Efficacy of Amoxicillin and or Enrofloxacin Against Mixed Infection with Escherichia coli and Salmonella enteritidis In vitro and In vivo
Research Article
Efficacy of Amoxicillin and/ or Enrofloxacin Against Mixed Infection with Escherichia coli and Salmonella enteritidis In vitro and In vivo
Mai A Fadel1*, Ahmed M Elmahdy1, Jihan Mostafa Badr2, Mohammed AM Saleh3, Mona A.A. AbdelRahman3
1Pharmacology and Pyrogen Unit, Biochemistry, Toxicology, Feed Deficiency Department, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Dokki P.O. Box 246, Giza 12618, Egypt; 2Department of Poultry Diseases, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Dokki P.O. Box 246, Giza 12618, Egypt; 3Reference Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC). Nadi El-Seid Street, Dokki P.O. Box 246, Giza 12618, Egypt.
Abstract | The present research investigated the efficacy of amoxicillin and enrofloxacin when being used alone, in a half-dose combination, and in a full-dose combination against Escherichia coli and Salmonella enteritidis mixed infection in broiler chickens. One-hundred and five chickens were equally divided into 7 groups. Group one was used as a non-treated non-infected group (negative control). Group two was used as an infected with Escherichia coli and Salmonella enteritidis (mixed infection), non-treated group (positive control). Group three was infected with the mixed infection and treated with amoxicillin 13.1 mg/kg b.wt. Group four was infected with the mixed infection and treated with enrofloxacin 10 mg/kg b.wt. Group five was infected and treated with a full treatment dose of amoxicillin and enrofloxacin. Group six was infected and treated with a half-dose combination of amoxicillin and enrofloxacin. Group seven was non-infected and treated with a full-dose combination of amoxicillin and enrofloxacin. Antimicrobial sensitivity test, minimum inhibitory concentration, minimum bactericidal concentration, and fractional inhibitory concentration were determined. High Performance Liquid chromatography (HPLC) method was developed to quantify the concentrations of enrofloxacin and amoxicillin in serum and different tissues of chickens. The pharmacokinetics and pharmacodynamics indices of both antibiotics were calculated and statistically analyzed. Aspartate aminotransferase, alanine aminotransferase, urea, and creatinine serum level were also estimated. From our findings, we concluded that the usage of a half-dose combination of enrofloxacin and amoxicillin is safer and more efficient in the treatment of Escherichia coli and Salmonella enteritidis mixed infection in broiler chickens than the usage of these antibiotics in a full-dose combination or solely.
Keywords | Amoxicillin, Enrofloxacin, E. coli, Salmonella, Mixed infection.
Received | July 27, 2022; Accepted | August 30, 2022; Published | September 25, 2022
*Correspondence | Mai A Fadel, Pharmacology and Pyrogen Unit, Biochemistry, Toxicology, Feed Deficiency Department, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Dokki P.O. Box 246, Giza 12618, Egypt; Email: dr.mai87@yahoo.com
Citation | Fadel MA, ELmahdy AM, Badr JM, Saleh MAM, AbdelRahman MAA (2022). Efficacy of amoxicillin and/ or enrofloxacin against mixed infection with escherichia coli and salmonella enteritidis in vitro and in vivo. Adv. Anim. Vet. Sci. 10(10): 2252-2264.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.10.2252.2264
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Escherichia coli and Salmonella enteritidis are the most worldwide spread bacterial infections in poultry causing dramatic economic losses in poultry (Gast and Porter, 2020; Nolan et al., 2020). Moreover, poultry is one of the most commonly used sources of animal protein in the Egyptian market, and therefore it may act as a potential source for human food poising through consumption of poultry and its by-products. OIE reported that 60% of pathogens responsible for human diseases arise from domestic animals or wildlife (OIE, 2022). Furthermore, we shall take into consideration the rapid emergence of antimicrobial resistance (AMR) through the uncontrollable use of antibiotics in animal sectors (OIE, 2022), such as fluoroquinolones (Li et al., 2017).
The combination of the two antibacterial drugs amoxicillin and enrofloxacin is a well-known mixture in the veterinary field. In this mixture, a synergistic effect has been demonstrated in vitro between quinolones and β-lactams. This drug is indicated for treating Gram-positive and Gram-negative bacterial infections in the digestive tract, as well as respiratory and urinary infections in poultry (Lashev and Haritova, 2012).
Amoxicillin (α-amino-hydroxy benzylpenicillin) is broad-spectrum penicillin categorized under the β-lactam class of antibiotics. It is a semi-synthetic antibiotic derived from a precursor molecule called 6-aminopenicillanic acid. Amoxicillin is bactericidal in action and interferes with cell wall synthesis in bacteria by inhibiting cross-linking of peptidoglycan molecules, which is a cell wall component in Gram-positive (major) and Gram-negative bacteria (Manzoor et al., 2011). It has good absorption and penetration into tissues with rapid bactericidal activity (Khatun et al., 2020). Many Gram-negative strains, including Escherichia coli and Salmonella enteritidis which were previously susceptible to amoxicillin, become recently resistant (Kuznetsova et al., 2020).
Enrofloxacin is a quinolone carboxylic acid derivative with antimicrobial action. It is effective against a broad spectrum of Gram-negative bacteria and is indicated for infections of the respiratory, gastrointestinal, and urinary tracts in cattle and poultry. Enrofloxacin is bactericidal through the inhibition of DNA-gyrase (Brahmareddy et al., 2015).
Poultry products that are being produced by farmers in the country are hardly screened for antibiotic residues which may result in serious health problems for consumers such as the development of antibiotic resistance and hypersensitivity reaction. Thus, monitoring antibiotic residues in food products becomes crucially important to guarantee food safety and to promote regulatory oversight over imported and locally produced food supplies to ensure that residues do not exceed maximum residue limits (Batrawi et al., 2017).
The combined use of pharmacokinetics (PK) and pharmacodynamics (PD) helps us improve the use of an antimicrobial agent. The incorporation of PK and PD data provides an essential basis for understanding various dosage regimens on the specific pathway of drug responses, information on effective doses, and suitable duration for treatment. Moreover, the pharmacokinetic/pharmacodynamic studies confirm the importance of dose detection to avoid antimicrobial resistance (Nielsen et al., 2011).
The aim of the present study was to investigate the effectiveness of a combination of a full dose and a half dose of enrofloxacin and amoxicillin against mixed infection of Escherichia coli and Salmonella enteritidis in vitro and in vivo taking into consideration their disposition kinetics, pharmacodynamic, tissue residues, and hepatic and renal function status in chickens.
Materials and Methods
Drugs
Amoxicillin was obtained as the brand name Amoxicillin 20%® which is manufactured by UCCMA, Egypt, in a form of a water-soluble powder, where each gram contains 200 milligrams of amoxicillin trihydrate.
Enrofloxacin was obtained as the brand name Nutri Flox 10%® which is manufactured by Nutrex, Holland, in a form of an oral solution, where each milliliter contains 100 milligrams enrofloxacin base.
Chemical Reagents
Amoxicillin standard (≥95%) was purchased from LKT Laboratories, Inc., USA. Enrofloxacin (99%) and ciprofloxacin (≥98%) standards were purchased from Sigma Aldrich, Germany. Acetonitrile (analytical grade) was purchased from Chem-Lab, Belgium. Methanol, acetonitrile, water (HPLC grade), ammonium hydroxide, and phosphate buffer were purchased from Fisher Scientific, Leicestershire, United Kingdom.
Bacteria
The bacterial strains (S. Entritidis and E. coli) which have been used in vivo were recovered from an avian origin and bacteriologically and serologically identified.
S. Entritidis is serotyped through the use of Salmonella antisera (Sifin Co., Japan) according to ISO 6579-3: 2014 and Kauffman–White scheme (Grimont and Weill, 2007), while E. coli serotype O2 is serotyped through the use of E. coli antisera (Sifin Co., Japan) (Ewing, 1986).
The susceptibility breakpoints of enrofloxacin and amoxicillin were interpreted based on CLSI 2008 and CLSI 2014, respectively.
Preparation of Challenge Inoculum
The inoculums were diluted according to McFarland standard in normal saline to be inoculated once orally with 1 ml containing 1.3x108 CFU for S. Entritidis (Ishola and Holt, 2008) and 0.5 ml containing 3x108 CFU for E. coli O2 (Dahshan and Mohamed, 2016).
Experimental Design
Ethical and Study Protocol Approval: This experiment was approved by the Research Committee of the Animal Health Research Institute (ARC-AH-1932), Ministry of Agriculture, Giza, Egypt.
Chickens: In the current study, one-hundred and five, fifteen-day-old, apparently healthy broiler chickens were purchased from a commercial poultry farm. All the chickens were checked via examining cloacal and tracheal swabs, swabs from housing cages, and a representative sample from the used ration by conventional isolation methods (Abd El-Ghany et al., 2012) to ensure that the chickens and the used ration are free from Salmonella and E. coli before the beginning of the study protocol. Chickens fed on commercial balanced antimicrobial free ration and water ad-libitum were reared under field conditions and were left for 15 days as an acclimatization period and to ensure the complete excretion of any drug from their bodies.
Grouping: A total number of one-hundred and five, thirty-day-old, chickens were divided equally into 7 groups. Group 1 was used as a non-treated non-infected group (negative control). Group 2 was used as an infected non-treated group (positive control). Group 3 was infected with the mixed infection (both Salmonella and E. coli stains), and after the appearance of the infection symptoms, it was treated orally with amoxicillin at a dose of 13.1 mg/kg b.wt. for 3 consecutive days (EMEA, 2004). Group 4 was infected the same and treated orally with enrofloxacin at a dose of 10 mg/kg b.wt. for 3 consecutive days (EMEA, 2018). Group 5 was infected and treated with a full treatment dose of amoxicillin and enrofloxacin for 3 consecutive days. Group 6 was infected and treated with half doses of amoxicillin and enrofloxacin in combination for 3 consecutive days. Group 7 was non-infected and treated with a full dose of each drug.
Experimental Samples: Blood samples were taken from a wing vein into tubes at 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 h after antibiotics administration for pharmacokinetic study. Blood samples were centrifuged at 2000 rpm for 10 min to yield serum which was stored at -20°C until analysis. Controlled and treated broilers were slaughtered at the end of the third day of repeated oral administration. Then, muscle, liver, kidneys, and blood samples were taken for the determination of tissue residues and the biochemical analysis and stored at -20°C till analysis.
Enumeration of Salmonella and E. coli strains was performed through the collection of cecal and liver samples for counting S. Entritidis and E. coli, respectively (Thushani et al., 2003; Marien et al., 2005). The samples were collected at 1, 2, and 3 days after antibiotic administration from the treated groups to measure the efficacy of amoxicillin and enrofloxacin antibiotics in treating Salmonella and E. coli infection. Samples were serially diluted, and then, 1 ml from each dilution was transferred on XLD and MacConkey agar plates, respectively.
Antimicrobial Sensitivity Test (AST)
An AST was conducted for S. Entritidis and E. coli O2 strains through the use of a disk-diffusion test on Mueller–Hinton agar, as previously described by WHO (2003) against 2 antibiotics (Himedia®), which were as follows: amoxicillin which was interpreted according to CLSI (2014) and enrofloxacin which was interpreted according to CLSI (2008).
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
Susceptibility testing was performed according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2022) guidelines using the broth dilution method. The drugs were diluted in Mueller Hinton broth tubes to give the final concentrations ranging from 5 to 0.02 μg/ml. Two-fold serial dilutions of antibiotic were utilized in Mueller Hinton broth with 5x105 CFU/ml of S. Entritidis and E. coli O2. A control negative tube was used containing broth without antibiotics. The tubes were incubated at 37°C for 24 h. MIC was defined as the lowest concentration of the tested antibiotics which did not give any visible bacterial growth (turbidity). MBC was determined through the inoculation of 10 µl from each clear tube and subcultured on Mueller Hinton agar. The plates were incubated at 35 ̊C for 18 hours. The lowest concentrations showing no growth gave the MBC value, denoting 99.5% killing of the original inoculum (Amita et al., 2013).
Checkerboard Method for the Antibiotics Combination
The combinations of amoxicillin and enrofloxacin were examined for determining fractional inhibitory concentration (FIC). Amoxicillin at 1/2 MIC was used with enrofloxacin concentrations ranging from 1/32 MIC to 2 MIC and vice versa (Jarrar et al., 2010). FIC index was calculated using the following formulas: FICamx = (MICamx in combination)/(MICamx alone), FICenro = (MICenro in combination)/(MICenro alone), and the FIC index = FIC amx + FIC enro. FIC index was utilized for characterizing the interactions of antibiotics as follows. Synergy: when FIC value of antibiotics combination is <0.5, it increases the inhibitory activity (decrease in MIC) for one or both compounds compared to each one alone. Additivity or indifference: when compounds combination results in a FIC value of 0.5–4, there is no increase in inhibitory activity or a slight increase in inhibitory activity from the additive effect of both compounds combined. Antagonism: when the combination of compounds results in a FIC value of > 4, it increases MIC or lowers the activity of the compound (Magryś et al., 2021).
Chromatographic Assay
High-performance liquid chromatography (HPLC) was utilized for determining amoxicillin and enrofloxacin concentrations in serum and tissue (muscle, liver, and kidney) samples.
Standard Preparation
A stock solution was prepared in methanol: water (50:50 v/v). From this stock solution, an intermediate solution was prepared at a concentration of 100 mg/L. The intermediate solution was used in the preparation of working standards in blank serum, muscle, liver, and kidneys (control group) at a range of 10 to 1000 µg/L for the three antibiotics. Preparation of quality control samples at 3 levels [0.5 MRL, 1 MRL, and 2 MRL] was conducted according to USP (2021) for each item established by the European Union (2010). The working standards and QC sample were extracted as mentioned below.
Apparatus and Chromatographic Conditions
HPLC system (Agilent 1200 series, Software, Agilent Chemistation Version B.040.01) SP1 (Agilent Technologies, Germany), and chromatographic column (Agilent C18, 100Å (4.6 × 250 mm, 5 μm) were utilized. The mobile phase consisted of buffer and acetonitrile (75% buffer: 25% acetonitrile) Batrawi et al. (2017). The buffer was prepared by mixing 75 mL of methanol with 425 mL of 0.02 M KH2PO4 and was then adjusted to pH 5.0 with 2 M H3PO4. The mobile phase was run gradient as illustrated in Table 1. The flow rate was 0.8 mL/min, the column temperature was set at 40 °C, and the injection volume was 100 μL. Analytes were monitored at 230 nm through the use of an ultraviolet detector (UV).
Table 1: Gradient table of mobile phase
| Time |
Buffer |
| 0-1 | 95% |
| 1-6 | 75% |
| 6-10 | 95% |
Extraction and Clean-Up
Extraction procedure has been carried out as reported by Oyedeji et al. (2021). This method was validated according to USP (2021) via determining method precision, recovery, linearity, limit of detection, and quantification.
Pharmacokinetics/Pharmacodynamics Determination
The pharmacokinetic parameters were calculated according to equations integrated by Baggot (1977), Baggot (1978a) and Baggot (1978b). AUC/MIC and the Cmax/MIC ratios were determined in order to detect the efficacy of enrofloxacin which is a concentration-dependent antibiotic. On the other hand, amoxicillin is a time-dependent antibiotic (Mckellar et al., 2004) which is determined through the value of T>MIC%. It is calculated using the following formula (Turnidge, 1998):
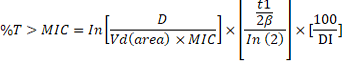
where D is the proposed dose; Vd (area) is the volume of distribution; t1/2β is the terminal elimination half-life; DI is the dosing interval.
Liver and Kidney Enzymes
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were quantitatively estimated following the method described by Reitman and Frankel (1957) and Young (1997). Serum urea level was estimated according to Wybenga et al. (1971). Meanwhile, serum creatinine level was determined according to Tietz (1986).
Statistical Analysis
The obtained results were statistically analyzed using IBM SPSS. Results were expressed as the mean values ± standard error (SE) and compared by one-way ANOVA (P≤0.05) and independent t-test (Kim, 2014). The pharmacokinetic variables were determined through the use of PK Solver: an add-in program for Microsoft Excel, version 2 (Zhang et al., 2010).
Results
Mortality%, clinical signs and PM examination
All infected groups showed dullness, depression, diarrhea and pasty vent before the onset of treatment. These signs were ameliorated with the half dose enrofloxacin and amoxicillin combination at the 2nd day of treatment (Group 6). At the 3rd day of treatment, group 5 (full dose enrofloxacin and amoxicillin combination) and group 6 were recovered. The mortality rate was 53.3% for groups 3 and 4 while groups 5 and 6 recorded much lesser mortalities which was 6.6%. On the other hand, all infected groups at the 1st day of treatment showed numerous lesions as liver congestion, congestion and hemorrhage of lung, internal visceral hemorrhage (septicemic picture), unabsorbed yolk sac, pericarditis with petechial hemorrhage on heart and enlarged cecum. This necropsy was the same till the 3rd day of treatment in groups 3 and 4 while in groups 5 and 6 appeared in semi-normal condition.
Enumeration of Salmonella enteritidis and E. coli Count
Count of Salmonella enteritidis in cecal samples showed a lack of growth so they were omitted from the statistical analysis, while E. coli count in liver samples grown on MacConkey medium was found at the 1st and 2nd days after treatment with significantly low count in Group 6 (half dose of amoxicillin and enrofloxacin combination) in comparison with that in Group 5 (full dose of amoxicillin and enrofloxacin antibiotics).
There was a significant difference between the four groups of treatment (p<0.05) for the mean E. coli enumeration in the 1st, 2nd, and 3rd days after treatment as shown in Table 2 and Figure 1.
Table 2: E. coli counts in liver samples on 1, 2 &3 days post-treatment on MacConkey medium:
|
1st day |
2nd day |
3rd day |
|
|
Group 1 |
- ve |
-ve |
-ve |
| Group 2 |
5.5±0.2 c |
5.5±0.8c |
5.4±0.9 c |
| Group 3 |
2.60206± 0.2a |
1.9±0.1b |
1±0.1a |
| Group 4 |
3.079181±0.08b |
1.9±0.6b |
1.3±0.9b |
| Group 5 |
3±0.9b |
2.0791812±0.13b |
1.1±0.2b |
| Group 6 |
2.778151±0.1a |
1.3±0.2a |
1.3±0.83b |
| Group 7 | -ve | -ve |
-ve |
Data are presented as mean ±SE. Means with different superscript small letters indicate significantly different in the same column between groups at P < 0.05 using one-way ANOVA test.
Determination of MIC and MBC for Enrofloxacin and Amoxicillin
MIC of enrofloxacin for S. Entritidis and E. coli O2 was 0.06 µg/ml and 0.04 µg/ml, respectively, while MIC of amoxicillin was 0.08 µg/ml and 2.4 µg/ml, respectively. Furthermore, MBC of enrofloxacin was tested to be 0.12 µg/ml and 0.08 µg/ml, respectively, while that of amoxicillin was 0.16 µg/ml and 4.8 µg/ml, respectively.
Interaction between Enrofloxacin and Amoxicillin Antibiotics Assessed by Checkboard Method
Based on FIC index, the antibacterial activity of amoxicillin was highly influenced by the combination with enrofloxacin against Salmonella enteritidis and E. coli O2. It was calculated as 0.2 and 0.4, respectively. These were indicated as a synergistic effect of the combination against the tested strains.
Results of Method Validation
The method was accurate with high recovery (92–110 %) of good linearity (˃ 0.99) with a low LOD and LOQ, as LOD was 4.2, 1.5, and 2.1 µg/L and LOQ was 12.8, 4.6, and 6.3 µg/L for amoxicillin, enrofloxacin, and ciprofloxacin in progress, respectively. Specificity and selectivity were illustrated in Figure 2 with the following retention times 4.2, 5.7, and 6.7 minutes.
Pharmacokinetic/Pharmacodynamics Model
Cmax/MIC and AUC/MIC ratios of enrofloxacin were calculated for Groups 4, 5, and 6. Moreover, T˃MIC value % was calculated for Groups 3, 5, and 6. Data are tabulated in Tables 3 and 4.
Enrofloxacin pharmacodynamics in Groups 4, 5, and 6 showed effective Cmax/MIC and AUC/MIC ratios against S. Entritidis and E. coli O2 infection. Meanwhile, amoxicillin achieved the optimal bactericidal effect against S. Entritidis and E. coli O2 in Groups 5 and 6 but failed with a dosing interval of 24 hrs in Group 3 against E. coli O2 infection. Enrofloxacin and amoxicillin combination in Group 6 recorded a dramatic decrease in vivo E. coli count.
Table 3: Pharmacokinetic/pharmacodynamic model of enrofloxacin and amoxicillin against S. Entritidis in presence of E. coli O2:
| Parameters |
Group 3 (Amoxicillin) |
Group 4 (Enrofloxacin) |
Group 5 (Full dose combined) |
Group 6 (Half dose combined) |
Acceptance criteria |
| Cmax/MIC | ---- | 36.7 | 45 | 28.3 |
≥10 |
| AUC/MIC | ---- | 381.7 | 481.7 | 276.7 | ≥100-125 |
|
T˃MIC% (Dose interval 12hr) |
297.5 | ---- | 391.5 | 291.8 | ≥50% |
|
T˃MIC% (Dose interval 24hr) |
148.8 | ---- | 195.9 |
145.95 |
Table 4: Pharmacokinetic/pharmacodynamic model of enrofloxacin and amoxicillin against E. coli O2 in presence of S.entritidis:
| Parameters |
Group 3 (Amoxicillin) |
Group4 (Enrofloxacin) |
Group 5 (Full dose combined) |
Group 6 (Half dose combined) |
Acceptance criteria |
| Cmax/MIC | ---- | 52.5 | 60 | 39 |
≥10 |
| AUC/MIC | ---- | 532.5 | 692.5 | 370 | ≥100-125 |
|
T˃MIC% (Dose interval 12hr) |
71.8 | ---- | 99.9 | 102.02 | ≥50% |
|
T˃MIC% (Dose interval 24hr) |
35.9 | ---- | 50.02 | 51 |
Table 5: Individual and combined kinetic parameters of enrofloxacin and amoxicillin in broilers after a single oral dose at 10 and 13.1 mg/kg b.wt. (Full dose) and (5mg and 6.55mg/kg b.wt.(half dose):
| Kinetic parameters |
Group 7 (Individual Healthy) |
Individual Diseased |
Group 5 (Full dose combined) |
Group 6 (Half dose combined) |
||||
| Enro | Amox | Group4 (Enro) | Group3 (Amox) | Enro | Amox | Enro | Amox | |
|
t1/2ka (h) |
0.6±0.1a |
0.7±0.2b |
1.3±0.1b |
0.55±1.3b |
1.1±0.6b |
0.5±0.4a |
1.3±0.5b |
0.5±0.1a |
|
t1/2Beta (h) |
6.1±0.2b |
6.6±0.1b |
7.4±0.3a |
5.5±0.3c |
7.4±0.02a |
7.1±0.3a |
7.3±0.09a |
8.5±0.2a |
| V/F (mg) (µg/ml) |
3.3±0.1a |
1.5±0.3b |
2.1±0.5b |
1.85±0.2a |
1.9±0.2b |
1.7±0.3b |
1.3±0.3c |
1.01±0.1c |
| CL/F(mg) (µg/ml)/hr |
0.4±0.3c |
0.3±0.2a |
0.4±0.8c |
0.4±0.5c |
0.32±0.4b |
0.36±0.5b |
0.27±0.1a |
0.23±0.2a |
|
Tmax (h) |
2.3±0.1a |
1.6±1.1b |
2.7±0.3bc |
1.3±0.5a |
2.4±0.45a |
1.2±0.8a |
2.5±0.3b |
1.3±0.4a |
|
Cmax (μg/ml) |
2.3±0.1b |
3.3±0.2b |
2.2±0.2b |
3.9±0.7ac |
2.7± 0.1a |
4.01±0.2a |
1.9±0.4b |
3.6±0.1b |
|
AUC0-24 (μg h/ml) |
23.5±0.1b |
36.9±0.1a |
22.9±0.5b |
28.7±0.6c |
28.9 ± 0.4a |
33.1±0.1b |
16.8±0.2c |
27.7±0.3c |
| MRT (h) |
9.2±0.2b |
9.4±0.2a |
10.5±0.35a |
7.9±0.1b |
10.9±0.8a |
10.1±0.2a |
9.9±0.9ab |
9.8±0.5a |
Enro: Enrofloxacin; Amox: Amoxicillin
* Values are the mean ±SD (n = 5). Means with different superscript small letters indicate significantly different in the same row between groups at P < 0.05 using one-way ANOVA test.
t1/2ka: absorption half-life, t1/2Beta: elimination half-life, V/F: apparent volume of distribution, CL/F: apparent total clearance of the drug from serum, Tmax: time to reach maximum serum concentration, Cmax: maximum serum drug concentration, AUC0-24: area under the serum concentration-time curve from time zero to time 24 hs, MRT: mean residence time.
Table 6: Tissue residues of enrofloxacin with its metabolite and amoxicillin after repeated oral administration of different doses in different groups of broiler chicken (n=3):
| Tissues |
days |
Individual Healthy |
Individual Diseased |
Group 5 (Full dose combined) |
Group 6 (Half dose combined) |
||||||||
| Enro | Cipro | Amox | Enro | Cipro | Amox | Enro | Cipro | Amox | Enro | Cipro | Amox | ||
| Muscle |
1st
|
0.6± 0.02bc |
0.05± 0.003c |
0.7± 0.03 c |
0.5± 0.02 b |
0.04± 0.003 b |
0.6± 0.02 b |
0.5± 0.01 b |
0.04± 0.002b |
0.6± 0.02 b |
0.3± 0.01 a |
0.02± 0.002 a |
0.3± 0.02a |
|
3rd |
0.05± 0.01bc |
ND |
0.3± 0.03b |
0.04± 0.004b |
ND |
0.3± 0.02b |
0.04± 0.002b |
ND |
0.3± 0.01b |
0.02± 0.002a |
ND |
0.1± 0.01a |
|
|
5th |
0.026± 0.02bc |
ND | ND |
0.02± 0.002b |
ND | ND |
0.02± 0.1b |
ND | ND |
0.01± 0.01a |
ND | ND | |
|
7th |
ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
|
Kidney |
1st
|
0.8± 0.02b |
0.6± 0.04c |
2.2± 0.2c |
0.7± 0.2b |
0.4± 0.01b |
1.8± 0.2b |
0.7± 0.2b |
0.4± 0.01b |
1.8± 0.2b |
0.4± 0.1a |
0.26± 0.01a |
1.1± 0.1a |
|
3rd |
0.07± 0.2c |
0.04± 0.01b |
1.57± 0.2c |
0.05± 0.02b |
0.02± 0.01a |
1.3± 0.1b |
0.05± 0.2b |
0.02± 0.03a |
1.3± 0.1b |
0.03± 0.1a |
0.01± 0.02a |
0.7± 0.07a |
|
|
5th |
0.05± 0.04c |
0.01± 0.1bc |
0.8± 0.04bc |
0.03± 0.03b |
0.008± 0.001b |
0.7± 0.03b |
0.03± 0.03b |
0.008± 0.1b |
0.7± 0.03b |
0.01± 0.004a |
0.005± 0.1a |
0.4± 0.02a |
|
|
7th |
ND | ND |
0.29± 0.02b |
ND | ND |
0.2± 0.01ab |
ND | ND |
0.2± 0.1ab |
ND | ND |
0.15± 0.01a |
|
| Liver |
1st
|
1.2± 0.02c |
0.84± 0.03c |
1.1± 0.1c |
0.9± 0.02b |
0.6± 0.02b |
0.8± 0.1b |
0.9± 0.05b |
0.6± 0.2b |
0.8± 0.1b |
0.5± 0.02a |
0.4± 0.2a |
0.5± 0.03a |
|
3rd |
0.08± 0.004c |
0.07± 0.01c |
0.6± 0.04c |
0.06± 0.003b |
0.02± 0.004b |
0.5± 0.03b |
0.06± 0.1b |
0.02± 0.04b |
0.5± 0.3b |
0.03± 0.01a |
0.01± 0.03a |
0.3± 0.05a |
|
|
5th |
0.04± 0.01bc |
ND | ND |
0.03± 0.004b |
ND | ND |
0.03± 0.2b |
ND | ND |
0.02± 0.02a |
ND | ND | |
|
7th |
ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
ND |
|
Enro: Enrofloxacin; Cipro: Ciprofloxacin; Amox: Amoxicillin
*Values are the mean ±SD. Means with different superscript small letters indicate significantly different in the same row between groups at P < 0.05 using one-way ANOVA test.
Table 7: AST,ALT,UREA,and Creatinine concentration in serum of different tested groups (n=3) (liver and kidney function test ).
| Parameters |
Group 1 Not infected not treated |
Group 2 infected not treated |
Group 3 (Amoxicillin) |
Group 4 (Enrofloxacin) |
Group 5 (Full dose combined) |
Group 6 (Half dose combined) |
Group 7 (Not infected full dose combined) |
| AST (U/L) |
95.01±4.36a |
113.00±2b |
94.01±4.58a |
115.02±5.57b |
118.00±6.56b |
98.00±8.19a |
100.2±3.25a |
| ALT(U/L) |
5.00±0.56a |
10.01±0.82b |
6.04±0.98a |
11.02±1.73b |
12.02±1.8b |
7.04±0.9a |
7.1±0.1a |
| Urea (mg/dl) |
17.00±0.50a |
23.00±1.32c |
18.02±1.7ab |
19.02±1.35ab |
20.02±1.32b |
18.03±0.87ab |
18.5±0.56ab |
|
Creatinine (mg/dl) |
0.73±0.05a |
0.97±0.13b |
0.73±0.07a |
0.77±0.06a |
0.83±0.08a |
0.75±0.02a |
0.75±0.04a |
AST: Aspartate aminotransferase ALT: Alanine aminotransferase
Data are presented as mean ±SD. Means with different superscript small letters indicate significantly different in the same row between groups at P < 0.05 using one-way ANOVA test.
Pharmacokinetics parameters are illustrated in Table 5 and Figures 3 and 4. There was a significant decrease in t1/2 beta, MRT, Cmax, and AUC0-24 values in Group 6 (half dose) for both enrofloxacin and amoxicillin in comparison with the other groups. On the other hand, there was a significant increase in V/F (volume of distribution) and CL/F (clearance time) values for enrofloxacin in Group 6 in comparison with the other groups. Amoxicillin in a half-dose combination (Group 6) recorded a significant decrease in V/F and CL/F values compared to the other groups.
Tissues Residues
Data shown in Table 6 demonstrated a significant reduction in the residue level of muscle, kidney, and liver tissues for Group 6 (half dose) in comparison with the other treated groups. Generally, all examined tissues of all treated groups can be consumed after the 1st day and on the 5th day of cessation of enrofloxacin and amoxicillin administration, respectively. These estimations were based on the maximum residual limits (MRLs) of the sum of enrofloxacin and ciprofloxacin residues (100 µg/kg, 200 µg/kg, and 300 µg/kg) for muscles, liver, and kidney, respectively. Furthermore, the MRLs of amoxicillin were 50 µg/kg for all tissues.
Biochemical Analysis
Data shown in Table 7 indicated a significant increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels in Groups 2, 4, and 5 in comparison with Groups 1, 3, 6 and 7. There was a significant increase in urea and creatinine serum level in Group 2 in comparison with Group 1 and all the other treated groups (3, 4, 5, 6 and 7). Moreover, there was no significant difference in creatinine levels between Group 1 and all the other treated groups (3, 4, 5, 6 and 7), the same also occurred for urea level except for group 5.
Discussion
Antibiotics were used for many years in controlling bacterial diseases in poultry flocks, either in therapeutic doses, in subtherapeutic doses as growth promotors, or for prophylaxis. All the forementioned ways lead to the emergence of antibiotic resistance and consequently produce inadequate efficacy of the used antibiotics (Gast and Porter, 2020). Thus, we had to determine the precise dose for any used antibiotic through PK and PD properties to avoid the previously stated problems (Belew et al., 2015). Fighting antimicrobial resistance phenomena is continuing through various ways of treatment. The combination of antibiotics is one of the most effective methods (Uchil et al., 2014).
In the current study, there was a gradual reduction in E. coli count in liver samples in all groups, despite a sharp reduction found in the 2nd group, and there was no count detected for E. coli on the last day in all groups which is similar to the result reported by Chantziaras et al. (2017), who orally treated broilers with half-dose enrofloxacin 5 mg/kg. On the other hand, amoxicillin in Groups 5 and 6 has a more powerful effect on E. coli count from liver when combined with enrofloxacin. These results were consistent with the idea of combination of amoxicillin with other products having an antimicrobial effect as surfactin (Liu et al., 2019). In Groups 4, 5, and 6, enrofloxacin used in treatment in either full or half doses and administrated solely or in combination with amoxicillin can eliminate Salmonella enteritidis instantly and in an effective away. These findings agreed with those reported by Li et al. (2017), who treated Salmonella typhimurium by enrofloxacin. Moreover, Barrow et al. (1998) treated Salmonella enteritidis but in a different regimen. On the same way, amoxicillin was effective in treating Salmonella typhi in mice solely or in combination with cassia (Ali et al., 2007).
The reported MIC of enrofloxacin against E. coli was 0.04 µg/mL which is consistent with that of EMEA (1998) which was determined at an inoculum density of 107 CFU/ml, while in our study the MIC was determined at an inoculum density of 105 CFU/ml. This data proved the progressive microbial resistance.
Through our study, we detected the synergistic action between enrofloxacin and amoxicillin combination. This was in the same line with Al-Hasan et al. (2009), who confirmed in vitro and in vivo synergy of fluoroquinolone with a beta-lactam combination against extended-spectrum beta-lactamase-producing Escherichia coli.
With special consideration for antibiotics with time-dependent killing (amoxicillin), the optimal responses occur when the time that the drug remains above the MIC is equal to or greater than 50% of the dosing interval. For agents with concentration-dependent killing (enrofloxacin), the best responses occur when the concentrations (Cmax) are ≥ 10 times above the MIC for their target organism(s) at the site of infection. Moreover, AUC/MIC should be ≥100–125 for more killing power. However, excessive AUC/MIC ratios may result in adverse reactions by disrupting the normal gastrointestinal flora and organ dysfunction (McKinnon and Davis, 2004). In accordance with the latter point, AUC/MIC ratio of Group 6 was the least recorded ratio over the recommended when compared with Groups 4 and 5. This indicates good bactericidal effect with less harm to the bird.
Enrofloxacin absorption half-life time (t1/2 ka) was significantly shorter in Group 7 than in the other treated groups (4, 5, and 6); otherwise, amoxicillin achieved shorter t1/2 ka in Groups 5 and 6 than in Groups 3 and 7. These data might be explained by Bardal et al. (2011). They reported that drugs of similar structure may compete for binding sites and therefore affect their pharmacokinetics.
The elimination half-life (t1/2 β) was longer in Group 6 (combination half dose) for enrofloxacin (7.3±0.09 h) than in Group 7 and for amoxicillin (8.5±0.2 h) than in Groups 3, 4, and 7. Mean residence time (MRT) was more significantly increased for both enrofloxacin and amoxicillin combination in Group 5 (10.9±0.8 and 10.1±0.2 h) and Group 6 (9.9±0.9 and 9.8±0.5 h) than Groups 3 and 4. Current values were differing from those of Atef et al. (2020), who determined similar t1/2 β (2.7±0.2 h) and lower Cmax (0.6±0.07 µg/ml) with slightly higher MRT (12.4±0.8 h) in their experimental trial with enrofloxacin 10 mg/kg to combat Eimeria infection in broilers. These differences have been explained by the different infective agents and post-antibiotics effect (PAE) (Sykes, 2013). For enrofloxacin (concentration-dependent antibiotics), administration of the total daily dose as a single dose every 24 hours is preferred over a smaller divided dose in order to maximize Cmax or AUC over MIC value (Levison and Levison, 2009). This is consistent with the data in Group 6 which recorded a good pharmacokinetic profile with regards to serum concentrations for enrofloxacin and amoxicillin at 24 h which were lower than the detected MIC for S. Entritidis and E. coli. This may be attributed to concentration-dependent post-antibiotic effect (PAE) which helps in the continuation of bactericidal action for a while after the level of antibiotic falls below the MIC.
On the other hand, Anadón et al. (1996) had estimated near values of t1/2 β and MRT of amoxicillin (9.1±0.6 h and 12.2±0.8 h, respectively). Clearance time (CL) was faster in Group 6 (combination half dose) for both enrofloxacin and amoxicillin (0.27±0.1 and 0.23±0.2 (µg/ml)/hr, respectively). Significant elongation of t1/2 β for both enrofloxacin and amoxicillin (Group 6) was reflected in lower values of its clearance time (Anadón et al., 1996). Moreover, a long T > MIC for amoxicillin is achieved by a long half-life reflecting dose interval (Sykes, 2013) which was achieved in Group 6 in comparison with Group 3 which recorded less T > MIC at 24 hrs (35.9%). These data with creatinine levels proved that the use of a half-dose combination is better than the other treated groups (3, 4, and 5).
The levels of enrofloxacin and amoxicillin combination in serum of infected chickens are significantly lower than their counterparts in healthy ones, and this is reflected in the pharmacokinetic parameters: a significant decrease in Cmax and AUC0-24, with a significant increase in V/F and CL/F. This observation could be due to faster extravascular distribution and the high ability of the combination to reach the diseased tissues (McCafferty and Scott, 2019).
The tissue concentrations following oral administration of amoxicillin were widely distributed in selected tissues (liver, kidney, and muscle). The concentrations were high initially and then decreased over time. This indicates that penetration of amoxicillin into tissues was good.
Residues of amoxicillin were the highest in the kidney, followed by the liver and then muscle, indicating that amoxicillin is an appropriate drug for treating urinary infection associated with septicemia caused by susceptible organisms like E. coli and Salmonella. No amoxicillin residues were detected in tissues after 7 days of stopping drug administration. The results demonstrated that, for daily oral administration of amoxicillin at 13.1 mg/kg per day in broiler chickens of all tested groups, a preslaughter withdrawal time must be more than 7 days as the drug is still detected in kidney till the 7th day in order to ensure that the drug is eliminated from tissues. The MRL (maximum residual limit) values for amoxicillin have been established (WHO/FAO codex, 2018). Similar findings of high therapeutic concentrations of amoxicillin in different tissues of broiler chickens were reported by Bhar et al. (2010).
Enrofloxacin and its metabolite ciprofloxacin were widely distributed into edible tissues liver, kidney, and muscle samples. Liver and kidney have the highest concentrations of both enrofloxacin and its metabolite ciprofloxacin. These findings are in line with (Intorre et al., 1997; Papich and Riviere, 2009; Sureshkumar and Sarathchandra, 2021). Enrofloxacin concentration was higher in the liver than in muscles at all the time points examined in the present study. The results obtained following the earlier findings regarding the distribution of enrofloxacin in the liver and muscle as reported by (Petrovic et al., 2006; Sureshkumar and Sarathchandra, 2021). Enrofloxacin is metabolized in the liver and transformed into the main metabolite, that is, ciprofloxacin, and some minor metabolites such as oxociprofloxacin, enrofloxacin amide, N-formyl ciprofloxacin, dioxociprofloxacin, destethylene enrofloxacin, desethylene ciprofloxacin, oxoenrofloxacin, and hydroxy oxoenrofloxacin (Prescott et al., 2000). The level of enrofloxacin transformation to ciprofloxacin in the present study is not so high. This was in agreement with Sureshkumar and Sarathchandra, (2021), who detected a lower ciprofloxacin concentration than the parent drug enrofloxacin. The metabolic conversion of enrofloxacin to ciprofloxacin was observed in all the tissue samples obtained from enrofloxacin-treated chickens. The descending order of tissue levels of enrofloxacin and its metabolite ciprofloxacin residues found in broiler tissues is as follows: liver> kidney> muscle. Enrofloxacin was efficiently distributed to most tissues, as evidenced by its detection in all the tissues, at all-time points studied during the withdrawal period. This is attributed to its lipophilicity and low protein binding capacity (Papich and Riviere, 2009).
Half dose of amoxicillin and enrofloxacin combination recorded the lowest tissue residues in all treated groups (3, 4, 5, and 7). These findings might be explained by the fact that β-lactam antibiotic activities have been classified as being mostly time-dependent (fT>MIC) (Gustafsson et al., 2001), while the effects of fluoroquinolones are correlated to either fCmax/MIC or fAUC/MIC (Andes and Craig, 2002).
Alanine transaminase and aspartate aminotransferase are two enzymes that have traditionally been utilized for detecting acute liver cell damage and pathological manifestations of liver failure. When liver cells are damaged, ALT and AST are released into the bloodstream, and the increase in their concentrations usually indicates liver injury. Oxidative stress (OS) is caused by an imbalance between reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are produced by various endogenous and exogenous processes, resulting in molecular and cellular damage (Liguori et al., 2018; Tan et al., 2018). ROS are produced as a protective mechanism against xenobiotics, cytokines, or bacterial invasion during mitochondrial oxidative metabolism (Ray et al., 2012).
Enrofloxacin-induced hepatic injury may be attributed to the mode of action, which involves the suppression of DNA gyrase and topoisomerase IV catalytic functions, a phenomenon that impacts several nucleic acid processes. Three main reasons may be highlighted for explaining that as follows. The first reason is that long-term oxidative stress destroys mitochondrial DNA and newly synthesized proteins, starting to form cytochrome complexes that completely release electrons and cause oxidative stress. The second reason is that highly stable fluoroquinolone protein and cation complexes remain in cells for many years and interrupt energy production and epigenetics. Finally, epigenetic alterations in gene regulation persist for many years even when fluoroquinolone is not found in the cell (El-Badawy et al., 2019). A significant increase in AST and ALT levels were found in groups 2, 4 and 5 when compared with groups 1, 3, 6 and 7. These results may be attributed to the effect of mixed infection either alone (group 2) or with the effect of full dose of enrofloxacin in (groups 4 and 5). Other reasons may also participate in the aforementioned result such as the absence of mixed infection harmful effect on the liver in groups 1 and 7; and finally, the absence or the reduction of previously mentioned enrofloxacin hepatic harmful effect in group 3 and group 6 respectively.
Our results agreed with Raini (2016), who found an increase of 1–3% in liver enzymes including ALT, AST, and ALP in patients receiving ciprofloxacin, norfloxacin, and ofloxacin. The results also agreed with Fitriana et al. (2020), who mentioned a non-significant increase in ALT in one-day-old chick groups treated with a combination of tylosin and enrofloxacin at a dose of 1 gram in 2 liters of drinking water and a dose of 2 grams in 2 liters of drinking water. In our results, a significant increase in AST, ALT, urea, and creatinine levels were found in Group 2 compared to Group 1 which might be attributed to the septicemic effect of S. Entritidis and E. coli mixed infection on the liver and kidneys. Such results were consistent with Muna et al. (2016), who found congestion, hemorrhage, focal degeneration, and complete necrosis in some areas where debris replaced hepatocytes in the liver of S. Entritidis infected broiler chicks. Moreover, Abalaka et al. (2017) demonstrated that E.coli-infected 5-week-old broiler chickens showed diffuse congestion and multifocal coagulative necrosis within the liver, locally extensive congestion, and hemorrhage within the kidney.
Significant increase in urea and creatinine levels in group 2 in comparison with other treated groups (group 3, 4, 5, 6, and 7) and control group (group 1) may be attributed to the renal harmful effect of the mixed infection with the absence of the treatment in that group.
According to the present findings, amoxicillin and enrofloxacin combination at half dose did not cause any hepatic or renal injuries with no significant change in AST, ALT, urea, and creatinine levels when compared with negative control group (group 1).
Conclusion
From our previously discussed results, we concluded that using the half-dose combination of enrofloxacin and amoxicillin in treating mixed pathogenic bacteria (E. coli and S. Entritidis) is more effective than using each of them alone or in a full-dose combination. Not only does the half-dose combination cost less money, it also does not have any harmful effect on internal metabolism and excretion organs (liver and kidneys). For all those reasons, we recommend the usage of the half-dose combination to benefit from the influential synergistic combination to overcome the infection with pathogenic bacteria, decrease antibiotics tissue residues that affect human health, and reduce poultry farming expenses in our Egyptian poultry ranches.
Acknowledgments
We thank Professor Hesham Salah Taha at Biochemistry department, Animal Health Research Institute (AHRI), Dokki, Giza, Egypt for his help in the pharmacological section of the study.
Conflict of interest
No conflict of interest was declared.
novelty statement
This research paper is the first study to declare the synergistic effect of combined antibiotics (enrofloxacin and amoxicillin) to face a mixed infection of different pathogens even at their half doses. At the same time, it declared the safety of such combination on both animals and human’s health status. The authors declared that these results have been published for the first time.
Authors contribution
Mai A. Fadel: designed the experiment, MIC, MBC and FIC evaluation, HPLC method validation data analysis, analyzed the data statistically and analysis the samples for pharmacokinetics on HPLC. Ahmed M. El-mahdy: analyzed the data statistically, participated in pharmacokinetic analysis on HPLC, MIC and MBC evaluation. Biochemical parameters data analysis. Mahmmad A.M Saleh: Performed HPLC method validation, detection of tissues residues analysis on HPLC. Jihan M. Badr and Mona A.A. AbdelRahman: performed AST and E.coli and Salmonella counting in vivo. All authors participated in execution of the experiment, wrote and revised the full manuscript.
References:
Abalaka SE, Sani NA, Idoko IS, Tenuche OZ, Oyelowo FO, Ejeh SA, Enem SI (2017). Pathological changes associated with an outbreak of colibacillosis in a commercial broiler flock. Sokoto J. Vet. Sci. 15 (3):95-102 https://doi.org/10.4314/sokjvs.v15i3.14
Abd El-Ghany WAA, El-Shafii SA, Hatem ME, Dawood RE (2012). A trial to prevent Salmonella Enteritidis infection in broiler chickens using autogenous bacterin compared with probiotic preparation. J. Agric. Sci. 4 (5): 91- 108. https://doi.org/10.5539/jas.v4n5p91
Al-Hasan MN, Wilson JW, Lahr BD, Thomsen KM, Eckel-Passow JE, Vetter EA, Baddour LM (2009). β-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob. Agents Chemotherap. 53(4): 1386-1394 https://doi.org/10.1128/AAC.01231-08
Amita M, Shashank K, Abhay K (2013). Scientific validation of the medicinal efficacy of Tinospora cordifolia. Scientific. World J. 1-8. https://doi.org/10.1155/2013/292934
Anadón A. Martinez Larrañaga MR, Diaz MJ, Bringas P, Fernandez MC, Martinez MA, Fernandez Cruz, ML (1996). Pharmacokinetics of amoxicillin in broiler chickens. Avian Pathol. 25(3): 449-458. https://doi.org/10.1080/03079459608419154
Andes D, Craig WA (2002). Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chem. 46:1665–1670. https://doi.org/10.1128/AAC.46.6.1665-1670.2002
Atef M, El-Banna HA, Elzorba HY, Soliman AM (2020). Pharmacokinetics and tissue residue of enrofloxacin in healthy, Eimeria-infected broiler chickens and those pre-treated with amprolium and toltrazuril. Int. J. Vet. Sci. Med. 8(1). 31-38. https://doi.org/10.1080/23144599.2020.1765720
Baggot JD (1978a). Some aspects of clinical pharmacokinetics in veterinary medicine II. J. Vet. Pharmacol. Therapeut. 1: 111-118. https://doi.org/10.1111/j.1365-2885.1978.tb00314.x
Baggot JD (1978b). Some aspects of clinical pharmacokinetics in veterinary medicine. I. J. Vet. Pharmacol. Therapeut. 1: 5-18 https://doi.org/10.1111/j.1365-2885.1978.tb00300.x.
Bardal SK, Waechter JE, Martin DS (2011). Applied pharmacology. Elsevier Health Sciences. https://doi.org/10.1016/B978-1-4377-0310-8.00013-0
Barrow PA, Lovell MA, Szmolleny G, Murphy CK (1998). Efect of enrofoxacin administration on excretion of Salmonella ententidis by experimentally infected chickens and on quinolone resistance of their Escherichia coli fora. Avian Pathol. J. of the W.V.P.A. 27: 586–590. https://doi.org/10.1080/03079459808419388
Batrawi N, Wahdan S, Al-Rimawi F (2017). A validated stability- indicating HPLC method for simultaneous determination of amoxicillin and enrofloxacin combination in an injectable suspension. Scient. Pharmaceut. 85(1): 6. https://doi.org/10.3390/scipharm85010006
Belew S, Kim JY, Hossain MA, Park JY, Lee SJ, Park YS, Suh JW, Kim JC, Park SC (2015). Pharmacokinetics of marbofloxacin after intravenous and intramuscular administration in Hanwoo Korean native cattle. J. Vet. Med. Sci.77:327-329. https://doi.org/10.1292/jvms.14-0221
Bhar MK, Datta BK, Patra PH, Dash JR, Sar TK, Chakraborty AK, Mandal TK (2010). Disposition kinetics of amoxicillin in healthy hepatopathic and nephropathic conditions in chicken after single oral administration. Vet. Res. Forum. 1,(3),:150-156.
Brahmareddy DR, Reddy DPK, Konda B (2015). Method Development and Validation for Estimation of Enrofloxacin By RP-LC In Marketed Formulations. Int. J. Pharm. Sci. 5:624–626.
Chantziaras I, Smet A, Haesebrouck F, Boyen F, Dewulf J (2017). Studying the effect of administration route and treatment dose on the selection of enrofloxacin resistance in commensal Escherichia coli in broilers. J. Antimicrob. Chemotherap. 72(7): 1991–2001. https://doi.org/10.1093/jac/dkx104
Clinical and Laboratory Standards Institute (CLSI) (2014). Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fourth Informational Supplement. CLSI Document M100-S24. Wayne. 34(1).
Dahshan MAH, Mohamed AA (2016). Vaccination against some E. coli Serotypes Isolated from Diseased Broiler Chickens with Chronic Respiratory Disease (CRD). J. Vet. Med. Res. 23(2): 243-248. https://doi.org/10.21608/jvmr.2016.43247
El-Badawy MF, Alrobaian MM, Shohayeb MM, Abdelwahab SF (2019). Investigation of six plasmid-mediated quinolone resistance genes among clinical isolates of Pseudomonas: A genotypic study in Saudi Arabia. Infect. Drug. Resist. 12: 915–923. https://doi.org/10.2147/IDR.S203288
EMEA (1998). Committee for veterinary medicinal products. Enrofloxacin (modification for bovine, porcine and poultry). Summary report (2). MRL/388/98-FINAL. European Medicines Agency.London.UK.
EMEA (2004): https://www.ema.europa.eu/documents/referral/solamocta-article-334-referral-annex-i-ii-iii_en.pdf
EUCAST (2022). The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. http://www.eucast.org
Ewing WH (1986). Edwards and Ewing’s Identification of Enterobacteriaceae. 4th ed. Elsevier. New York.
Fitriana I (2020). Antibiotics combination effects of tylosin and enrofloxacin on liver and renal functions of broiler. Indonesian J. Vet. Sci. 1(1).
Gast RK, PorterJr RE (2020). Salmonella infections.In: M. Bouliane, C.M. Logue, L.R. McDougald, V. Nair & D.L. Suarez (eds.), Dis. Poult. 719–753. Iowa,Wiley-Blackwell.
Grimont PAD, Weill FX (2007). Antigenic formulas of the Salmonella Serovars. 9th eds. WHO Collaborating Center for reference and research on Salmonella. Paris.
Gustafsson I, Lowdin E, Odenholt I, Cars O (2001). Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 45:2436–2440. https://doi.org/10.1128/AAC.45.9.2436-2440.2001
Intorre I, Mengozzi G, Bertini S, Bagliacca M, Luchetti E, Soldani G (1997). The plasma kinetics and tissue distribution of enrofloxacin and its metabolite ciprofloxacin in the muscovy duck. Vet. Res. Comm. 21: 127-136. https://doi.org/10.1023/A:1005773603905
Ishola OO, Holt PS (2008). Salmonella Enteritidis experimental infection in chickens: Effects of challenge dose on serum immunoglobulin G antibody response. African J. Biotechnol. 7(20).
Jarrar N, Adwan KM, Abu-Hijleh A (2010). Antibacterial activity of Rosmarinus officinalis L. alone and in combination with cefuroxime against methicillin resistant Staphylococcus aureus. Asian Pacific J. Trop. Med. 3(2): 121-123. https://doi.org/10.1016/S1995-7645(10)60049-1
Khatun MS, Das D, Akter MS, Faruk MAZ, Das S, Tuhin MRI, Islam MS (2020). Residual effect of amoxicillin in broiler. Int. J. Nat. Social Sci. 7(3): 51-58.
Kim HY (2014). Analysis of variance(ANOVA) companing means of more than two
groups. Restorat. Dentist. Endodont. ISSN 2234-7658. https://doi.org/10.5395/rde.2014.39.1.74
Kuznetsova MV, Gizatullina JS, Nesterova, LY, Starčič Erjavec M (2020). Escherichia coli isolated from cases of colibacillosis in Russian poultry farms (Perm Krai): sensitivity to antibiotics and bacteriocins. Microorganisms. 8(5): 741. https://doi.org/10.3390/microorganisms8050741
Levison ME, Levison JH (2009). Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. 23(4): 791-815. https://doi.org/10.1016/j.idc.2009.06.008
Li J, Hao H, Cheng G, Wang X, Ahmed S, Shabbir M, Liu Z, Dai M, Yuan Z (2017). The effects of different enrofloxacin dosages on clinical efficacy and resistance development in chickens experimentally infected with Salmonella Typhimurium. Scient. Rep. 7(1): 11676. https://doi.org/10.1038/s41598-017-12294-7
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D (2018). Oxidative stress, aging, and diseases. Clin. Interv. Aging. 13:757–772. https://doi.org/10.2147/CIA.S158513
Liu J, Wang X, Shi W, Qian Z, Wang Y (2019). Sensitization of avian pathogenic Escherichia coli to amoxicillin in vitro and in vivo in the presence of surfactin. PLoS One.12:14(9). https://doi.org/10.1371/journal.pone.0222413
McCafferty EH, Scott LJ (2019). Migalastat: a review in Fabry disease. Drugs. 79(5): 543-554. https://doi.org/10.1007/s40265-019-01090-4
McKinnon PS, Davis SL (2004). Pharmacokinetic and pharmacodynamic issues in the treatment of bacterial infectious diseases. Eur. J. Clin. Microbiol .Infect. Dis.23(4):271-88. https://doi.org/10.1007/s10096-004-1107-7
Magryś A, Olender A, Tchórzewska D (2021). Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Archiv. Microbiol. 203(5): 2257-2268. https://doi.org/10.1007/s00203-021-02248-z
Marien, M, Decostere A, Martel A, Chiers K, Froyman R, Nauwynck H (2005). Synergy between avian pneumovirus and Orni thobacterium rhinotracheale in turkeys. Avian Pathol. 34:204–211. https://doi.org/10.1080/03079450500096414
McKellar QA, Sanchez Bruni SF, Jones DG (2004). Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Therapeut. 27(6):503-514. https://doi.org/10.1111/j.1365-2885.2004.00603.x
Muna EA, Salih MH, Zakia AM, Halima MO, Abeer AM, Ameera MM, Idris SB (2016). Pathology of broiler chicks naturally infected with Salmonella enteritidis (S. Entritidis) and Salmonella typhimurium (S. typhimurium) during an outbreak in Sudan. J. Scient. Res. Rep. 10(1):1-8. https://doi.org/10.9734/JSRR/2016/23431
Nielsen EI, Cars O, Friberg LE (2011). Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob. Agents Chemotherap. 55(10):4619-4630. https://doi.org/10.1128/AAC.00182-11
Nolan LK, Vaillancourt JP, Barbieri NL, Logue CM (2020). Colibacillosis, In: Swayne D(eds). Diseases of Poultry 14th eds,Wiley-Blackwell, Hoboken, NJ, USA. 770- 830. https://doi.org/10.1002/9781119371199.ch18
OIE (2022). https://www.oie.int/en/what-we-do/global-initiatives/one-health
Oyedeji AO, Msagati TAM, Williams AB, Benson NU (2021). Detection and quantification of multiclass antibiotic residues in poultry products using solid-phase extraction and high-performance liquid chromatography with diode array detection. Heliyon. 7(12). https://doi.org/10.1016/j.heliyon.2021.e08469
Papich MG, Riviere JE (2009). Fluoroquinolone antibacterial drugs. In: Veterinary Pharmacology and Therapeutics. J.E. Riviere, and M.G. Papich, (9th eds), Wiley-Blackwell, Iowa State University Press, USA, 983-1011.
Petrovic J, Baltic M, Cupic V, Stefanovic S, Dragica S (2006). Residues of enrofloxacin and its main metabolite ciprofloxacin in broiler chickens. Acta Vet. (Beograd). 56: 497-506. https://doi.org/10.2298/AVB0606497P
Prescott JF, Baggot JD, Walker RD (2000). Fluoroquinolones. In: Antimicrobial Therapy in Veterinary Medicine. J.F. Prescott, J.D. Baggot and R.D. Walker (3rd eds)., Iowa State University Press, Iowa, 315-339.
Raini M (2016). Antibiotik Golongan Fluorokuinolon: Manfaat dan Kerugian.
Media Litbangkes. 26 (3): 163-174. https://doi.org/10.22435/mpk.v26i3.4449.163-174
Ray PD, Huang BW, Tsuji Y (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 24(5):981–990. https://doi.org/10.1016/j.cellsig.2012.01.008
Reitman S, Frankel S (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28(1): 56–63. https://doi.org/10.1093/ajcp/28.1.56
Sykes J E (2013). Canine and feline infectious diseases. Elsevier Health Sciences.
Sureshkumar V, Sarathchandra G (2018). A HPTLC-Fluorescent densitometry assay for simultaneous detection of enrofloxacin and ciprofloxacin in broiler chicken tissues. Food Anal. Methods.11: 1076-1085. https://doi.org/10.1007/s12161-017-1077-x
Tan BL, Norhaizan ME, Liew WPP, Rahman HS (2018). Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front Pharmacol. 9(1162):1162. https://doi.org/10.3389/fphar.2018.01162
Tietz NW (1986). Textbook of clinical chemistry. WB saunders, Philadelphia, pp. 1919.
Tietz NW (1995). Clinical guide to laboratory tests, WB saunders, Philadephia, 3rd (eds), pp. 268-273.
Turnidge JD (1998). The Pharmacodynamics of Beta-Lactams. Clic. Infect. Dis. 27: 10-22. https://doi.org/10.1086/514622
USP (2021). (1225) Validation of compendial procedures and (621) chromatography. Rockville, Rockville, MD, United State Pharmacopeia.
WHO/ FAO Codex (2018). Maximum Residue Limits (MRLS) and Risk Management Recommendations (RMRS) for residues of veterinary drugs in foods. CX/MRL 2-2018
WHO (2003). Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World, Geneva, Switzerland.
Wybenga DR, Digigorgio J, Piliggi VJ (1971). Automated method for urea measurement in serum. Clin. Chem. 97:891-895 https://doi.org/10.1093/clinchem/17.9.891
Young DS (1997). Effects of drugs on clinical laboratory tests. Ann. Clin. Biochem. 34(6): 579-581 https://doi.org/10.1177/000456329703400601
Zhang Y, Huo M, Zho J, Xie S (2010). PK Solver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Praograms Biomed. 99(3): 306-314. https://doi.org/10.1016/j.cmpb.2010.01.007
To share on other social networks, click on any share button. What are these?






