium bicarbonate, pH 9.6) and incubated for 2 h at 37°C in a shaking water bath. The plates were sealed with an adhesive tape and incubated overnight at 4°C to allow complete adsorption of the solid phase. Excess of the unbound antigens were removed by washing 3 times with the washing buffer (PBS PH 7.2 containing 0.05% Tween – 20). A volume of 200 µl of the blocking buffer (2% Bovine serum albumin (BSA, PH 7.2) was added to each well (to block the free binding sites) and the plates were incubated for 2 h at 37 °C with shaking. The plates were then washed 3 times with the washing buffer. The plates well were loaded with 100 µl/well of serially diluted tested serum samples, covered, and incubated for 1 h at 37°C. A volume of 100µl of the HRP-labeled anti-camel IgG (1:4000 dilutions in PBS, PH7.2) was added to each well, plates were then sealed, and left for 1 h at 37°C in a shaking water bath. The plates were then washed 3 times using the washing buffer and a volume of 100 µl of a substrate buffer containing TMB [(3,3’,5,5’-tetramethylbenzidine) 30 mg in 75ml Dw (Sigma chemical Co.)] was added to each well. The plates were incubated in the dark for 7 mins at RT. The reaction’s special coloration was detected. The reaction was stopped by adding 50 µl/well of the stopping solution (H2 SO4 -2.5M). The enzyme-mediated
Table 1: Semen quality parameters of male camels aged > 3 to ≤ 5 years
| Case-No. |
Breed |
Age/ year |
Volume ml |
Viscosity Of 5 |
Abnormality (%) |
Viability (%) |
Motility (%) |
Sperm cell count 106 / ml |
|
1 |
Majaheem | 4 | 2 | 4 | 15 | 65 | 80 | 500.000 |
| 2 | Majaheem | 3 | 8 | 4 | 40 | 40 | 60 | 120.000 |
| 3 | Waddah | 5 | 10 | 1 | 70 | 70 | 70 | 20.000 |
| 4 | Waddah | 5 | 6 | 3 | 0 | 0 | 0 | 0 |
| 5 | Waddah | 5 | 8 | 3 | 60 | 50 | 60 | 400.000 |
| 6 | Waddah | 4 | 7 | 3 | 30 | 40 | 70 | 80.000 |
| 7 | Waddah | 3 | 9 | 4 | 30 | 30 | 60 |
90.000 |
| 8 | Waddah | 5 | 8 | 3 | 35 | 40 | 50 | 70.000 |
| Mean±SD |
4.14 ±0.89 |
7.43 ±2.57 |
3.14 ±1.069 |
40.00 ±18.93 |
47.86 ±14.68 |
64.29 ±9.75 |
182.9 ±187.1 |
|
| P values | > 0.10 | 0.0526 | 0.0496* | > 0.10 | > 0.10 | > 0.10 |
0.0114* |
|
Mean ±SD. Azoospermic case No.4 was excluded. * Significant at P ≤ 0.05
Table 2: Semen quality parameters of male camels aged ≥5 to ≤7 years
| Case-No. |
Breed |
Age/ year |
Volume ml |
Viscosity Of 5 |
Abnormality (%) |
Viability (%) |
Motility (%) |
Sperm cell count 106 / ml |
| 9 | Majaheem | 6 | 7.5 | 1 | 0 | 0 | 0 | 10.000 |
| 10 | Majaheem | 7 | 5 | 0 | 0 | 0 | 0 | 0 |
| 11 | Majaheem | 6 | 5 | 1 | 0 | 0 | 0 | 0 |
| 12 | Majaheem | 7 | 7 | 1 | 90 | 70 | 80 | 200.000 |
| 13 | Majaheem | 5 | 5 | 2 | 20 | 40 | 30 | 70.000 |
| 14 | Majaheem | 5 | 6 | 5 | 15 | 25 | 25 | 60.000 |
| 15 | Waddah | 7 | 10 | 3 | 0 | 0 | 0 | 0 |
| 16 | Waddah | 6 | 5.5 | 3 | 20 | 1 | 10 | 80.000 |
| 17 | Waddah | 6 | 10 | 4 | 50 | 50 | 50 | 80.00 |
| 18 | Waddah | 6 | 4 | 3 | 30 | 50 | 50 | 200.000 |
| 19 | Waddah | 6 | 6 | 3 | 20 | 2 | 10 | 90.000 |
| 20 | Waddah | 6 | 8 | 2 | 30 | 3 | 20 |
120.000 |
| 21 | Waddah | 6 | 6 | 3 | 25 | 30 | 35 | 80.000 |
| Mean± SD |
5.90 ±0.56 |
6.50 ±1.70 |
2.700 ±1.25 |
30.00 ±24.61 |
27.10 ±25.15 |
31.00 ±23.90 |
99.00 ±59.90 |
|
| P values | 0.0004*** | > 0.10 | > 0.10 | 0.0111* | > 0.10 | > 0.10 |
0.0545 |
|
Mean ±SD. Azoospermic cases No.10;11;15 were excluded. *, and *** were significant at P ≤ 0.05 and P≤ 0.001, respectively.
Table 3: Semen quality parameters of male camels aged > 7 years
| Case-No. |
Breed |
Age/ year |
Volume (ml) |
Viscosity Of 5 |
Abnormality (%) |
Viability (%) |
Motility (%) |
Sperm cell count 106 / ml |
| 22 | Majaheem | 8 | 6 | 4 | 20 | 75 | 85 | 600.000 |
| 23 | Majaheem | 8 | 8 | 4 | 30 | 60 | 70 | 200.000 |
| 24 | Waddah | 11 | 6.5 | 1 | 0 | 0 | 0 | 0 |
| 25 | Waddah | 10 | 4.7 | 4 | 5 | 60 | 80 | 400.000 |
| 26 | Waddah | 8 | 6 | 2 | 80 | 10 | 20 | 150.000 |
| 27 | Waddah | 8 | 7 | 4 | 35 | 50 | 40 | 150.000 |
| 28 | Waddah | 8 | 7 | 3 | 40 | 50 | 60 | 180.000 |
| 29 | Waddah | 9 | 6 | 4 | 60 | 40 | 50 | 140.000 |
| Mean±SD |
8.42 ±0.78 |
6.38 ±1.05 |
3.57 ±0.78 |
38.57 ±24.95 |
49.29 ±20.50 |
57.86 ±23.07 |
260.0 ±175.0 |
|
| P values | 0.0004*** | > 0.10 | 0.0004*** | > 0.10 | > 0.10 | > 0.10 |
0.0103* |
|
Mean ±SD. Azoospermic case No.24 was excluded * and *** Significant at P ≤ 0.05 and P ≤ 0.0001, respectively.
Table 4: Age and breed-dependent relations to semen quality parameters of male camels
| Parameters |
Age (years) |
Breed |
|||||
| > 3 to ≤5 |
≥5 to ≤7 |
>7 |
P-value |
Majaheem | Waddah |
P-value |
|
| Age/ year |
4.143 ±0.89 |
5.900 ±0.56 |
8.429 ±0.78 |
5.300 ±1.25 |
6.429 ±1.95 |
||
| Volume (ml) |
7.429 ±2.57 |
6.500 ±1.70 |
6.386 ±1.05 |
> 0.10 |
6.100 ±1.77 |
6.871 ±1.64 |
> 0.10 |
| Viscosity Of 5 |
3.143 ±1.069 |
2.700 ±1.25 |
3.571 ±0.78 |
> 0.10 |
2.600 ±1.71 |
3.214 ±0.69 |
> 0.10 |
| Abnormality (%) |
40.00 ±18.93 |
30.00 ±24.61 |
38.57 ±24.95 |
0.0473* |
30.50 ±33.29 |
37.50 ±19.39 |
0.0653 |
| Viability (%) |
41.875 ±7.14 |
20.8461 ±6.38 |
43.125 ±8.57 |
0.0112* | 37.5±9.03 | 25.8±10.64 | 0.0350* |
| Motility (%) |
47.25 ±8.92 |
23.8461 ±6.36 |
50.62 ±9.64 |
0.0482* |
30.5 ±5.01 |
40.416 ±5.36 |
0.0357* |
|
Sperm cell count 106 x ml |
182.9 ±187.1 |
99.00 ±59.90 |
260.0 ±175.0 |
> 0.10 |
130.0 ±146.3 |
159.3 ±109.7 |
> 0.10 |
Mean ±SD. * Significant at P ≤0.05. P-values of column and raw factors were ≤ 0.0001 and 0.1073 respectively (Two-way ANOVA).
Table 5: Evaluated ELISA assay parameters of camel serum of infertile and control samples
| Parameters |
-ve |
+ve |
Age |
Breed |
|||||
| > 3 to ≤5 |
≥5 to ≤7 |
>7 |
P-value |
Majaheem | Waddah |
P-value |
|||
|
ODs at first dilution (1:1) |
0.042 ±0.001 |
1.221 ±0.038 |
0.83 ±0.18 |
0.83 ±0.24 |
0.98 ±0.14 |
0.0026** |
0.82 ±0.24 |
0.89 ±0.19 |
0.0657 |
|
Conc. at first dilution (1:1) ng/100µl |
13.74 ±2.19 |
406.1 ±11.71 |
174.5 ±109.7 |
176.6 ±116.3 |
245.7 ±86.91 |
0.0035** |
186.2 ±103.2 |
196.9 ±114.6 |
0.0557 |
|
ODs of cut-off values |
0.041 ±0.00 |
0.039 ±0.001 |
0.04 ±0.002 |
0.04 ±0.0027 |
0.03 ±0.003 |
> 0.10 |
0.04 ±0.002 |
0.040 ±0.003 |
> 0.10 |
|
Conc. at cut-off values (ng/100µl) |
13.10± 1.05 |
15.4 ±0.56 |
15.55 ±0.94 |
13.87 ±0.76 |
16.31 ±3.93 |
> 0.10 |
13.99 ±1.14 |
14.96 ±2.259 |
> 0.10 |
|
Antibody titer at cut-off values |
2.0 ±0.00 |
256 ±0.00 |
1/128.0 ±0.00 |
1/90.40 ±94.06 |
1/74.00 ±81.56 |
> 0.10 |
1/71.00 ±83.73 |
1/114.5 ±76.13 |
0.1466 |
|
Antibodies index (%) |
6.876 ±2.43 |
98.855 ±8.37 |
52.02 ±9.64 |
57.95 ±6,38 |
78.65 ±3.57 |
0.0026** |
56.3431 ±8.06 |
65.025± 5.001 |
0.0287* |
Mean ±SD. * and ** are significantly different at P ≤0.05, P ≤0.01, respectively (ANOVA-Kolmogorov-Smirnov test). P-values of column and raw factors were ≤ 0.0001 and ≤ 0.0170 respectively (Two-way ANOVA). False positive samples were excluded.
Table 6: Evaluated ELISA assay parameters of camel testicular seminal plasma of infertile and control samples
| Parameters |
Co. -ve |
Co. +ve |
Age |
Breed |
|||||
|
>3 to ≤5 |
≥5 to ≤7 | >7 |
P-value |
Majaheem |
Waddah |
P-value |
|||
|
ODs at first dilution (1:1) |
0.032 ±0.009 |
1.221 ±0.038 |
0.124± 0.051 |
0.5911 ±0.203 |
0.596 ±0.151 |
0.0026** |
0.528 ±0.154 |
0.5713 ±0.18 |
0.0027** |
|
Conc. at first dilution (1:1) ng/100µl |
14.001 ±2.909 |
406.1 ±11.71 |
4.328 ±1.767 |
90.74 ±73.85 |
93.79 ±56.40 |
0.0035** |
65.42 ±43.32 |
86.39 ±64.43 |
0.0615 |
|
ODs of cut-off values |
0.0331 ±0.006 |
0.039 ±0.0009 |
0.0039 ±0.0016 |
0.035 ±0.0026 |
0.035 ±0.0035 |
> 0.10 |
0.0363 ±0.003 |
0.035 ±0.0032 |
0.0024** |
|
Conc. at cut-off values ng/100µl |
13.9 ±2.333 |
15.4 ±0.565 |
2.799 ±1.143 |
14.04 ±0.704 |
16.35 ±1.36 |
> 0.10 |
14.47 ±0.977 |
15.43 ±2.135 |
> 0.10 |
|
Antibody titer at cut-off values |
1.00 ±0.0 |
256.0 ±0.0 |
1/197.4 ±80.58 |
1/82.67 ±14.9 |
1/25.0 ±15.46 |
> 0.10 |
1/24.00 ±19.04 |
1/89.50 ±39.3 |
0.0557 |
|
Antibodies index (%) |
1.2346 ±0.656 |
98.855 ±24.65 |
28.1431 ±5.32 |
33.048 ±6.72 |
46.8257 ±2,65 |
0.0028** |
30.307 ±2.12 |
38.226 ±4.54 |
0.0418* |
Mean ±SD. ** is significantly different at P ≤ 0.01 (ANOVA-Kolmogorov-Smirnov test). P-values of column and raw factors were ≤ 0.0048 and ≤ 0.0459, respectively (Two-way ANOVA). False positive samples were excluded.
reaction was measured at 492 nm wavelength using a microplate ELISA reader (BIO-TEK, INC., ELx, 800UV Winooski, VT, USA).
Statistical Analysis
Values of data were illustrated as means ± standard errors. Kolmogorov-Smirnov test was used after an ANOVA for statistical analysis, with P ≤ 0.05 is regarded as statistically significant. Statistical analysis was conducted with the Graphpad prism program 7.
RESULTS & DISCUSSION
To the best of our knowledge, ASAs have not been explored in male camels. Thus, the current research aimed to investigate the presence of ASAs in serum and seminal plasma samples obtained from infertile 29 male camels aging > 3 to ≤5, ≥5 to ≤7, and >7 years old and were of two different breeds. The diagnosis of infertility requires the detection of active spermatogenesis (Cobellis, et al., 2014). Thus, the semen characteristics including volume, viscosity, abnormality, viability, motility, and numbers, were evaluated for all the examined animals aging 3-5 years, 5-7 years, and over 7 years as shown in Tables 1, 2, and 3, respectively. Data demonstrated that the animals used in the current study were infertile as the values of their semen quality parameters were lower than the values of a normal camel semen previously reported (Waheed et al., 2018, Mahmood et al., 2020). As shown in Table 4, significant variations among the examined ages were seen only in sperm abnormality and viability (P ≤ 0.05). No significant differences were observed among the examined ages regarding sperm cell count, volume, viscosity, and motility. Data demonstrated no significant differences between the examined breeds in any of the examined semen quality parameters (Table 4). as shown in Table 4, no significant interactions were noted between the examined ages and breeds regarding any of the examined sperm quality parameters (P =0.1073).
Sperm quality has been shown to be negatively affected by ASAs (Ferrer and Miller, 2018; Gupta et al., 2021). Assessing ASAs production may enable overcoming problems with sperms ejaculation, sperms travel, or production (Marconi and Weidner 2017). Antibodies generated against specific sperm-antigens can be detected and examined for potential clinical diagnostic applications (Fu et al., 2016). ELISA is a diagnostic method frequently used in biological research to identify antibodies relating to a particular antigen. In the current study, we were able to generate an ELISA assay capable of detecting camel ASAs with 93.1 % sensitivity and 100% specificity in serum samples and 79.31 % sensitivity and 100% specificity in seminal plasma samples (Supplementary Tables, 1 & 2).
As shown in Table 5, the findings of the current study demonstrated significant differences among the examined ages and breeds regarding ASAs levels in the serum. Data showed significant differences among the examined ages with regard to the ODs at the first dilution (1:1), ASAs concentrations at the first dilution, and ASAs indices (P ≤ 0.01). The ODs of the cut-off value, ASAs concentrations at cut-off values, and ASAs titers at cut-off values were not significantly different among the examined three ages. Concerning the breed type effects on the serum levels of ASAs, the data demonstrated no significant differences between the examined breeds regarding except for the ASAs indices (P ≤ 0.05). Our data indicated significant interactions between the examined ages and breeds regarding the serum levels of ASAs (P ≤ 0.05).
As shown in Table 6, the findings of the current investigation revealed significant differences among the examined ages and breeds regarding the ASAs levels in the seminal plasma. The data demonstrated significant differences among the examined three ages with regard to the ODs at the first dilution (1:1), ASAs concentrations at the first dilution, and ASAs indices (P ≤ 0.01). However, ODs of the cut-off value, ASAs concentrations at cut-off values, and ASAs titers at cut-off values were not significantly different. Concerning the effect of the breed type on the seminal plasma levels of ASAs, the data demonstrated significant differences between the two breeds at the ODs at the first dilution (1:1) (P ≤ 0.01), ODs of the cut-off value (P ≤ 0.01), and ASAs indices (P ≤ 0.05). The data indicated significant interactions between the examined ages and breeds regarding the seminal levels of ASAs (P ≤ 0.05).
ASAs may sterilize the animals through their effects on sperm survival, motility, and interaction with the zona pellucida, as well as their effects on post-fertilization events such as early embryogenesis and fetal development (Vazquez-Levin et al., 2014). ASAs probably decrease sperm fecundities by agglutinating and/or immobilizing them. In humans, ASAs is found in 8 to 21% of infertile males, 9 to 36% of infertile couples, and 6 to 23% of infertile women (Naz RK. 2004). Infertile patients had ASAs concentrations that were higher (between 9 and 12 percent) (Bozhedomov et al., 2015). The effect of age on sperm viability and motility in relation to antibody indexes was examined after false positive cases were eliminated. As shown in Figure 2, the results showed that in the youngest group, the means of the sperm viability and motility values were 41.875% and 47.25%, respectively, compared to 20.8461% viability and 23.8461% motility in the ≥5 - ≤7 years old group. This demonstrates a significant decrease in sperm viability and motility in the ≥5 - ≤7 years old camels compared to the camel males aging 3-5 years (P ≤ 0.01), which associated with a significant increase in the serum and seminal plasma ASAs indexes (%) in the ≥5 - ≤7 years group compared to the younger group (P ≤ 0.05). Surprisingly, the viability and motility of the sperm in the male camels aging >7 years were 43.12% and 50.62%, respectively, significantly elevated as compared to that of the 3-5 years and 5-7 years groups (P ≤ 0.05 and P ≤ 0.01, respectively). In addition, the serum and seminal plasma ASAs indexes (%) were significantly elevated in the oldest group compared to the other two groups (P ≤ 0.05). The overall means of the viability % and motility % of the sperm was not affected with age. A previous report demonstrated that human sperm viability and motility decrease with increasing dose and duration of exposure to ASAs (Pujianto et al., 2018). This contradiction can be attributed to the in-vitro settings of the previous study. However, our data demonstrated that the levels of ASAs in the serum and seminal plasma increased with age, suggesting that age advancement could be a risk factor that contributes to immunological sterility in male camels.
After the exclusion of the false positive cases, we investigated the breed-dependent relations of sperm viability and motility to antibody indexes. As shown in Figure 3, the sperm viability of the Majaheem breed (39.5%) was significantly higher than that of the Waddah breed (30.5%) (P ≤ 0.05), while the sperm motility of the Waddah breed (40.416%) was significantly elevated compared to the Majaheem breed (33.8%) (P ≤ 0.05). The ASAS indexes (%) in the Waddah breed were 65.025% and 40.226% for the serum and seminal plasma, respectively, while in the Majaheem breed were 53.343% and 30.307% for the serum and seminal plasma, respectively. The results apparently exhibited significant differences between the two breeds with regard to sperm viability and motility. The results also demonstrated that the serum and seminal plasma ASAs indexes were significantly higher in the Waddah breed compared to the Majaheem breed (P ≤ 0.05) suggesting that the Waddah breed could be more prone to immunological sterility than the Majaheem breed. However, a study involving a large number of animals is needed to investigate the susceptibleness of the different camel breeds to ASAs-mediated sterility.
CONCLUSIONS
In summary, the current study was carried out to mainly to investigate ASAs in serum and seminal plasma of infertile dromedary male camels. Our results demonstrated for the first time the presence of ASAs in ≥ 80% of the examined animals. Furthermore, the data has provided some evidence of a positive correlation between the levels of ASAs and the age of the male camel. Interestingly, our data also suggested an influence for the breed type over the ASAs levels in dromedary male camels. The data presented in the current study highlight a potential role of ASAs in camel infertility; however, more work is needed to determine ASAs’ contribution to reproductive challenges in camels.
ACKNOWLEDGMENTS
The researchers would like to thank the Deanship of Scientific Research, Qassim University, for the support of this project. Thanks, are extended to the Qassim university veterinary hospital for kindly providing the semen and sera samples.
NOVELTY STATEMENT
To the best of our knowledge, the current study is the first study reporting the presence of ASAs in male camels with a history of infertility.
CONFLICT OF INTEREST
The authors have no relevant financial or non-financial interests to disclose.
REFERENCES
Archana SS, Selvaraju S, Binsila BK, Arangasamy A, Krawetz SA (2019). Immune regulatory molecules as modifiers of semen and fertility: A review. Mol. Reprod. Dev. 86(11):1485-504 https://doi.org/10.1002/mrd.23263.
Bauer C, Steng G, Prevot F, Dorchies P (2002). Seroprevalence of Oestrus ovis infection in sheep in southwestern Germany. Vet. Parasitol. 110, 137-143. https://doi.org/10.1016/S0304-4017(02)00317-5
Bello A, Bodinga HA (2020) Common reproductive problem associated with one humped camel (Camelus dromedarius) in West Africa. Insights Vet. Sci. 4: 001-003. https://doi.org/10.29328/journal.ivs.1001018
Bozhedomov VA, Nikolaeva MA, Ushakova IV, Lipatova NA, Bozhedomova GE, Sukhikh GT (2015). Functional deficit of sperm and fertility impairment in men with antisperm antibodies. J. Reprod. Immunol. 112, 95–101. https://doi.org/10.1016/j.jri.2015.08.002
Chiu WWC, Chamley LW (2004). Clinical associations and mechanisms of action of antisperm antibodies. Fertil. Steril. 82(3):529-35. https://doi.org/10.1016/j.fertnstert.2003.09.084
Classen DC, Morningstar JM, Shanley JD (1987). Detection of antibody to murine cytomegalovirus by enzyme-linked immunosorbent and indirect immunofluorescence assays. J. Clin. Microbiol. 25(4): 600-4. https://doi.org/10.1128/jcm.25.4.600-604.1987
Cobellis G, Noviello C, Nino F, Romano M, Mariscoli F, Martino A, Parmeggiani P, Papparella A (2014). Spermatogenesis and cryptorchidism. Front. Endocrinol. 5: 63. https://doi.org/10.3389/fendo.2014.00063
Cuia D, Han G, Shang Y, Liu C, Xia L, Li L, Yi S (2015). Antisperm antibodies in infertile men and their effect on semen parameters: A systematic review and meta-analysis. Clin. Chim. Acta. 444: 29-36. https://doi.org/10.1016/j.cca.2015.01.033
Doumas BT, Bayse DD, Carter RJ, Peters Jr T, Schaffer RA (1981). A candidate reference method for determination of total protein in serum. I. Develop. Validat. Clin. Chem. 27(10), 1642-1650. https://doi.org/10.1093/clinchem/27.10.1642
Esmailnejad A, Nikahval B, Mogheiseh A, Karampour R, Karami S (2020). The detection of canine anti-sperm antibody following parenteral immunization of bitches against homogenized whole sperm. Basic Clin. Androl. 30(1): 1-7. https://doi.org/10.1186/s12610-020-0100-z
Ferrer MS, Miller LMJ (2018). Equine sperm-bound antisperm antibodies are associated with poor semen quality. Theriogenology. 118: 212-218. https://doi.org/10.1016/j.theriogenology.2018.05.034
Fu J, Yao R, Luo Y, Yang D, Cao Y, Qiu Y, Song W, Miao S, Gu Y, Wang L (2016). Immune infertility should be positively diagnosed using an accurate method by monitoring the level of anti-ACTL7a antibody. Sci. Rep. 9;6:22844. https://doi.org/10.1038/srep22844
Gupta VK, Srivastava SK, Ghosh SK, Srivastava N, Singh G, Verma MR, Katiyar R, Muthu R, Bhutia L, Kumar A, Singh R (2021). Effect of endogenous hormones, antisperm antibody and oxidative stress on semen quality of crossbred bulls. Anim. Biotechnol. 1-8. https://doi.org/10.1080/10495398.2021.1905656
Kawase O, Jimbo M (2018). Detection of sperm-reactive antibodies in wild sika deer and identification of the sperm antigens. J. Vet. Med. Sci. 17-0660. https://doi.org/10.1292/jvms.17-0660
Kershaw-Young CM, Maxwell WMC (2011). Advancing artificial insemination in camelids, particularly the alpaca. Rural industrial research crop public, No. 11, Project No. R 1882, Aust. Govt, 63-64 P. https://doi.org/10.1016/j.anireprosci.2013.02.005
Khalil A. H., Abd Al-Galil A. S., Sabek A. A., Zeineldin M. M., Abo-Kora S. Y. (2019). Sedative, analgesic, behavioral and clinical effects of intravenous nalbuphine-xylazine combination in camels (Camelus dromedarius). J. Vet. Sci. 20(5). https://doi.org/10.4142/jvs.2019.20.e55
Kim CA, Parrish JJ, Momont HW, Lunn DP (1999). Effects of experimentally generated bull antisperm antibodies on in vitro fertilization. Biol. Reprod. 60 1285–12 91. https://doi.org/10.1095/biolreprod60.6.1285
López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Gude-Sampedro F (2014). OptimalCutpoints: an R package for selecting optimal cutpoints in diagnostic tests. J. Stat. Softw. 61, 1-36. https://doi.org/10.18637/jss.v061.i08
Lu SM, Li X, Wang SL, Yang XL, Xu YZ, Huang LL (2019). Success rates of in vitro fertilization versus intracytoplasmic sperm injection in men with serum anti-sperm antibodies: a consecutive cohort study. Asian J. Androl. 21:473–477. https://doi.org/10.4103/aja.aja_124_18
Marconi M, Weidner W (2017). Site and risk factors of antisperm antibodies production in the male population. Immun Infertil, Springer, Cham . pp. 133–147. https://doi.org/10.1007/978-3-319-40788-3_8
McLachlan RI (2002). Basis, diagnosis and treatment of immunological infertility in men. J. Reprod. Immunol. 57: 1–2, 35-45. https://doi.org/10.1016/S0165-0378(02)00014-1
Morton KMl, Thomson PC, Bailey K, Evans G, Maxwell WM (2010). Quality parameters for alpaca (Vicugna pacos) semen are affected by semen collection procedure. Reprod. Domest. Anim. 45(4): 637- 643. https://doi.org/10.1111/j.1439-0531.2008.01321.x
Naz RK (2004). Modalities for treatment of antisperm antibody mediated infertility: novel perspectives. Am. J. Reprod. Immunol. 51(5):390-7. https://doi.org/10.1111/j.1600-0897.2004.00174.x
Parsonson IM, Winter AJ, McEntee K (1971). Allergic epididymo-orchitis in guinea pigs and bulls. Vet. Pathol. 8 333–351. https://doi.org/10.1177/030098587100800405
Pujianto DA, Hajizah H, Mansur IG, Amarudin A (2018). Antisperm antibodies disrupt plasma membrane integrity and inhibit tyrosine phosphorylation in human spermatozoa. Med. J. Indones. 27(1), 3-11. https://doi.org/10.13181/mji.v27i1.1429
Restrepo B, Cardona-Maya W (2013). Antisperm antibodies and fertility association. Actas Urológicas Españolas (English Edition), 37(9): 571-578. https://doi.org/10.1016/j.acuroe.2012.11.016
Shibahara H, Chen Y, Yamaya A, Wakimoto Y, Fukui A, Hasegawa A (2022). Antisperm antibodies and reproductive failure. Immunol. Recurr. Preg. Loss Implant. Failure., 137-151. https://doi.org/10.1016/B978-0-323-90805-4.00003-1
Tabouret G, Pre´ vot F, Bergeaud JP, Dorchies P and Jacquiet P (2001). Oestrus ovis (Diptera: Oestridae): sheep humoral immune response topurified excreted/secreted salivary gland 28 kDa antigen complex from second and third instar larvae. Vet. Parasitol., 101: 53–66. https://doi.org/10.1016/S0304-4017(01)00501-5
Vazquez-Levin MH, Marin-Briggiler CI, Veaute C (2014). Antisperm antibodies: Invaluable tools toward the identification of sperm proteins involved in fertilization. Am. J. Reprod. Immunol. 72: 206–218. https://doi.org/10.1111/aji.12272
Waheed MM, Meligy AM, Dhalam SA (2018). The relationship between seminal plasma and serum trace elements and semen parameters of dromedary camels (Camelus dromedarius). Reprod. Domest. Anim. 53(6): 1367-1374. https://doi.org/10.1111/rda.13268
Wani NA, Billah M, Skidmore JA (2008). Studies on liquefaction and storage of ejaculated dromedary camel (Camelus dromedarius) semen. Anim. Reprod. Sci. 109:309–318. https://doi.org/10.1016/j.anireprosci.2007.10.011
Wright PJ (1980) Serum spermagglutinins and semen quality in the bull. Aust. Vet. J. 56 10–13. https://doi.org/10.1111/j.1751-0813.1980.tb02530.x
To share on other social networks, click on any share button. What are these?






