Effect of Restricted Feeding on Growth Performance, Carcass Traits, Blood Hematology, Internal Organs, and Intestinal Histomorphology of Broilers
Research Article
Effect of Restricted Feeding on Growth Performance, Carcass Traits, Blood Hematology, Internal Organs, and Intestinal Histomorphology of Broilers
1Department of Animal Production, Faculty of Animal Science, Universitas Gadjah Mada, Jl. Fauna, 55281, Yogyakarta, Indonesia; 2Departement of Animal Nutrition and Feed Science, Faculty of Animal Science, Universitas Gadjah Mada, Jl. Fauna, 55281, Yogyakarta, Indonesia; 3Department of Applied Animal Science, Kangwon National University, 24341, Chuncheon, South Korea.
Abstract | Feed restriction can be used in an attempt to save feed costs in the broiler business because it can encourage compensatory growth in broilers. This study was conducted to determine the effect of restricted feeding through the fasting method on growth performance, carcass traits, digestive organs, blood hematology, and intestinal histomorphology of broilers. A total of 240-day-old male New Lohmann broilers were randomly assigned to two treatments: ad libitum feeding (AF) and restriction feeding (RF). Restricted feeding is carried out by fasting method once a week after the brooding period at 10, 17, 24, and 31 d. Each treatment was replicated in 15 groups (each replicate consisted of 8 broilers). Data collected during the study were broiler growth performance, carcass traits, relative weight of digestive and immune organs, blood hematology, and intestinal histomorphology of broilers. The data obtained were statistically analyzed with an independent t-test analysis. The results showed RF decreased body weight gain at 21 d but was not different at 33 d than the AF group. The RF group had an increase in jejunum relative weight, jejunum villus width, and villus: Crypt ratio. In conclusion, feed restriction through the fasting method can be implemented without negative effects on final performance, carcass traits and immune system.
Keywords | Broiler, Blood hematology, Carcass trait, Growth performance, Intestinal histomorphology, Restricted feeding
Received | February 24, 2024; Accepted | April 06, 2024; Published | May 18, 2024
*Correspondence | Bambang Ariyadi, Department of Animal Production, Faculty of Animal Science, Universitas Gadjah Mada, Jl. Fauna, 55281, Yogyakarta, Indonesia; Email: bambang.ariyadi@ugm.ac.id
Citation | Islamiati P, Hanif MF, Sujiwo J, Nugroho A, Ariyadi B (2024). Effect of restricted feeding on growth performance, carcass traits, blood hematology, internal organs, and intestinal histomorphology of broilers. Adv. Anim. Vet. Sci., 12(7):1266-1272.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.7.1266.1272
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Demand for poultry meat is increasing at a faster pace due to its good quality, nutritive value, and reasonable price. With the growing demand for poultry meat, the demand for poultry feed is also increasing (Mallick et al., 2020). Feed is an important element in the poultry industry, accounting for about 70% of total costs (Etuah et al., 2021). One of the problems is the increasing price of broiler feed. This is due to the increasing price of cereal grains and legumes used to make poultry feed due to the increasing market that uses them as food and fuel (Muscat et al., 2020). The increase in feed prices that occurs can cause economic losses to farmers due to decreased profit margins (Phiri et al., 2023).
One of the efforts to reduce feed costs and increase efficiency in feed use is by limiting feeding through the fasting method (Qin et al., 2021). Feed restriction programs using the fasting method are one of the most widely proposed strategies to reduce the impact of excessive feed consumption in ad libitum feeding management (Banong and Hakim, 2011). In addition, feed restriction in male broilers tends to increase feed intake during the refeeding period, and feed restriction results in better feed conversion (Butzen et al., 2013). Moderate levels of feed restriction and longer refeeding periods are required to achieve adequate compensatory growth (Olukomaiya et al., 2015).
There is no research on the treatment of feeding restrictions by fasting once a week after the brooding period on broiler performance. This study aims to determine the growth performance of broilers treated with restrictions on feeding time by fasting once a week after the brooding period. The results of the study are expected to obtain ways to save feed use so that feed costs can be reduced, which in turn increases farmers’ income and does not interfere with production performance.
Materials and Methods
Ethical clearance
The Experiment was received prior authorization from the Ethical Commission from the Faculty of Veterinary Medicine, Universitas Gadjah Mada, Indonesia with No: 0009/EC-FKH/Eks./2021.
Birds, diets and housing
A total number of 240 one-day-old male New Lohmann broiler chickens (± 40-45 g) were used in the current study. The birds were divided into two feeding treatments, ad libitum feeding (AF) and restriction feeding (RF). Every treatment was replicated 15 times, with 15 pens (1 m x 1 m) and eight birds in each replicate per pen. Restricted feeding through the fasting method is carried out once a week after the brooding period at 10, 17, 24, and 31 days. The method of fasting is by satisfying broilers every Wednesday from 07.00 WIB until Thursday at 07.00. During feeding treatment, broilers are given drinking water ad libitum. The basal diet was a maize-soybean-based diet that contained 3000-3200 kcal of ME and 21-23% of crude protein provided by PT. Charoen Phokpand Indonesia. The feed composition of starter (0-21 d), and grower-finisher (22-33 d) diets were based on Table 1. The experiment was performed at the research barn (open house), Livestock Development Centre, Faculty of Animal Science, Universitas Gadjah Mada, Indonesia. The animal care practices employed in this study were conducted in accordance with the guidelines outlined in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010).
Table 1: Ingredients and nutrient composition of experimental broiler starter, grower, and finisher diets.
|
Starter |
Grower |
Finisher |
|
|
Moisture (%) |
Max 13 |
Max 13 |
Max 13 |
|
Crude protein (%) |
21-23 |
19-21 |
19-21 |
|
Extract ether (%) |
Min 5 |
Min 5 |
Min. 5 |
|
Crude fiber (%) |
Max 5 |
Max 5 |
Max 5 |
|
Ash (%) |
Max 7 |
Max 7 |
Max 7 |
|
Calcium (%) |
Min 0,9 |
Min 0,9 |
Min 0,9 |
|
Phosphor (%) |
Min 0,6 |
Min 0,5 |
Min 0,5 |
|
ME (kcal) |
3200 |
3000 |
3000 |
Growth performance and carcass trait
Body weight, feed intake, weight gain, feed conversion ratio, and performance index data were collected on days 0, 7, 14, 21, and 33. On day 33, one bird per replicate per treatment was randomly selected, weighed, and slaughtered by cutting the jugular vein in a professional slaughtering room. Broilers were harvested at 33 days of age following recommendations of Hidayat et al. (2023). The carcass weight of the samples was obtained by weighing the whole body of broilers after removing blood, feathers, neck, head, claws, and internal organs, except for the kidneys and lungs. Carcass were prepared and measured following Aprianto et al. (2023). Carcass percentage was obtained by calculating carcass weight divided by slaughter weight and then multiplied by 100%. Leg, wing, back, breast and abdominal weight was obtained from weighing the section produced by each chicken.
Blood hematology
One bird per replicate was collected for blood hematological analysis on day 33. Blood samples were taken from the brachial vein (wing) of chickens that had been fasted for 12 hours. Blood hematology examinations were measured following Karimy et al. (2017). The blood was collected into tube with 10% of EDTA as an anticoagulant. Hematological parameters tested were the number of red blood cells (RBC), white blood cells (WBC), hemoglobin, mean differential WBC including heterophiles, lymphocytes, and H/L ratio.
Internal organs and intestinal histomorphology
The weight of the internal organs divided by the weight of the living organism yields the relative weight of the internal organs. After separating each component, the length of the broiler birds’ digestive tracts was measured. In this investigation, 2-cm samples were obtained after the loop and used to quantify villi height, villi width, crypt depth, and the ratio of villi height to crypt depth in the duodenum, jejunum, and ileum. All intestinal segments were prepared and measured following Hanim et al. (2023). Samples of the small intestinal segment were fixed in 10% buffered formalin (100 ml 40% formalin, 4 g phosphate, 6.5 g dibasic sodium phosphate, and 900 ml distilled water), which was soaked for 24 to 48 hours. The samples were rapidly prepared by soaking them in alcohol at different percentages (70, 80, 90, and 100%) and dehydrating them. After being cleansed with xylol, the small intestine samples were infiltrated in paraffin wax (Leica Biosystems, Wetzlar, Germany). After infiltration in hot paraffin wax, the tissue samples were embedded in cassettes, which gave the final shape that will be sectioned in the next steps. The embedding media in this process is also hot melted paraffin wax. Using a rotary microtome (Yamato Kohki Industrial Co., Ltd., Saitama, Japan), the paraffin blocks were sectioned into 5 μm pieces and then stained with hematoxylin and eosin. Ultimately, a light microscope was used to examine the materials. Under a microscope with Optilab Viewer version 2.2 software (PT Miconos Transdata Nusantara, Yogyakarta, Indonesia) coupled to a monitor device, the sections were examined at a magnification of 4× PT. Miconos Transdata Nusantara, Yogyakarta, Indonesia) Image Raster software version 4.0.5 was used to quantify the generated photos of the histomorphology samples.
Statistical analysis
The pen was the experimental unit for statistical analyses of growth performance, carcass weight, internal organ weight, histomorphology of the small and large intestine (absolute, relative, and total weight), and histomorphology of the small intestine (villus height, crypt depth, villus height, and crypt depth ratio), which were analyzed using t-test following Kim (2019) in SPSS version 26 (SPSS Inc., Chicago, IL).
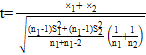
Description
x1 = Average of control broilers data; x2 = Average of fasting treatment broilers data; n1 = Number of control broilers; n2 = Number of samples of fasting treatment broilers; s1 = Standard deviation of control broilers; s2 = Standard deviation of fasting treatment broilers.
Results and Discussion
Growth performance and carcass trait
The result showed that feed intake, weight gain, feed conversion ratio and mortality were not significantly different between the two groups on day 33, but the RF group showed a decreased weight gain from the broiler on day 21 (P<0.05; Table 2). FR treatment on broilers has reduced the weight of legs (P<0.05; Table 3).
Table 2: Effect of feed restriction by fasting method on growth performance of broilers during 33 days.
|
Variables |
Group |
SEM |
p value |
|
|
AF |
RF |
|||
|
Day 0-21 |
||||
|
Feed intake (g) |
588 |
571 |
9.8 |
0.41 |
|
Body weight gain (g) |
420 |
389 |
7.0 |
0.03 |
|
Feed conversion ratio |
1.6 |
1.5 |
0.03 |
0.09 |
|
Mortality (%) |
1.1 |
1.3 |
0.32 |
0.23 |
|
Day 0-33 |
||||
|
Feed intake (g) |
2629 |
2521 |
31.9 |
0.10 |
|
Body weight gain (g) |
1745 |
1658 |
21.6 |
0.05 |
|
Feed conversion ratio |
1.6 |
1.6 |
0.01 |
0.80 |
|
Mortality (%) |
4.5 |
4.8 |
0.42 |
0.21 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Table 3: Effect of feed restriction by fasting method on carcass traits of male broilers at 33 days.
|
Variable |
Group |
SEM |
p value |
|
|
AF |
RF |
|||
|
Live weight (g) |
1802 |
1714 |
21.8 |
0.11 |
|
Carcass weight (g) |
1397 |
1350 |
22.2 |
0.31 |
|
Carcass yield (%) |
77.9 |
78.9 |
1.4 |
0.74 |
|
Breast weight (g) |
523 |
509 |
10.5 |
0.51 |
|
Breast yield (%) |
29.2 |
29.7 |
0.64 |
0.75 |
|
Leg weight (g) |
581 |
539 |
8.2 |
0.01 |
|
Leg yield (%) |
32.4 |
31.6 |
0.53 |
0.42 |
|
Wings weight (g) |
142 |
138 |
4.0 |
0.63 |
|
Wings yield (%) |
7.9 |
8.1 |
0.22 |
0.75 |
|
Back weight (g) |
150 |
163 |
14.0 |
0.64 |
|
Back yield (%) |
8.3 |
9.5 |
0.83 |
0.46 |
|
Abdominal fat weight (g) |
29.4 |
30.0 |
1.4 |
0.87 |
|
Abdominal fat yield (%) |
0.02 |
0.02 |
0.01 |
0.54 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Table 4: Effect of feed restriction by fasting method on the immune organs of male broiler at 33 days.
|
Variable |
Group |
SEM |
p value |
|
|
AF |
RF |
|||
|
Bursa fabricius (%) |
0.09 |
0.08 |
0.01 |
0.51 |
|
Spleen (%) |
0.07 |
0.07 |
0.01 |
0.64 |
|
Thymus (%) |
0.28 |
0.30 |
0.02 |
0.55 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Blood hematology and immune organs
RF treatment did not affect broiler blood hematology (Table 5). Moreover, Table 4 shows the relative weight of the immune organs of broilers on day 33. The dietary treatments did not influence the relative weight of the immune organs of broilers.
Table 5: Effect of feed restriction by fasting method on blood hematology of male broiler during 33 days.
|
Variables |
Group |
SEM |
p value |
|
|
AF |
RF |
|||
|
Red blood cells (106/µl) |
2.7 |
2.6 |
0.08 |
0.86 |
|
White blood cell (103/µl) |
20.3 |
16.9 |
1.4 |
0.26 |
|
Hemoglobin (g/dl) |
7.1 |
7.4 |
0.24 |
0.58 |
|
Heterophile (103/µl) |
12 |
12 |
1.02 |
0.30 |
|
Lymphocytes (103/µl) |
5.0 |
4.9 |
0.49 |
0.93 |
|
H/L ratio |
2.6 |
2.2 |
0.29 |
0.58 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Digestive organs and histomorphology of intestine
Restricted feeding improved the weight of crop, ventriculus, and jejunum of broilers on day 33 d (P<0.05, Table 6). The length gastrointestinal tract of broilers was not affected by the restricted feeding treatments (Table 7). RF group showed a wider villus than the AF group in the jejunum (P<0.05, Table 8). The highest ratio of villi and crypts in the jejunum was shown in the RF group.
Table 6: Effect of feed restriction by fasting method on relative weight of male broiler digestive organ at 33 days.
|
Variable |
Group |
SEM |
p-value |
|
|
AF |
RF |
|||
|
Oesophagus (%) |
0.25 |
0.29 |
0.01 |
0.06 |
|
Crop (%) |
0.25 |
0.34 |
0.01 |
0.01 |
|
Proventriculus (%) |
0.35 |
0.36 |
0.01 |
0.67 |
|
Ventriculus (%) |
1.4 |
1.6 |
0.04 |
0.04 |
|
Liver (%) |
2.0 |
2.0 |
0.07 |
0.92 |
|
Heart (%) |
0.48 |
0.50 |
0.01 |
0.52 |
|
Pancreas (%) |
0.17 |
0.18 |
0.01 |
0.45 |
|
Duodenum (%) |
29.2 |
29.7 |
0.64 |
0.75 |
|
Jejunum (%) |
581 |
539 |
8.2 |
0.01 |
|
Ileum (%) |
32.4 |
31.6 |
0.53 |
0.42 |
|
Cecum (%) |
142 |
138 |
4.0 |
0.63 |
|
Colon (%) |
7.9 |
8.1 |
0.22 |
0.75 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Feed restriction will not affect broiler performance because it triggers compensatory growth so that it is no different from chickens that are not restricted. Feeding restriction through the fasting method in broilers showed a decrease in body weight gain on day 21 but did not affect the body weight gain on day 33. These results are in line with the research of (Nurhayati, 2007), feed restriction up to 10% in broilers from the age of 6-14 days resulted in slaughter weights that were not different from the control (fed ad libitum), while feed restriction of more than 10% resulted in lower slaughter weights than the control. Urdaneta-Rincon and Leeson, (2002) conducted feed restriction on male broilers by providing feed at 90% of ad libitum consumption from the age of 5-10 days, 5-15 days, 5-20 days, and 5-30 days. Feeding at 90% of the amount of ad libitum feed consumption proved to produce body weight (35 days of age) which was not different from the control group of 1696, 1725, 1727, 1734, and 1676 g/ birds with the control group of 1744 g/ birds.
Table 7: Effect of feed restriction by fasting method on length of intestinal tract of male broiler at 33 days.
|
Variable |
Group |
SEM |
p-value |
|
|
AF |
RF |
|||
|
Duodenum (cm) |
25.4 |
26.6 |
0.69 |
0.41 |
|
Jejunum (cm) |
62.9 |
63.7 |
1.6 |
0.79 |
|
Ileum (cm) |
62.9 |
63.8 |
1.4 |
0.79 |
|
Cecum (cm) |
32.6 |
32.42 |
0.81 |
0.91 |
|
Colon (cm) |
9.6 |
8.9 |
0.43 |
0.40 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Table 8: Effect of feed restriction by fasting method on intestinal histomorphology of male broiler at 33 days.
|
Variable |
Group |
SEM |
p-value |
|
|
AF |
RF |
|||
|
Duodenum |
||||
|
Villus height |
753 |
890 |
45 |
0.20 |
|
Villus width |
86.2 |
105 |
9.7 |
0.43 |
|
Crypt |
111 |
97.8 |
7.5 |
0.47 |
|
Villus: crypt |
7.0 |
9.2 |
0.70 |
0.16 |
|
Jejunum |
||||
|
Villus height |
587 |
699 |
38.7 |
0.21 |
|
Villus width |
51.9 |
75.7 |
5.6 |
0.02 |
|
Crypt |
68.2 |
68.4 |
3.6 |
0.98 |
|
Villus: crypt |
8.6 |
10.3 |
0.34 |
0.01 |
|
Ileum |
||||
|
Villus height |
680.9 |
559.4 |
40.2 |
0.19 |
|
Villus width |
62.3 |
71.1 |
4.5 |
0.43 |
|
Crypt |
76.9 |
77.5 |
3.0 |
0.94 |
|
Villus: crypt |
8.9 |
7.3 |
0.60 |
0.27 |
AF: ad libitum feeding; RF: restricted feeding by fasting method; SEM: standard error of mean.
Decreased body weight can occur due to limited consumption of energy and protein, which causes the need to maximize tissue growth not to be met (Azis et al., 2011). Factors that cause a decrease in body weight during the feeding restriction period include the limited supply of nutrients and energy to support tissue growth (Hornick et al., 2000). Cabel and Waldroup (1990) stated that broiler chickens that were treated with satiation at the beginning of their growth resulted in unfulfilled protein intake and energy intake so their growth was disrupted. Hardini (2013) stated that broilers catch up with growth due to feeding restrictions if the right feeding restriction method is used. The energy and protein that support this catch-up growth comes from a decrease in maintenance needs during the period of re-feeding after a period of feeding restriction.
Normal feeding after the filler feed is intended to spur body cells to repair growth, resulting in compensatory growth. An interesting phenomenon is that the length of time for growth recovery or growth compensation determines the final body weight (Haryadi and Wihandoyo, 2005). During feed restriction, there will be an increase in the rate of stomach juice and contribute to the reduction of feed and nutrients eaten. In addition, it indicates that the longer the chicken gets filler feed, the growth is inhibited with the result that the body size also decreases and the development of body organs, especially the digestive organs, cannot be optimal or smaller because of the need for feed and the volume of feed that can be accommodated becomes less (Rumiyani et al., 2011).
In addition, feeding restriction through the fasting method does not affect the broiler’s immune system as indicated by the absence of changes in immune organs and blood haematology. Previous studies that discussed the abnormalities of broiler immune function mentioned that the weight of the thymus, bursa Fabricius, and spleen decreased significantly under stress conditions (Calefi et al., 2016; Ohtsu et al., 2015; Quinteiro-Filho et al., 2010). In addition, the number of heterophils and lymphocytes, and consequently the ratio of heterophils to lymphocytes, can vary depending on the condition (Simitzis et al., 2012). There are consistent reports of increased H/L ratios in stressed poultry species (Ajakaiye et al., 2010; Osti et al., 2017; Scanes, 2016). According Emadi and Kermanshahi (2006) the level of body resistance in poultry can be determined by the value of the H/L ratio, around 0.2 (low), 0.5 (normal) and 0.8 (high) for environmental adaptation. Heckert et al. (2002) proved in their research that a decrease in the weight of the Fabricius bursa in broiler chickens reared with high cage density can reduce the number of lymphocytes so that antibodies, including gama globulin, which are important in the immune system, are low. Thus, this indicates that the feed restriction treatment did not cause stress in broilers at day 33. Other results may be obtained if blood collection is conducted on day 21 when weight gain has decreased, which may be stressful due to feed restriction.
Several other studies have shown that feed restriction does not affect the blood hematology of broilers. The skip-a-day method of feeding restricted feed to broilers at weeks 2, 3 and 4 and returning to full feed thereafter did not affect the blood hematological condition of the chickens at day 56 blood collection (Akinsola et al., 2019). Felicia and Olutoye (2020), showed that feed restriction in the 5th week, 29-35th day group, 6th week, 36-42nd day group and 7th week, 43-49th day group had no effect on blood hematology of broiler on the 56th day of testing. Olukomaiya et al. (2014) explained that feed restriction affected the blood hematology of chickens on the 56th day of testing.
In our study feed restricted increased villus width and villus and crypt ratio in jejunum. Azouz et al. (2019), an increase in villi height and crypt depth in broilers exposed to feed restrictions. An increase in the height of the villi in the jejunum, which is the main site for nutrient uptake, and the ileum, which plays a key role in the re-absorption of water and electrolytes, may indicate an increase in absorption of nutrients at the re-feeding period and this is associated with gastrointestinal hypertrophy (Yu and Robinson, 1992). The main mechanisms of compensatory growth depend not only on increasing FI, but also on increasing digesta load, influencing maintenance needs, and adaptation of digestive organs. The digestive adaptation was one important factor contributing to growth compensation (Sahraei, 2012). The compensatory growth could be achieved by promoting the improvement of intestinal villus development and feed consumption when broiler chickens were fed frequently (Chaiyasing et al., 2024).
The feed restriction of chickens at 6 weeks of age caused an increase in the jejunal villus height, which was regarded as an adaptive strategy to maximize nutrient uptake once feeding (Thompson and Applegate, 2006). Yamauchi and Tarachai (2000) showed rapid recovery of villus height through increased epithelial cell area and cell mitosis after 1 d of refeeding in chickens. A decrease in metabolic rate could lead to a reduction in the energy required to maintain gastrointestinal turnover. In fact, feed restriction affects intestinal villus height, cell area, cell proliferation, and mitosis rate (Shamoto and Yamauchi, 2000).
Conclusions and Recommendations
In conclusion, restricted feeding through fasting once a week after the brooding period for 33 days of maintenance does not affect final growth performance, carcass trait, and immune system. Contrastingly, feed restriction increases the histomorphology of broiler jejunum. Feed restriction can be used as a way to reduce feed costs without causing negative effects. In future studies, we suggest looking at the effect of feed restriction through fasting on the tight junctions of the small intestine.
Acknowledgments
The authors are grateful to Universitas Gadjah Mada, Yogyakarta, Indonesia for financial support through the Rekognisi Tugas Akhir (RTA) research scheme in 2021.
Novelty Statement
This is the first study to report the effects of feed restriction through the fasting method once a week after the brooding period on days 10, 17, 24, and 31 on carcass characteristics, digestive and immune organ profiles and small intestine histomorphology.
Author’s Contribution
BA study conception and design. PI, MFH, JS draft manuscript preparation. PI, AN data collection. MFH analysis and interpretation of results. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest
The authors declare no conflict of interests with any financial and organization related to the materials discussed in the paper.
References
Ajakaiye JJ, Pérez-Bello A, Cuesta-Mazorra M, García-Díaz JR, Mollineda-Trujillo Á (2010). Effects of vitamin C and E dietary supplementation on erythrocyte parameters of layer chickens reared in high ambient temperature and humidity. Rev. Bras. Cienc. Avic., 12(3): 205–209. https://doi.org/10.1590/S1516-635X2010000300010
Akinsola KL, Nathaniel J, Obasi EN, Obike OM (2019). Effects of strains and skip-a-day feed restriction on haematological parameters of broiler chickens. Niger. J. Anim. Sci. Tech., 2(2): 12–22.
Aprianto MA, Muhlisin, Kurniawati A, Hanim C, Ariyadi B, Anas MA (2023). Effect supplementation of black soldier fly larvae oil (Hermetia illucens L.) calcium salt on performance, blood biochemical profile, carcass characteristic, meat quality, and gene expression in fat metabolism broilers. Poult. Sci., 102(10). https://doi.org/10.1016/j.psj.2023.102984
Azis A, Abbas H, Heryandi Y, Kusnadi E (2011). Pertumbuhan kompensasi dan efisiensi produksi ayam broiler yang mendapat pembatasan waktu makan. Med. Petern., 34(1): 50–57. https://doi.org/10.5398/medpet.2011.34.1.50
Azouz HMM, Gadelrab SS, El-Komy HM (2019). Effects of late feed restriction on growth performance and intestinal villi parameters of broiler chicks under summer conditions. Egypt Poult. Sci., 39(4): 935-951. https://doi.org/10.21608/epsj.2019.67514
Banong S, Hakim MR (2011). The effect of age and duration of fasting on performance and carcass characteristics of broiler chicken. Jurnal Ilmu dan Teknologi Peternakan. 1(2): 98-106.
Butzen FM, Ribeiro AML, Vieira MM, Kessler AM, Dadalt JC, Della MP (2013). Early feed restriction in broilers. I–Performance, body fraction weights, and meat quality. J. Appl. Poult. Res., 22(2): 251–259. https://doi.org/10.3382/japr.2012-00639
Cabel MC, Waldroup PW (1990). Effect of different nutrient-restriction programs early in life on broiler performance and abdominal fat content. Poult. Sci., 69(4): 652–660. https://doi.org/10.3382/ps.0690652
Calefi AS, de Siqueira A, Namazu LB, Costola-de-Souza C, Honda BBT, Ferreira AJP, Quinteiro-Filho WM, da Silva Fonseca JG, Palermo-Neto J (2016). Effects of heat stress on the formation of splenic germinal centres and immunoglobulins in broilers infected by Clostridium perfringens type A. Vet. Immunol. Immunopathol., 171: 38–46. https://doi.org/10.1016/j.vetimm.2016.02.004
Chaiyasing R, Srinontong P, Aengwanich W, Promsatit S, Cahyadi DD, Wandee J (2024). Effects of different feeding frequencies on broiler chickens’ growth performance and intestinal villus development. Trends Sci., 21(2). https://doi.org/10.48048/tis.2024.7353
Emadi M, Kermanshahi H (2006). Effect of turmeric rhizome powder on the activity of some blood enzymes in broiler chickens. Int. J. Poult. Sci., 6(1): 48–51. https://doi.org/10.3923/ijps.2007.48.51
Etuah S, Mensah J O, Aidoo R, Musah EF, Botchwey F, Oppong AL, Owusu K (2021). Financial viability of processing broiler chicken into cut parts in Ashanti region of Ghana. Cogent Food Agric., 7(1). https://doi.org/10.1080/23311932.2021.1917742
Federation of Animal Science Societies (2010). Guide for the care and use of agricultural animals in research and teaching. Champaign (IL): Feder. Anim. Sci. Soc. Champaign (IL, USA).
Felicia AO, Olutoye OO (2020). Effects of strain and skip-a-day feed restriction on haematological parameters of broiler chickens at finisher stage. Sumerianz J. Agric. Vet., 312: 174–177. https://doi.org/10.47752/sjav.312.174.177
Hanim C, Dono ND, Ariyadi B, Habibi MF, Al Anas M, Hanif MF (2023). Effect of protected sodium butyrate on growth performance, carcass traits, relative weight of digestive organs and intestinal histomorphology of broilers. J. Anim. Feed Sci., 32(4): 413–419. https://doi.org/10.22358/jafs/166080/2023
Hardini D (2013). Penghematan biaya produksi melalui pembatasan pakan pada ayam broiler. Jurnal Pengkajian dan Pengembangan Teknologi Pertanian. 16(1): 39-44.
Haryadi FT, Wihandoyo (2005). Studi kelayakan ekonomi dan produksi pemanfaatan pakan pengisi dan phenomena compensatory growth pada peternakan ayam pedaging. Bull. Petern., 29(1): 26–34. https://doi.org/10.21059/buletinpeternak.v29i1.1159
Heckert RA, Estevez I, Russek-Cohen E, Pettit-Riley R (2002). Effects of density and perch availability on the immune status of broilers. Poult. Sci., 81(4): 451–457. https://doi.org/10.1093/ps/81.4.451
Hidayat F, Afnan R, Fadilah R (2023). Pengaturan suhu brooding terhadap performa ayam broiler pelanggan PT New Hope Indonesia. J. Ilmiah Pendidikan Indonesia, 28(4): 599-606. https://doi.org/10.18343/jipi.28.4.599
Hornick JL, Van Eenaeme C, Gérard O, Dufrasne I, Istasse L (2000). Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol., 19(2): 121–132. https://doi.org/10.1016/S0739-7240(00)00072-2
Karimy MF, Sutrisno B, Agus A, Suryani AE, Istiqomah L, Damayanti E (2017). Aflatoxin effect on erythrocyte profile and histopathology of broilers given different additives. Abbreviation: IOP Conf. Ser. Earth Environ., 101(1). https://doi.org/10.1088/1755-1315/101/1/012033
Kim HY (2019). Statistical notes for clinical researchers: The independent samples t-test. Restor. Dent. Endod., 44(3). https://doi.org/10.5395/rde.2019.44.e26
Mallick P, Muduli K, Biswal JN, Pumwa J (2020). Broiler poultry feed cost optimization using linear programming technique. J. Operat. Strat. Plann., 3(1): 31–57. https://doi.org/10.1177/2516600X19896910
Muscat A, de Olde EM, de Boer IJM, Ripoll-Bosch R (2020). The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Sec., 25: 100330. https://doi.org/10.1016/j.gfs.2019.100330
Nurhayati (2007). Pengaruh pembatasan pakan awal terhadap kualitas karkas broiler. Agritek, 15(2): 243–245.
Ohtsu H, Yamazaki M, Abe H, Murakami H, Toyomizu M (2015). Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci., 52(4): 282–287. https://doi.org/10.2141/jpsa.0150062
Olukomaiya OO, Adeyemi OA, Sogunle OM, Abioja MO, Ogunsola IA (2015). Effect of feed restriction and ascorbic acid supplementation on growth performance, rectal temperature and respiratory rate of broiler chicken. J. Anim. Plant Sci., 25(1): 65–71.
Olukomaiya OO, Adeyemi O, Sogunle O, Abioja MO, Iwuchukwu PO, Emuveyan UP (2014). Effects of feed restriction and ascorbic acid supplementation on haematological parameters of Marshall broiler chickens. Int. J. Infect. Dis., 3(2): 18-22.
Osti R, Bhattarai D, Zhou D (2017). Climatic variation: Effects on stress levels, feed intake, and bodyweight of broilers. Rev. Bras. Cienc. Avic., 19(3): 489–496. https://doi.org/10.1590/1806-9061-2017-0494
Phiri PT, Ruzhani F, Madzokere F, Madududu P (2023). Factors affecting the profitability of smallholder broiler production in Mutare district, Manicaland Province, Zimbabwe: A quantile regression approach. Cogent Econ. Finance, 11(2): 2242660. https://doi.org/10.1080/23322039.2023.2242660
Qin Z, Wang Z, Shah A M, Ning Z, Tian Y, Zhu Q, Wang Y, Yin H, Zhang Z, Zhang L, Ye L, Li D, Shu G, Zhao X (2021). Feed restriction influences growth performance and blood glucose in faster- and slower- growing chickens. Pak. J. Zool., 54(1): 307-314. https://doi.org/10.17582/journal.pjz/20190403070443
Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sá LRM, Ferreira AJP, Palermo-Neto J (2010). Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci., 89(9): 1905–1914. https://doi.org/10.3382/ps.2010-00812
Rumiyani T, Wihandoyo, Sidadolog JHP (2011). The effect of stuff feeding during 22-28 days of ages on growth and percentage of meat and abdominal fat pad of broiler. Bull. Petern., 35(1): 38–49. https://doi.org/10.21059/buletinpeternak.v35i1.589
Sahraei, M., 2012. Feed restriction in broiler chickens production: A review. Glob. Vet., 8(5): 449–458.
Scanes CG (2016). Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci., 95(9): 2208–2215. https://doi.org/10.3382/ps/pew137
Shamoto K, Yamauchi K (2000). Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult. Sci., 79(5):718-723. https://doi.org/10.1093/ps/79.5.718
Simitzis PE, Kalogeraki E, Goliomytis M, Charismiadou MA, Triantaphyllopoulos K, Ayoutanti A, Niforou K, Hager-Theodorides AL, Deligeorgis SG (2012). Impact of stocking density on broiler growth performance, meat characteristics, behavioural components and indicators of physiological and oxidative stress. Br. Poult. Sci., 53(6): 721–730. https://doi.org/10.1080/00071668.2012.745930
Thompson KL, Applegate TJ (2006). Feed withdrawal alters small-intestinal morphology and mucus of broilers. Poult. Sci., 85(9):1535–1540. https://doi.org/10.1093/ps/85.9.1535
Urdaneta-Rincon M, Leeson S (2002). Quantitative and qualitative feed restriction on growth characteristics of male broiler chickens. Poult. Sci., 81(5): 679–688. https://doi.org/10.1093/ps/81.5.679
Yamauchi K, Tarachai P (2000). Changes in intestinal villi, cell area and intracellular autophagic vacuoles related to intestinal function in chickens. Br. Poult. Sci., 41(4):416-423. https://doi.org/10.1080/00071660050194902
Yu MW, Robinson FE (1992). The application of short-term feed restriction to broiler chicken production: A review. J. Appl. Poult. Res., 1(1): 147–153. https://doi.org/10.1093/japr/1.1.147
To share on other social networks, click on any share button. What are these?






