Effects of Selective Breeding on the Nutritional Composition of Chinese Soft-Shelled Turtles, Trionyx sinensis
Effects of Selective Breeding on the Nutritional Composition of Chinese Soft-Shelled Turtles, Trionyx sinensis
Fen Wang1,2, Lin Zuo1,2, Xiang Zhou1,2, Zhu Chen1,2, Xiao Na Xu1,2, Suo Fei Ji1,2, Guan Jun Hou1,2, Cheng Jun Zhu2, Ye Zhang3, You Feng Su4, Gendong Jin5,
Jia Jia Wang1,2, Yuan Gao6, Guang Tong Song1,2* and Ye Lin Jiang1,2*
1Key Laboratory of Aquaculture and Stock Enhancement for Anhui Province, Fisheries Research Institute of Anhui Academy of Agricultural Sciences, S.40, NongKe Road, HeFei, 230031, AnHui Province, China.
2Anhui Engineering Research Center for Turtle Farming Technology, Anhui Xijia Agricultural Development Co., Ltd, Bengbu, 233701, China
3Ma′anshan Chunsheng Ecological Agriculture Co., Ltd, Ma′anshan, 243000, China
4Tongcheng sushi Agricultural Development Co., Ltd, Tongcheng, 231440
5Tongcheng Fishery Service Center, Tongcheng, 231400
6Lingbi Yuanda Aquaculture Technology Co., Ltd, Suzhou, 234200
Fen Wang and Lin Zuo contributed to the work equally and should be regarded as co-first authors.
ABSTRACT
A comparative study of two types of third-generation Chinese soft-shelled turtles (Trionyx sinensis) from the Yangtze River (i.e., Type A with black spots on the carapace and Type B without spots) was conducted to evaluate the effect of selective breeding on their nutritional composition. Our nutrient analysis results indicated that both types were high-quality aquatic products with high protein and low fat contents. The amino acid contents in the muscle of type A were significantly higher than those of type B (P < 0.05), but no significant differences were observed in the calipash. Additionally, the amino acid score (AAS) in the muscle of type A was >1, which was significantly higher than that of type B (P<0.05). The contents of all fatty acids and EPA+DHA were significantly higher in Type A than in type B (P<0.05), whereas there were no significant differences in the fatty acid composition of the calipash of the two types (P > 0.05). The muscle atherogenicity index (AI) and thrombogenicity index (TI) of the two types were 0.42-0.45 and 0.33-0.34, respectively, which were significantly lower than that of the calipash (P<0.05). The hypocholesterolemic/ hypercholesterolemic fatty acid ratio (HH) was 3.91–3.89, which was significantly higher than that of the calipash (P<0.05). Additionally, the collagen content of type A calipash was significantly higher than that of type B (P<0.05). Collectively, our findings indicated that selective breeding could improve the nutritional value of T. sinensis from the Yangtze River.
Article Information
Received 20 September 2022
Revised 18 October 2022
Accepted 01 November 2022
Available online 05 February 2024
(early access)
Published 20 February 2024
Authors’ Contribution
FW and LZ designed the experiment, performed experimental work and wrote the manuscript. XZ, ZC, XNX, SFJ, GJH, CJZ, YZ, YFS, GJ, JJW and YG helped collect the samples and perform the analysis of data. GTS, YLJ contributed reagents and materials, and edited the manuscript.
Key words
Trionyx sinensis, Amino acids, Fatty acids, Collagen, Yangtze River
DOI: https://dx.doi.org/10.17582/journal.pjz/20220920090925
* Corresponding author: [email protected], [email protected]
0030-9923/2024/0002-0937 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The Chinese soft-shelled turtle (Trionyx sinensis) is a freshwater turtle species belonging to the Trionychidae family, and is widely cultured in China, where its breeding history can be traced back to 460 BC. owing to the substantial economic benefits (Zhang et al., 2017), T. sinensis harvests have steadily increased each year. In 2020, the annual output of T. sinensis was approximately 332,616 metric tons, 1.25 times that of 10 years prior, accounting for 1.03% of the freshwater aquaculture output in China (Bureau of Fisheries et al., 2011, 2021). This increase in production has led to a shift in consumer demographics, from wealthy people and upscale restaurants to the public in general.
The main reason for this phenomenon is the large market demand for this product. T. sinensis have long been prized for their medicinal properties. a highly appreciated part of many dishes of the East and Southeast Asian cuisine. According to the Compendium of Materia Medica written by 400 years ago, as well as previously published records on Chinese medicinal herbs and their medicinal properties and other studies on Chinese traditional medicine, the carapace, muscle, and eggs of T. sinensis possess unique therapeutic properties (Chu et al., 2007). Early studies have found that consuming soft-shelled turtles can inhibit the growth of solid tumors by activating the immune system (Feng et al., 1996). In recent years, several studies have confirmed that soft-shelled turtles have a high nutritional value, particularly due to their wide variety and content of amino acids and fatty acids. T. sinensis is a high-quality aquatic product with abundant essential amino acids and fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (He et al., 2013).
In China, T. sinensis are farm-bred in vast numbers for the food trade, but the wild populations gradually decline year by year. Therefore, currently all taxa are subsumed under Pelodiscus sinensis, which is listed as vulnerable by the IUCN red list of threatened species (Fritz et al., 2010). For centuries, Pelodiscus turtles have been bred and traded in high numbers, resulting in many populations established outside their native range. The middle and lower reaches of the Yangtze River are inhabited by T. sinensis, an indigenous species of soft-shelled turtles that is often referred to as plum-blossom soft-shelled turtle or Yangtze River soft-shelled turtle by the locals due to their bluish-black plum-shaped spots on their carapace. However, with the development of aquaculture and increased transportation rates, the hybridization between various strains of Chinese soft-shelled turtle is becoming increasingly common. This hybridization process has resulted in Yangtze River soft-shelled turtles without their characteristic spots on the carapace, whose growth performance is not as good as the original Yangtze River soft-shelled turtles (Chen et al., 2006). Therefore, there are currently ongoing efforts to purify and rejuvenate Yangtze soft-shelled turtle lineages through selective breeding.
In this study, the effects of selective breeding on the nutritional value of Chinese soft-shelled turtles were studied by evaluating the nutrient composition of two third-generation (F3) lineages, Type A with spots and Type B without spots.
Materials and Methods
Experimental design
The selective breeding of T. sinensis were conducted in Ma’anshan City in the Yangtze River Basin in 2010. The carapace of the original T. sinensis parental strain was covered in black spots, and approximately 79% of the third-generation had black spots covered on the carapace (Wang et al., 2021). Two types of T. sinensis (type A: with black spots; type B: without black spots) were cultured separately in the pond with a lotus planting area of 30% and a stocking density of 400 individuals/m2. The turtles were fed twice a day with a commercial formulated diet on sunny days from the end of May to mid- and late-September each year. The commercial feed consisted of 48% crude protein. The feeding amount was adjusted to 4% of the body weight of the turtles. To obtain tissue samples, three type A and B turtles were anesthetized with ether, then decapitated and bled to death. The leg muscle and calipash of each turtle were collected and crushed, after which the muscle and calipash of each sample were placed in a polyethylene sealed bag and frozen at -20°C for subsequent experiments. All analyses of meat and calipash for each sample were performed in triplicate.
Detection methods
A total of 30 samples for each of the two types were measured. Crude protein contents were determined following the methods of the association of official analytical chemists (AOAC, 2005). Amino acid and fatty acid analyses were conducted as indicated by the GB 5009.124-2016 and GB/T 5009.168-2016 guidelines, which were issued by the National Health and Family Planning Commission of China and the Chinese Food and Drug Administration (National Health and Family Planning Commission of T.R.C., 2016). The amino acid contents of the processed samples were determined with an automatic amino acid analyzer (Hitachi, L-8900) and the reference standards were purchased from Sykam (Beijing) Scientific Instrument Co. Ltd. For fatty acid determination, the processed samples were analyzed with a Shimadzu GC-2010 Plus gas chromatograph, and the fatty acid methyl ester reference standards were purchased from Sigma Aldrich (Shanghai) Trading Co., Ltd.
The collagen content was estimated via hydroxyproline analysis. The hydroxyproline content was determined by LC-MS (Shimadzu lc-ms-8050). Before determination, the sample was subjected to acidolysis using 6 M HCl at 110 ± 2 ℃ for 6 h (Wang et al., 2022; Khong et al., 2016).
Evaluation of amino acids (AA) and fatty acids
The amino acids score (AAS), atherogenicity index (AI), thrombogenicity index (TI), and hypocholesterolemic/ hypercholesterolemic fatty acid ratio (HH) were calculated using the equations below:

*AAS, amino acid scoring standard model recommended by the FAO/WHO (1973).
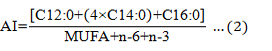
MUFA, monounsaturated fatty acid.
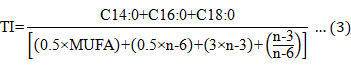
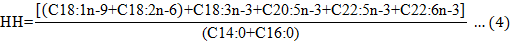
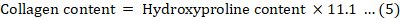
Data analysis
All results were expressed as mean ± standard deviation (mean ± SD) and the evaluations were conducted via one-way analysis of variance (ANOVA) and Duncan multiple comparison analysis using SPSS 22.0. Differences were considered to be statistically significant at a P-value <0.05.
Results
General nutrients
Table I shows the general nutrient compositions of the leg muscle and calipash of the two types of soft-shell turtles. The nutrient compositions of these two parts had no significant differences (P>0.05). The muscle exhibited significantly higher ash and crude fat levels than the calipash (P < 0.05). In contrast, the crude protein of the calipash (approximately 21%) was significantly higher than that of the muscle (about 16%) (P < 0.05). The crude fat content of types A and B were approximately 0.8% and 0.2%, respectively.
Amino acid analysis and evaluation
Figure 1 shows the kinds and contents of amino acids in the muscle and calipash of the two types of soft-shell turtles. A total of 17 amino acids were detected, including eight essential amino acids (EAA), four flavor-enhancing amino acids (FEAA), and eight functional amino acids (FAA). The compositions and contents of amino acids in the same part were consistent. Glutamic (Glu) and glycine (Gly) contents in muscle and calipash were the highest, respectively, while cysteine (Cys) was the lowest in both parts.
Table II shows the classification and contents of amino acids of two types. The content of total amino acids (TAA) in the muscle of type A was significantly higher than that of type B (P<0.05), whereas the content in calipash, was slightly higher than that of type B (P>0.05). Moreover, the content of TAA in muscle was significantly lower than in calipash (P<0.05).
Eight types of EAA were detected in both muscle and calipash. The EAA content of type A muscle was significantly higher than that of type B (P<0.05), while no significant difference was found in the calipash of two types (P>0.05). The EAA/TAA ratios in muscles of type A and B were 42.91% and 41.08%, respectively, and the EAA/NEAA ratios were 86.64% and 87.43%, respectively. The EAA/TAA ratios in calipash of type A and B were 22.17% and 20.42%, respectively, and the EAA/NEAA ratios were 33.83% & 30.50%, respectively.
Table I. Proximate composition of muscle and calipash of the two types of soft-shell turtles (g 100g-1).
|
Muscles |
Calipash |
|||
|
Type A |
Type B |
Type A |
Type B |
|
|
Ash |
0.95±0.01b |
0.93±0.02b |
0.62±0.01 a |
0.61±0.01a |
|
Moisture |
76.71±0.71a |
76.38±1.23a |
77.66±0.81a |
77.55±1.41a |
|
Crude protein |
16.62±1.03a |
16.31±1.23a |
21.31±1.81b |
20.78±2.01b |
|
Crude fat |
0.82±0.07b |
0.81±0.04b |
0.24±0.01 a |
0.23±0.01a |
Different superscripts in the same row indicate the significant differences (P<0.05).
Table II. The variety of amino acids in muscle and calipash of the two types of soft-shell turtles (dry weight, g 100 g-1).
|
Amino acids |
Muscle |
Calipash |
||
|
Type A |
Type B |
Type A |
Type B |
|
|
EAA |
30.94±0.49 c |
26.77±2.15 b |
19.23±0.65 a |
18.06±0.44 a |
|
FAA |
39.24±0.95 b |
34.62±2.92 a |
42.73±1.28 c |
41.69±1.23 c |
|
NEAA |
35.71±2.03 b |
30.63±2.48 a |
56.85±2.56 c |
59.31±2.44 c |
|
FEAA |
28.58±1.07 b |
25.71±2.20 a |
48.79±1.48 c |
46.06±1.84 c |
|
TAA |
72.10±2.17 b |
65.17±5.07 a |
86.73±2.74 c |
88.53±2.85 c |
|
EAA/TAA (%) |
42.91±1.24 b |
41.08±1.03 b |
22.17±0.41 a |
20.42±0.80 a |
|
FEAA/TAA (%) |
39.64±0.93 a |
39.45±0.49 a |
56.26±0.25 b |
52.01±0.53 b |
|
FAA/TAA (%) |
54.42±0.37 b |
52.10±0.41 b |
49.27±0.04 a |
47.10±11.20 a |
|
EAA/NEAA (%) |
86.64±4.65 b |
87.43±3.23 b |
33.83±0.77 a |
30.50±1.53 a |
EAA, essential amino acids; FAA, functional amino acids; NEAA, non-essential amino acids; FEAA, flavor-enhancing amino acids; TAA, total amino acids. Different superscripts in the same row indicate the significant differences (P<0.05).
Table III. Amino acid score (AAS) of the two types of soft-shelled turtles.
|
Amino acids |
Muscle |
Calipash |
FAO/WHO |
||
|
Type A |
Type B |
Type A |
Type B |
||
|
Thr |
1.34±0.02 d |
1.23±0.11 c |
0.97±0.02 b |
0.86±0.01 a |
250 |
|
Val |
1.02±0.01 d |
0.83±0.06 c |
0.60±0.02 b |
0.54±0.01 a |
340 |
|
Met+Cys |
1.01±0.06 b |
0.83±0.11 b |
0.51±0.01 a |
0.50±0.08 a |
220 |
|
Iso |
1.53±0.04 d |
1.33±0.12 c |
0.75±0.03 b |
0.69±0.03 a |
250 |
|
Leu |
1.28±0.02 d |
1.23±0.10 c |
0.75±0.03 b |
0.67±0.02 a |
440 |
|
Phe+Tyr |
1.56±0.03 b |
1.61±0.11 c |
0.93±0.03 a |
0.90±0.03 a |
330 |
|
Lys |
1.68±0.04 c |
1.78±0.14 d |
0.94±0.03 b |
0.88±0.03 a |
340 |
Different superscripts in the same row indicate the significant differences (P<0.05).
two types (P>0.05). There were no significant differences between the FEAA content in the calipash of the two types, which ranged between 46.06% and 48.79%, and accounted for 52.01%–56.26% of the TAA content. However, these values were significantly higher than those observed in the muscle (P<0.05). Particularly, the contents of glycine (Gly) and alanine (Ala) were approximately five and two times higher than in the muscle, respectively, suggesting that the calipash has a more pleasant flavor than the muscle.
The FAA content in the muscle was significantly lower than that in the calipash. Moreover, the FAA/TAA ratio in muscle was significantly higher than that in the calipash of both types (P<0.05). There were no significant differences in the FAA content or the FAA/TAA ratio of the calipash of the two types of turtles. However, the FAA content in the muscle of type A was significantly higher than that of type B (P < 0.05).
Table III summarizes the muscle and calipash amino acid score (AAS) of types A and B according to the FAO standard. The AAS content in the muscle of type B was higher than 1, except for valine (Val) and methionine + cysteine (Met+Cys). The AAS of type A muscle was also higher than 1, indicating that the muscle of the two types can provide a large amount of EAA.
Fatty acid analysis and evaluation
As shown in Figure 2, a total of 13 fatty acids were detected in the muscle. The fatty acid composition of each part is shown in Table IV, including four kinds of saturated fatty acids (SFAs), five kinds of polyunsaturated fatty acids (PUFAs), and four kinds of monounsaturated fatty acids (MUFAs). Among these types of fatty acids, the PUFA content was the highest, whereas the MUFA content was the lowest. The SFA, MUFA, and PUFA content in type A were significantly higher than those in type B (P<0.05) but there was no significant difference in the proportion of TFA between the two types (P>0.05). Furthermore, a total of 11 fatty acids were detected in the calipash, among which SFAs (4 kinds) exhibited the highest contents and MUFAs (3 kinds) had the lowest content. There was no significant difference in the proportion and contents of SFA, MUFA, and PUFA between the calipash of type A and B turtles (P>0.05). Except for PUFA/TFA, the fatty acid content, SFA/TFA, and MUFA/TFA in muscle were significantly higher than that of calipash (P<0.05).
The SFA content of the muscle of type A turtles were significantly higher than that of type B (P<0.05), but there was no significant difference in the proportion of SFA/TFA (P>0.05). The SFA contents in the muscle of the two types were significantly higher than that of the calipash (P<0.05). In contrast, the SFA/TFA ratios in muscle were significantly lower than those in the calipash (P<0.05), with values of 29% and 41% for the type A and B turtles, respectively.
UFA accounts for approximately 70% and 59% of TFA in muscle and calipash, respectively. The MUFA contents in the muscle and calipash of the type A and B turtles were between 23.70% and 29.48%. No significant differences in MUFA or MUFA/TFA ratios were observed between the tissues of types A and B turtles (P>0.05). The only exception to the aforementioned result was that the MUFA content of the muscle tissues of type A turtles was significantly higher than that of type B turtles (P<0.05). Moreover, the muscle MUFA content was significantly higher than that of the calipash, and the MUFA/TFA ratio of the muscle was significantly lower than the calipash (P<0.05).
As summarized in Table IV, the PUFA contents and the proportion of PUFA/TFA in the muscles of the type A and B turtles were significantly higher than in the calipash (P<0.05). Particularly, the PUFA content in the muscles of type A (567.90 mg 100 g-1) was approximately 1.7 times that of type B, and approximately 6.6 times and 8.7 times that of the calipash of type A and B, respectively. Among these PUFAs, C18:2n6 had the highest content in muscles, which was significantly higher than that of calipash (P<0.05). Moreover, the type A muscle had the highest C18:2n6 content, which was significantly higher than that of the type B muscle (P<0.05). Furthermore, C18:3n3 was only detected in the muscle and not in the calipash.
Table IV. Fatty acid composition of the two types of soft-shell turtles (dry weight, mg 100 g-1).
|
Fatty acids |
Muscle |
Calipash |
||
|
Type A |
Type B |
Type A |
Type B |
|
|
TFA |
1238.53±267.21 c |
694.74±55.07 b |
293.88±58.66 a |
203.84±12.58 a |
|
SFA |
368.25±84.15 c |
202.16±18.52 b |
116.84±21.76 a |
81.96±10.01 a |
|
UFA |
870.28±183.08 c |
492.59±37.11 b |
169.17±44.17 a |
121.88±3.21 a |
|
MUFA |
302.38±93.05 c |
164.34±9.44 b |
87.80±24.64 a |
56.96±2.32 a |
|
PUFA |
567.90±91.83 c |
328.25±28.22 b |
85.94±15.57 a |
64.91±1.41 a |
|
EPA+DHA |
261.56±32.26 c |
133.48±12.79 b |
17.76±4.31 a |
12.46±1.40 a |
|
n-3 PUFA |
269.20±34.12 c |
137.92±13.16 b |
17.76±4.31 a |
12.46±1.40 a |
|
n-6 PUFA |
298.70±59.46 c |
190.32±16.81 b |
65.89±15.33 a |
52.45±0.51 a |
|
SFA/TFA(%) |
29.66±0.43 a |
29.07±0.64 a |
41.20±1.74 b |
40.08±2.49 b |
|
MUFA/TFA(%) |
24.01±2.23 a |
23.70±0.74 a |
29.48±2.27 b |
28.01±1.45 b |
|
pufa/tfa(%) |
46.33±2.58 c |
47.23±0.53 c |
29.32±1.33 a |
31.91±1.26 b |
|
PUFA/SFA |
1.54±0.11 b |
1.62±0.04 b |
0.72±0.04 a |
0.80±0.08 a |
|
n-3/n-6 |
1:1.10±0.10 a |
1:1.38±0.09 a |
1:3.81±0.76 b |
1:4.25±0.51 c |
|
AI |
0.42±0.01 a |
0.45±0.01 a |
0.79±0.08 b |
0.86±0.05 c |
|
TI |
0.33±0.03 a |
0.34±0.01 a |
0.91±0.08 b |
0.88±0.06 b |
|
HH |
3.91±0.08 b |
3.89±0.15 b |
2.21±0.10 a |
2.15±0.16 a |
TFA, total fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; AI, Atherogenicity index; TI, Thrombogenicity index; HH, Hypocholesterolemic/hypercholesterolemic fatty acid ratio.
Value with different superscripts in the same row are significantly different(P<0.05).
For P. sinensis, the PUFA/SFA ratio ranged from 0.72 (calipash of type A) to 1.62 (muscle of type B). The ratio in the muscle was roughly double that of the calipash, but there were no significant differences between the levels in the two tissues of the types A and B turtles (P>0.05).
The n-3 and n-6 PUFA contents in the muscle were higher than in the calipash. Particularly, the n-3 and n-6 PUFA contents in the type A muscle were significantly higher than that in the type B muscle (P<0.05). However, there was no significant difference between the n-3 and n-6 PUFA contents in the calipash of type A and B turtles (P>0.05). The n-3/n-6 ratio in muscles (1:1.10–1:1.38) was significantly lower than that in calipash (1:3.81–1:4.25) (P<0.05). However, there was no significant difference between the two evaluated tissues of the type A and B turtles (P>0.05).
The content of EPA and DHA in type A muscle was 261 mg 100 g-1, approximately two times that of type B muscle, and 15 times that of calipash.
There were no significant differences in AI, TI, and HH values in the evaluated tissues of the type A and B turtles (P > 0.05), and the overall trends were consistent. The AI and TI values in the muscle were significantly higher than in the calipash, and the HH value was significantly lower than in the calipash (P<0.05).
Collagen content of calipash
The collagen contents calculated based on the hydroxyproline content of the soft-shelled belongs to type I collagen (Table V). The calipash collagen content of type A is 75.48±4.60%, which was significantly higher than that of type B at 70.34±3.27% (P<0.05).
Table V. Collagen content in calipash of the two types of soft-shell turtles (dry weight, %).
|
Type A |
Type B |
|
|
Collagen content (%) |
75.48±4.60 |
70.34±3.27 |
Discussion
General nutrients
It is worth noting that the crude protein content in the calipash was significantly higher than that in the muscle by over 30%. Moreover, the content of crude protein in the calipash was higher than that of silkworms (approximately 16%) (Longvah et al., 2011), marine fish (12%–20%) (Fernandes et al., 2014; Usydus et al., 2011), and rainbow trout (19.93%–20.09%) (Yao et al., 2020). The crude fat contents of both types were less than the Japanese soft-shelled turtle strain (muscle: 0.96%, calipash: 0.32%) and the Qingxi Hua soft-shelled turtle strain (muscle: 1.05%, calipash: 0.27%) (Chen et al., 2015). Aquatic products with a fat content below 2% are considered lean (Mohanty et al., 2019). The crude fat content of both the type A and B turtles was below the aforementioned threshold, and therefore P. sinensis can be classified as a low-fat aquatic product. The contents of crude protein in the muscles of type A (16.62%) and type B (16.31%) were similar to that of the Japanese strain (16.50%), less than the Qingxi strain (17.83%) and Huaihe strain (18.05), and slightly higher than that of the Yellow River strain (15.86%). The crude protein contents in the calipash of type A (21.31%) and type B (20.78%) were lower than that of the Japanese strain (26.93%), Yellow River strain (27.93%), and Huaihe strain (31.34%), but higher than the Qingxi strain (20.07%) (Chen et al., 2015; Liang et al., 2018). Therefore, similar to other strains, both type A and B soft-shell turtles were confirmed to be high-protein and low-fat food sources, with no significant differences between them (P>0.05).
Evaluation of amino acid content
Amino acids are proteins’ basic building blocks and essential life-sustaining nutrients. In addition to largely determining the nutritional value of food, amino acids also influence flavor profiles (Huang et al., 2021). The nutritional value of protein mainly depends on the kind, quantity, and composition of EAA (Zhang et al., 2018). The standard for evaluating high-quality protein proposed by FAO/WHO in 1991 is that the ratio of EAA/TAA should be approximately 40%, whereas the ratio of EAA/NEAA must exceed 60% for protein sources to be labelled as high-quality protein (FAO, 2013). FAAs are involved in and regulate several key metabolic pathways, which can promote the health, growth, and reproduction of organisms. Therefore, FAAs hold great promise in the prevention and treatment of metabolic diseases such as obesity, diabetes, cardiovascular diseases, intestinal and neurological disorders, and viral infections (Mohanty et al., 2019; Wu, 2013).
In our study, the contents of TAA in muscle and calipash were similar to other Chinese soft-shelled turtle strains (Liang et al., 2018). Besides, the muscles of both two types were high-quality protein, whereas the calipash did not meet the FAO/WHO standard. The AAS content in the muscle of type B was higher than 1, except for valine (Val) and methionine + cysteine (Met+Cys). The AAS of type A muscle was also higher than 1, indicating that the muscle of the two types can provide a large amount of EAA. Our findings thus demonstrated that P. sinensis meat is a high-quality protein source, especially the muscle of type A. However, the AAS contents in the calipash of the two types were lower than 1 and were significantly lower than that of the muscles (P < 0.05). Met+Cys had the lowest AAS in the muscles and calipash of the two types and was therefore the most limiting amino acid in the analyzed turtles (Yangtze River Strain). The amino acid composition and content of food are important indicators of nutritional value (Dong et al., 2018). Particularly, the nutritional value of protein is greatly influenced by the EAA composition. The muscle tissues of the two types of turtles met the FAO standard, and most of the AAS was greater than 1. In contrast, only the AAS in the calipash was less than 1, which is lower than the FAO standard. The FEAA contents in the calipash of the two types were significantly higher than that in the muscles (P < 0.05), suggesting that the calipash has a better flavor than the muscles. The EAA, FAA, and EAA/TAA and FAA/TAA ratios in the muscle were significantly higher than those in the calipash (P<0.05), indicating that the muscle has a higher nutritional value than the calipash. Based on the above-described amino acid analysis, it can be preliminarily concluded that purification and rejuvenation can significantly increase the contents of various amino acids in the muscle of Chinese soft-shelled turtles (Yangtze River strain). However, the proportion of total amino acids did not significantly increase. Moreover, the muscles of both types of turtles were more nutritious than the calipash, despite the fact that the latter presumably has a better flavor.
Fatty acid analysis and evaluation
SFA increases low-density lipoprotein cholesterol levels in the blood and increases the risk of heart disease. Therefore, the World Health Organization (WHO) recommends reducing the intake of SFA (Fernandes et al., 2014). The SFA contents in muscle were significantly higher than in calipash, while the SFA/TFA ratio showed a contrasting result.
The nutritional value of fatty acids is mainly determined by UFA. MUFAs can effectively improve the blood glucose and blood lipid metabolism of diabetic patients. Additionally, MUFAs can also decrease the likelihood of coronary heart disease due to their hypolipidemic effects (Liu et al., 2019; He et al., 2013). The MUFA contents in the muscle and calipash were consistent with the MUFA content of freshwater fish (23%–33%) (Hameed et al., 2017). High PUFA contents can not only significantly improve the flavor of food but also increases the juiciness of the meat. Additionally, PUFAs also possess hypolipidemic effects and can lower blood pressure, in addition to inhibiting platelet aggregation and regulating immune function, which can reduce the incidence of cardiovascular diseases (Shi et al., 2013). C18:2n6 and C18:3n3 cannot be synthesized by the human body and are therefore considered essential PUFAs. Therefore, type A muscle was supposed to be a good source of C18:2n6 and C18:3n3 from the soft-shelled turtle.
As we described above, excessive SFA intake has been linked to increases in total cholesterol and LDL-cholesterol levels in serum (Rincón-Cervera et al., 2019). Additionally, the PUFA/SFA ratio is another relevant nutritional indicator. Previous studies have indicated that a PUFA/SFA ratio higher than 0.4 promotes cardiovascular health (Ospina-E et al., 2012). Our findings thus confirmed that P. sinensis is an excellent food source with a good balance between PUFA and SFA, especially the muscle.
The ratio of n-3/n-6 PUFAs is an important indicator of the quality of fatty acids. High n-6 PUFA contents can increase the pathogenesis of many diseases such as various types of cancer, as well as inflammatory and autoimmune diseases, whereas n-3 PUFAs have the opposite effect (Mohanty et al., 2019). Therefore, foods with a high n-3/n-6 ratio can help reduce the incidence of cancer and cardiovascular disease (Murillo et al., 2014). The n-3/n-6 ratio recommended by the Chinese Nutrition Association is 1:4–6. However, the n-3/n-6 ratios in the muscle and calipash of soft-shelled turtles were lower than the recommended level. Nevertheless, the dietary n-3/n-6 ratio of most Chinese people has exceeded 1:10 in the past 10 years (Meng et al., 2017). In consequence, soft-shelled turtles can be supposed to help with a more balanced nutrition intake for Chinese people.
EPA and DHA are essential fatty acids for the growth of humans and animals, which can effectively prevent the occurrence of cardiovascular diseases and have been found to promote the growth of brain cells (Zhang et al., 2018). According to international recommendations, the daily intake of EPA and DHA for healthy adults is at least 250 mg. Therefore, a weekly intake of 675 g of type A muscle can meet the minimum recommended level of EPA and DHA.
Ulbricht and Southgate (1991) proposed the concept of AI and TI in 1991 to evaluate the effect of food on the incidence of coronary heart disease. Both AI and TI are indicators of the potential for platelet aggregation. Lower values are indicative of protective effects against coronary artery disease (Fernandes et al, 2014). HH is related to cholesterol metabolism and, from a nutritional point of view, higher HH values translate to more health benefits. Based on our findings, the muscle of Chinese soft-shelled turtles could protect against coronary artery disease and promote cholesterol metabolism. Therefore, the consumption of Chinese turtle muscle and calipash is encouraged.
Collagen content of calipash
Hydroxyproline is a characteristic amino acid in collagen and is therefore used as an indicator to evaluate collagen content (Khong, 2016). Type I collagen which was detected in calipash has excellent biological properties, has a good hemostatic effect, and promotes wound healing (Tian et al., 2013; Yang et al., 2016). Currently, collagen is mainly obtained from the skin and bones of pigs and cows. However, Chinese soft-shelled turtles are a promising source of high-quality collagen because the collagen content of P. sinensis is far higher than that of pigs and cows.
Conclusions
After purification and rejuvenation, there was no significant difference in the crude protein, crude fat, moisture, and ash content of the two types F3 generation Chinese soft-shelled turtles (Yangtze River water system). Similar to other strains of Chinese soft-shelled turtles, both the A and B types are high-quality aquatic products with high protein levels and low fat contents.
The EAA/TAA and EAA/NEAA ratios and AAS in the muscle of the two types indicated that muscle is a better source of high-quality protein than calipash. Particularly, the AAS of both the muscle and calipash of type A Chinese soft-shelled turtle with spots on the back was higher than 1. However, the calipash contains an FEAA rate of approximately 52%–56%, which means it tastes better than the muscle.
There were no significant differences in the SFA/TFA, MUFA/TFA, PUFA/TFA, n-3 /n-6, AI, TI, and HH of the tissues of the two types of turtles. However, the content of EPA+DHA in type A was twice that in type B. Moreover, our findings indicated that the muscles of the two types of Chinese soft-shelled turtles possess more health-promoting properties than the calipash, and therefore regular consumption is encouraged.
In general, purification and rejuvenation had subtle but positive effects on the F3 generation. Collectively, our findings provide insights into the effects of selective breeding on the nutritional properties of Chinese soft-shelled turtles (Yangtze River strain), as well as which parts of the turtle possess the strongest health-promoting effects.
Acknowledgments
Authors are grateful to Mr. Bin Xu (Fisheries Research Institute, Anhui Academy of Agricultural Sciences, Hefei, China) for euthanizing soft-shelled turtles.
Funding
The study was financially supported by Major Science and Technology Projects in Anhui Province (18030701171, 202003a0602006), Key Technologies R & D Program of Anhui Province (202104b11020024); Engineering Center (Wanke[2010]123) and Industry Technology System of Aquaculture in Anhui Province (Wannongke (2021) 711).
IRB approval and ethical statement
The study was approved by the Animal Experimentation Welfare Ethics Committee of Anhui Academy of Agricultural Sciences (AAAS) (No. AAAS2023-5). The Departmental Ethical Committee approved the experiment before this experiment.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
AOAC, 2005. Official Methods of Analysis of AOAC International, 18th. Gaithersburg, MD, USA.
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs of the PRC, National Fisheries Technology Extension Center and China Society of Fisheries, 2011. China fisheries statistical yearbook. Volume II: Aquaculture, 1st edn. Agricultural Press, Beijing, China. pT. 37.
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs of the PRC, National Fisheries Technology Extension Center and China Society of Fisheries, 2021. China fisheries statistical yearbook. Volume II: Aquaculture, 1st edn. Agricultural Press, Beijing, China. pT. 35.
Chen, S.S., Xue, J., He, Z.Y., Wang, H.H., He, X., and Dai, Z.Y., 2015. Analysis and comparison of nutritional components of two different strains of Chinese soft-shelled turtle. Fd. Sci., 18: 114-119.
Chen, Y.B., Ma, L.P., and Shan, X.L., 2006. One of the techniques of soft-shelled turtle culture: A new technology for cultured soft-shelled turtle in lake cages. China Aquat. Prod., 1: 37-39.
Chu, J.H., Chen, S.M., and Huang, C.H., 2007. Effect of dietary iron concentrations on growth, hematological parameters, and lipid peroxidation of soft-shelled turtles, Pelodiscus sinensis. Aquaculture, 269: 532-537. https://doi.org/10.1016/j.aquaculture.2007.03.004
Dong, Z., Zhang, M., Wei, S., Ge, H., Li, L., Ni, Q., Ling, Q., and Li, Y., 2018. Effect of farming patterns on the nutrient composition and farming environment of loach, Paramisgurnus dabryanus. Aquaculture, 497: 214-219. https://doi.org/10.1016/j.aquaculture.2018.07.061
FAO, 2013. Dietary protein quality evaluation in human nutrition. In: Report of an FAO expert consultation. FAO Food and Nutrition Paper 92. Food and Agriculture Organization of the United Nations, Rome, Italy.
FAO/WHO, 1973. Energy and protein requirements. World Health Organization Technical Report Series No. 522, Geneva, Switzerland
Feng, H., Yamazaki, M., Matsuki, N., and Saito, H., 1996. Anti-tumor effects of orally administered soft-shelled turtle powder in mice. Biol. Pharm. Bull., 19: 367-368. https://doi.org/10.1248/bpb.19.367
Fernandes, C.E., da Silva Vasconcelos, M.A., de Almeida Ribeiro, M., Sarubbo, L.A., Andrade, S.A.C., and de Melo Filho, A.B., 2014. Nutritional and lipid profiles in marine fish species from Brazil. Fd. Chem., 160: 67-71. https://doi.org/10.1016/j.foodchem.2014.03.055
Fritz, U., Gong, S., Auer, M., Kuchling, G., Schneeweiss, N., and Hundsdörfer, A.K., 2010. The world’s economically most important chelonians represent a diverse species complex (Testudines: Trionychidae: Pelodiscus). Org. Divers. Evol., 10: 227-242. https://doi.org/10.1007/s13127-010-0007-1
Hameed, A., Hussain, S.A., Shabbir, M.A., Pasha, I., and Song, Y., 2017. Nutritional and fatty acids profile analyses of commonly consumed fresh water fish species in Pakistan. Am. J. Biochem. Biotechnol., 13: 15-26. https://doi.org/10.3844/ajbbsp.2017.15.26
He, R., Xie, J., Huang, S.L., Shi, J.B., Gao, Z.L., and Xiong, Q., 2013. Comparison of nutritional composition of Chinese soft-shelled turtles (Pelodiscus sinensis) grown in greenhouse and imitative ecological farming conditions. Fd. Sci., 34: 234-238. (in Chinese with English abstract).
Huang, X.L., He, X.F., Yang, Q., Gu, W.C., Zhou, X.D., Zhang, H., and Zhou, N., 2021. Determination and analysis of 17 amino acids in horst from different origins. Fd. Sci., 2: 255-261.
Khong, N.M.H., Yusoff, F.M.D., Jamilah, B., Basri, M., Maznah, I., Chan, K.W., and Nishikawa, J., 2016. Nutritional composition and total collagen content of three commercially important edible jellyfish. Fd. Chem., 196: 953-960. https://doi.org/10.1016/j.foodchem.2015.09.094
Liang, H., Tong, M., Cao, L., Li, X., Li, Z., and Zou, G., 2018. Amino acid and fatty acid composition of three strains of Chinese soft-shelled turtle (Pelodiscus sinensis). Pakistan J. Zool., 50: 1061-1069. https://doi.org/10.17582/journal.pjz/2018.50.3.1061.1069
Liu, S.Y., Wang, G.Y., Gu, D.H., Wang, X.F., Xu, Z.Q., Fan, J.T., Pu, Y.H., Ge, C.R., and Liao, G.Z., 2019. Comparative analysis of free fatty acid contents in five kinds of Yunnan ham. Fd. Ferment. Ind., 2: 207-213.
Longvah, T., Mangthya, K., and Ramulu, T.J.F.C., 2011. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Fd. Chem., 128: 400-403. https://doi.org/10.1016/j.foodchem.2011.03.041
Meng, S., Zhang, H., Sun, J., and Liu, Q., 2017. Study on nutritional value of instant sea cucumber processed by the new processing approach. Am. J. Biochem. Biotechnol., 13: 51-57. https://doi.org/10.3844/ajbbsp.2017.51.57
Mohanty, B.T., Mahanty, A., Ganguly, S., Mitra, T., Karunakaran, D., and Anandan, R., 2019. Nutritional composition of food fishes and their importance in providing food and nutritional security. Fd. Chem., 293: 561-570. https://doi.org/10.1016/j.foodchem.2017.11.039
Murillo, E., Rao, K.S., and Durant, A.A., 2014. The lipid content and fatty acid composition of four eastern central Pacific native fish species. J. Fd. Compos. Anal., 33: 1-5. https://doi.org/10.1016/j.jfca.2013.08.007
National Health and Family Planning Commission of T.R.C. and China Food and Drug Administration, 2016. Determination of amino acid in food (in Chinese).
Ospina-E, J.C., Sierra-C, A., Ochoa, O., Pérez-Álvarez, J.A., Fernández-López, J., 2012. Substitution of saturated fat in processed meat products: A review. Crit. Rev. Fd. Sci., 52: 113-122. https://doi.org/10.1080/10408398.2010.493978
Rincón-Cervera, M.Á., González-Barriga, V., Valenzuela, R., López-Arana, S., Romero, J., and Valenzuela, A., 2019. Profile and distribution of fatty acids in edible parts of commonly consumed marine fishes in Chile. Fd. Chem., 274: 123-129. https://doi.org/10.1016/j.foodchem.2018.08.113
Shi, P.S., Zhu, Y.T., Wang, Q., Gu, Q.H., and Xiong, B.X., 2013. Comparison of nutrition compositions of juvenile paddlefish (Polyodon spathula) fed with live feed and formula feed. Turk. J. Fish. aquat. Sci., 13: 271-279.
Tian, Y.T., Shen, H.H., Song, W., Mao, Y.T., Zhou, Y.R., Peng, Q., Li, C.Y., and Qian, G.Y., 2013. Extraction process optimization and structural characterization of turtle-derived collagen. Jiangsu agric. Sci., 12: 265-269.
Ulbricht, T.L.V., and Southgate, D.A.T., 1991. Coronary heart disease: Seven dietary factors. Lancet, 338: 985-992. https://doi.org/10.1016/0140-6736(91)91846-M
Usydus, Z., Szlinder-Richert, J., Adamczyk, M., and Szatkowska, U., 2011. Marine and farmed fish in the Polish market: Comparison of the nutritional value. Fd. Chem., 126: 78-84. https://doi.org/10.1016/j.foodchem.2010.10.080
Wang, F., Chen, Z., Cheng, Y.S., Hou, G.J., Ji, S.F., Zhang, Y., Li, J., Zhu, C.J., Wu, Y.C., Song, G.T., and Jiang, Y.L., 2021. Nutritional evaluation of two strains of Chinese soft-shelled turtle, Pelodiscus sinensis. J. Fd. Comp. Anal., 101: 103971. https://doi.org/10.1016/j.jfca.2021.103971
Wang, F., Chen, Z., Song, G.T., Fang, G.X., Zhu, C.J., Zhou, X., and Jiang, Y.L., 2022. Two methods to determine the content of collagen in the calipash of Chinese soft-shelled turtle under different breeding modes. Phys. Test. Chem. Anal. B: Chen. Anal., 5: 558-560.
Wu, G., 2013. Functional amino acids in nutrition and health. Amino acids, 45: 407-411. https://doi.org/10.1007/s00726-013-1500-6
Yang, Y.N., Li, C.Y., and Qian, G.Y., 2016. Extraction and purification of soft-shelled turtle collagen and its application in biomaterials. Chin. J. Biol. Eng., 6: 819-830.
Yao, L., Zhang, A., Zhang, H., Shao, J., Wen, M., Wang, C., Jiang, H., and Li, M., 2020. Effects of dietary aqueous extract from Eucommia ulmoides Oliver on growth, muscle composition, amino acid composition and fatty acid composition of rainbow trout (Oncorhynchus mykiss). Pakistan J. Zool., 53: 603-611. https://doi.org/10.17582/journal.pjz/20191219081232
Zhang, J., Chen, L., Yu, T., Zhou, Q.Y., Yang, X.L., Zhou, W.S., Shen, B.T., Su, S.T., Wan, Q., and Gui, J.F., 2018. Analysis and comparison of nutritional components of four strains of Chinese soft-shelled turtle. Chin. J. aquat. Biol., 4: 770-778.
Zhang, J., Wang, F., Jiang, Y.L., Hou, G.J., Cheng, Y.S., Chen, H.L., Li, X., 2017. Modern greenhouse culture of juvenile soft-shelled turtle, Pelodiscus sinensis. Aquacult. Int., 25: 1607-1624. https://doi.org/10.1007/s10499-017-0137-y
To share on other social networks, click on any share button. What are these?









