Effect of Inducement, Dilution Ratio and Freezing Rate on the Quality of Cyprinus carpio (Linnaeus, 1758) Spermatozoa
Effect of Inducement, Dilution Ratio and Freezing Rate on the Quality of Cyprinus carpio (Linnaeus, 1758) Spermatozoa
Kenyum Lollen1, Judith Betsy C1*, Cheryl Antony2 and Stephen Sampath Kumar J3
1Department of Aquaculture, Fisheries College and Research Institute, Tuticorin, Tamil Nadu, India.
2Department of Aquaculture, Dr. M.G.R. Fisheries College and Research Institute, Ponneri, Tamil Nadu, India.
3Directorate of Sustainable Aquaculture, Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, Tamil Nadu, India.
ABSTRACT
Cyprinus carpio is a widely cultured species and exploitation of natural stock due to increased demand in production can be averted by hormonal inducements which further affects the quality of gametes. Cryopreservation can aid in the conservation of gene pool and bestow superior quality sperm. Freezing and thawing rate are the most critical factor in the cryopreservation of milt. In the present study, variability between spermatological parameters of induced and non-induced C. carpio was documented for a period of 7 months. Further, the non-induced milt was stored for 24 h at three dilution ratio (1:10, 1:20 and 1:40) and spermatological parameters were analysed. Then the milt was cryopreserved with the best dilution ratio using 3 freezing protocols with two step cooling profile in programmable freezer. The spermatological properties of milt obtained from non-induced fishes were superior which was further used for short term preservation. When experimented with dilution ratio, the highest motility duration of 74.3±2.16 s was obtained at 1:10 dilution ratio. The best results on motility duration (62.28 ± 2.12 s), fertilizing ability (77.3±1.63 %) and hatching rate (61.6±2.44 %) was recorded when freezing protocol I was followed and the values were statistically significant. Hence the results revealed that for successful cryopreservation of C. carpio milt, use of non-induced milt at 1:10 dilution ratio using slow freezing rate will give the best results in terms of motility, fertilization and hatching rate.
Article Information
Received 30 March 2022
Revised 15 September 2022
Accepted 21 October 2022
Available online 12 January 2023
(early access)
Published 16 February 2024
Authors’ Contribution
KL execution of the experiment, data analysis, manuscript writing and reviewing. CJB conceptualization, design and execution of the experiments, data analysis, manuscript writing and reviewing. CA manuscript correction. JSSK conceptualization and design of the experiment. All authors contributed critically to the drafts and gave final approval for publication.
Key words
C. carpio, Inducement, Dilution ratio, Freezing rate, Fertilization
DOI: https://dx.doi.org/10.17582/journal.pjz/20220330110350
* Corresponding author: [email protected]
0030-9923/2024/0002-0903 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTODUCTION
The total world fisheries and aquaculture production has reached 179 million tonnes in 2018 which was recorded as the highest of all time and its value has been estimated at USD 401 billion (FAO, 2020). The aquaculture sector was the main driver that has helped the increase in production of aquatic animals. The global aquaculture production was dominated by carps. Common carp (Cyprinus carpio) is a widely cultured freshwater species and is an exhaustively researched Cyprinid. It is one of the most important fish species in global aquaculture with a share of 7.7% (4189.5 thousand tonnes) to the total global freshwater aquaculture production (FAO, 2020).
However, due to the emergence of intensive aquaculture of carps and development of commercial aquaculture, there is a need to acquire high quality seed for grow-out of marketable size under a given period of time. As it is difficult to meet such a high demand of seed through the natural breeding of carp, hormonal manipulations are used as a management tool to enhance the efficiency of egg production, increase spermiation and facilitate hatchery operations (Mylonas et al., 2010). The seed production of cultivable carps are mainly based on the induced breeding through hormones like carp pituitary extract, luteinizing hormone releasing hormone, human chorionic gonadotropin, Wova-FH, Ovaprim and Ovatide and the success largely depends upon the availability of well-maintained broodstock (Gupta et al., 1990). Though hormonal inducement can make the fish to breed, the sperm quality plays an important role in determining the fertilization success and it is known that the hormonal inducement affect the milt quality and motility of spermatozoa (Clemens and Grant, 1965). Belova (1981) stated that milt obtained from captive fish is low in volume and high in sperm concentration but the milt obtained as a result of hormonal stimulation had increased milt volume and low sperm concentration.
Although hatchery produced seeds are available for C. carpio, the gamete quality, purity and genetic quality of the seeds is a big question because of continuous hormonal manipulations, inbreeding and unintentional intraspecific hybridization practiced by the hatchery operators. It is, therefore, necessary to conserve the gene pool of wild stocks for quality brood production (Khan et al., 2015). In the above light, cryopreservation of fish gametes and application in the seed production is considered to be the simplest and most inexpensive method to preserve genomes that can be used to conserve the wild gene pool (Rafiquzzaman et al., 2007). First experiments on the cryopreservation of common carp milt were reported by Moczarski (1977) and the topic has been studied extensively since then. Despite successful cryopreservation of more than 200 fish species (Tsai and Lin, 2012), the quality of post-thaw spermatozoa are reported to deteriorate remarkably which straight away affects the rate of fertilisation and hatching (Liu et al., 2007).
The freeze-thaw step is the most crucial aspect of cryopreservation which needs to be optimized in order to decrease the impairment of spermatozoa occurred during the freezing process (Yoon et al., 2015). Several authors (Cloud and Patton, 2008; Dziewulska and Domagala, 2013) observed increased efficiency of spermatozoa when an effective dilution ratio in combination with cryoprotectants was manoeuvred during cryopreservation. Lahnsteiner et al. (2003) mentioned that at a ratio of 1:10, the osmolality of the extender–water mixture was high enough to stabilize sperm viability. He also stated that too low dilution ratio did not activate full sperm motility and too high ratios resulted in insufficient low sperm concentrations in the fertilization solution. Contrary to that, Linhart et al. (2000) reported 1:25 ratio as the optimal ratio of milt to fertilization medium and Magyary et al. (1996) found 1:20 as the optimal ratio for C. carpio. Sultana et al. (2009) observed that the best ratio for the milt and cryodiluents for the C. carpio spermatozoa was at 1:9 considering both pre-freeze and post-thaw motilities of spermatozoa.
The conventional freezing methods are known to have ill effects called cryoinjuries on the spermatozoa cryopreserved (Bozkurt et al., 2005). Thus, the choice and concentration of cryoprotectants, rate of freezing and cooling must be optimized for each species as the basis for any protocol development and successful cryopreservation process (Agrawal, 2011). The formation of intracellular ice crystal or spermatozoa dehydration can be circumvented by following an appropriate cooling rate during freezing (Cloud and Patton, 2008). Hence in the present study, the variations in the spermatological properties of induced and non-induced fishes were documented. Also, the best dilution ratio along with freezing rate was studied to minimise cryo injuries during the cryopreservation of C. carpio milt.
MATERIALS AND METHODS
Experimental fish
Adult male C. carpio brooders (50 nos) with an average body weight (ABW) of 468±0.5 g were procured and maintained in 2 cement tanks of 9000 l capacity each. During the period of rearing, the fishes were provided with supplementary feed @ 2-3% BW. The faecal matters and uneaten feeds were siphoned out daily from the tanks. The water was exchanged once in 2 days at 70% level.
Milt collection
Milt from male fishes was collected from the donor with mild oozing milt. Milt collection was done by gentle stripping as described by Lubzens et al. (1997). The genital pore region was cleaned with absorbent cotton and double ply tissue paper to remove any moisture or mucus in that area. The evacuation of urine was done by mild gentle press near the genital pore anteriorly, that could also press out the faecal matter if any to avoid contamination of the milt and the milt was collected in a sterile, pre-labelled vial (Betsy et al., 2019).
Experimental design
The study consisted of three independent trials. The aim of Trial 1 was to evaluate the effect of inducement on the spermatological properties. Trial 2 was performed to assess the effect of different dilution ratios on the milt quality before cryopreservation. In Trial 3, the influence of different freezing rate was assessed with best dilution ratio based on (i) sperm motility and (ii) fertilizing capacity.
Effect of hormonal inducement on spermatological properties
After acclimatization of fishes, once in a week, 5 male brooders were administered with single dose of Wova-FH (Biostadt Agrisciences, Wockhardt Life Sciences, Mumbai, India) @ 0.5 ml/kg BW using a 1 ml graduated syringe intramuscularly at an angle of 45⁰ in the dorsal side (Basudha et al., 2017). The brooders induced with hormone was maintained in FRP tanks till milt collection. Milt samples were collected from both induced and non-induced fishes. During every collection, 5 number of brooders were used each for induced and non-induced treatment. Milt was collected from induced fishes after 12 h of hormone administration.
Effect of dilution ratios on the milt quality
Based on the results obtained in the first trial, milt collected from non-induced fishes alone was used to evaluate the effect of dilution ratio. Milt from 10 fishes were collected for each treatment and pooled together. The collected milt was diluted with Freshwater Fish Saline (FWFS) (7.5g NaCl, 0.2g KCl, 0.2g NaHCo3, 0.2g CaCl2 per 100 ml of DW) as extender and DMSO at 10% (v/v) as cryoprotectant. Hence the ratio of extender to cryoprotectant was 9:1 (Betsy et al., 2017). All chemicals were of AR grade purchased from Merck, Germany. The milt dilution was performed inside the cold handling unit in 10 ml glass beakers. The freshly collected milt was diluted with extender and cryoprotectant at three different dilution ratios viz., 1:10, 1:20 and 1:40. Extender was added to the sample drop by drop during constant gentle mixing to avoid an osmotic shock of spermatozoa by cryoprotectant (Boryshpolets et al., 2017). The diluted spermatozoa was refrigerated at 4 ⁰C for 24 h after which the spermatological properties were evaluated.
Effect of freezing rate on the milt quality
For the purpose of 3rd trial, milt was again collected from 10 fishes for each treatment and pooled together and was diluted with the best dilution ratio obtained from Trial 2. The diluted milt was equilibrated for a period of 10 min at 4⁰C in the cold handling unit. After equilibration, the diluted milt was loaded in cryovials of 2 ml volume. The loaded cryovials were transferred to controlled rate freezer (PLANER, Kryo 560-16) programmable by Planer’s MRV controller system. Three sets of three step freezing protocol (FP) were used such as, FP I: 5⁰C to -4⁰C (at the rate of 4⁰C/min) (Ramp 1 min) and from -4⁰C to -80⁰C (at the rate of 10⁰C/min) and held for 10 min and direct transfer to liquid nitrogen (LN2); FP II: 5⁰C to -4⁰C (at the rate of 5⁰C/min) (Ramp 1 min) and from -4⁰C to -80⁰C (at the rate of 10⁰C/min) and held for 10 min and direct transfer to liquid nitrogen LN2 and FP III: 5⁰C to -4⁰C (at the rate of 10⁰C/min) (Ramp 1 min) and from -4⁰C to -80⁰C (at the rate of 10⁰C/min) and held for 10 min and direct transfer to liquid nitrogen LN2.
Once the curve programming was complete and the temperature reached -80°C, the straws were immediately transferred to a BA11 Cryocan (IBP, India) for storage at -196˚ C in canister containing goblet. The BA11 Cryocan was maintained with LN2 to a depth of 20 to 23 cm from the bottom that was checked every week with the help of a dip stick. The quality of cryopreserved spermatozoa maintained in the BA11 cryocan with LN2 was analyzed once in 10 days for a period of 30 days (Judycka et al., 2017).
During each sampling, cryopreserved vials were taken out from the BA11 cryocan and thawed at 25⁰C for 1-2 min as recommended by Lubzens et al. (1997) in serological water bath. The vials were thawed rapidly to avoid recrystallization (Lahnsteiner et al., 2000). After thawing, the water was wiped off the vials and the sample was taken for observation. The sample was examined for the parameters as that of the pre- frozen milt and the data obtained was recorded.
Assessment of sperm motility and fertilizing ability
Sperm motility
The collected milt was analysed under phase contrast microscope (NIKON E360) at X 200 magnification for its motility duration which was evaluated through the observation of their motility by placing 1 µl of milt sample and 10 µl of tap water on glass slide and observing under microscope (Akcay et al., 2004). The motility assessment was carried out before and after dilution. The motility score was assessed following Betsy and Kumar (2014).
Sperm density
The density of spermatozoa was determined by counting in a standard hemocytometer with an area of 1/25+1/400 mm2 and depth of 0.1 mm (Naubaeur, Germany). The sperm was diluted 100 folds with freshwater fish saline as described by Betsy et al. (2019). A droplet of the diluted milt was placed on a haemocytometer slide and counted using light microscope.
The number of spermatozoa per ml of milt was determined for each sample by using the following formula given by Aramli et al. (2013) and spermatozoa density was expressed as × 109 cells/ml.
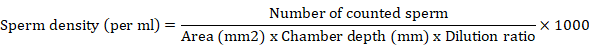
Percentage of live and dead spermatozoa
The percentage of live and dead spermatozoa was assessed using Eosin-Nigrosin stain as described by Chutia et al. (1998). On grease free glass slides, 1 μl of diluted milt was mixed with 1 μl of Eosin-Nigrosin stain placed on the corner of the slide. With the help of cover glass, the milt and the stain were dragged front and back for proper mixing. The smears were dried at 40⁰C in hot plate (5 MLH-DX, Remi Equipment) for 10 to 20 s. The percentage of live and dead spermatozoa was calculated by the concept of stain exclusion by the living cells. This was ascertained by microscopic observations (Nikon E360).
Fertilization ability
Dry method of in vitro fertilization was practiced following Sultana et al. (2009) and Aliniya et al. (2013). Fresh milt was collected from the fishes and diluted with same diluents and dilution ratios used for frozen sperm to minimize error. Eggs that were fertilized with the fresh milt were maintained as Control. Fertilisation was done in dry plastic dishes (Betsy et al., 2019). Cryopreserved spermatozoa were thawed for 2 min and added over the eggs immediately and gently mixed with feather. After thorough mixing, water was added to the milt egg mixture and mixed for one minute (Sultana et al., 2009). Then the eggs were transferred to nylon filaments. The eggs that were attached with the filaments were placed in hapa for incubation. Fertilization parameters such as fertilization rate (Brommage and Cumaranatunga, 1988) and hatching rate (Hanjavanit et al., 2008) were studied for all the samples. The calculation for fertilization and hatching rate was done as follows:
Fertilization rate = [Number of fertilized eggs/ Total eggs] × 100
Hatching rate = [Number of healthy fertilized egg/ Number of fertilized eggs] × 100
Statistical analysis
Data were obtained in triplicates and expressed as mean ± SD. Analysis of statistical significance between treatments was performed using one way ANOVA (p<0.05) and paired sample t test (p<0.001). All analysis were carried out using SPSS for windows version 22.0.
RESULTS AND DISCUSSION
Effect of hormonal inducement on spermatological parameters of non-induced milt
Motility duration
The motility duration of spermatozoa obtained from non-induced milt exhibited high variations. The highest mean motility duration (79±8.28 s) was obtained during February, 2021 (Table I). Uniform mean motility duration of 78 ±2.49 s and 78 ±6.23 s was documented during the month of November and December, 2020, respectively. The milt collected during the months of January, March and May 2021 reflected mean motility duration of 77±7.84 s, 75±3.74 s and 68±1.29 s, respectively (Table I). There was a decreasing trend of motility duration from February to April. The lowest mean motility duration was recorded during the month of April 2021 (62±3.29 s) (Table I). These values complies with that of Rahman et al. (2011) for fresh milt of Hypophthalmichthys molitrix and Bighead carp (H. nobilis) which had mean motility duration of 95.78±0.49 s and 95.11±0.35 s, respectively during the early spawning seasons. According to Yaron et al. (2009), the early spawning season of C. carpio was in the month of February which corresponds with the present work where the highest motility duration was obtained during the month of February. The values obtained was in accordance with Bozkurt et al. (2008) who reported 67.68±4.32 s as average motility duration in grass carp (Ctenopharyngodon idella). The motility score recorded in all samples of non-induced milt was 10.
The fluctuating motility durations and concentrations at the beginning and end of spawning may be due to factors such as age, length and weight and rearing conditions of brood stock (Faruk and Zafer, 2005). It is also to be noted that the repeated use of the same brooder over seasons, nutritional requirement, stocking density, and environmental changes together or individually may also affect the sperm quality that changes between spawning seasons (Rahman et al., 2011). The parameters of milt varies between conspecific males, between species and across the reproductive season (Rahman et al., 2011).
Sperm density
The highest spermatozoa density of 3.25 x109 Cells/ml was obtained during the month of January 2021 whereas, the lowest value of 1.96x109 Cells/ml was recorded during May 2021 (Table I). This is in accordance with Rahman et al. (2011) who reported higher sperm density of 3.15 x109 Cells/ml during early spawning seasons for Silver carp and lower density of spermatozoa of 2.42 x109 Cells/ml during late spawning season. Although May month was not the late spawning season for common carp, in the present study lowest mean motility duration was recorded during May which could be due to other factors such as age of the brooder and rearing condition (Faruk and Zafer, 2005). Milt collected during February and April 2021 exhibited almost similar spermatozoa density (Table I).
The observed difference in the sperm concentration across spawning seasons could have resulted from discontinuous spermatogenesis (Piros et al., 2002), may be due to changing endocrine conditions that affect spermatozoa maturation (Alavi et al., 2008). The spermatozoa concentration were found to decrease for rainbow trout, Onchorhynchus mykiss (Buyukhatipoglu and Holtz, 1984) and C. carpio (Christ et al., 1996) as the spawning season advanced.
Effect of hormonal inducements on spermatological parameters of induced milt
Motility duration
The motility duration of spermatozoa significantly changed over the study period and there was no particular increasing or decreasing trend in motility duration. From Table I, it can be observed that the highest mean motility duration obtained for induced C. carpio spermatozoa was 70±5.43 s during the month of April 2021. The milt collected during the month of November 2020 and February 2021 exhibited similar mean motility duration of 63±3.09 s and 63±2.62 s, respectively. The lowest mean motility duration of 59±7.03 s was recorded during January 2021 (Table I). The motility score recorded in all samples of induced milt was 8.
Table I. Spermatological parameters of induced and non-induced milt.
|
Treatment |
Mean motility duration (s) |
Sperm density (x109 Cells/ml) |
|
Induced milt |
||
|
November,2020 |
63 ±3.09e |
1±0.06g |
|
December, 2020 |
69±5.09b |
2.14±0.36c |
|
January, 2021 |
59±7.03g |
2.5 ±0.69a |
|
February, 2021 |
63±2.62e |
1.75± 0.49f |
|
March,2021 |
67±2.86 d |
2.21± 0.60b |
|
April, 2021 |
70±5.43a |
1.89± 0.04d |
|
May, 2021 |
68±0.81c |
1.8 ±0.16e |
|
Non-induced milt |
||
|
November,2020 |
78 ±2.49b |
2.96± 0.10c |
|
December, 2020 |
78 ±6.23b |
2.18±0.04f |
|
January, 2021 |
77±7.84d |
3.25±0.08a |
|
February, 2021 |
79±8.28 a |
2.21±0.03e |
|
March,2021 |
75±3.74e |
3.05±0.13b |
|
April, 2021 |
62±3.29 g |
2.25±0.02d |
|
May, 2021 |
68±1.29 f |
1.96±0.05g |
Data expressed as Mean ± SE (n=5, r=3); Mean values in same column with different subscript differ significantly (p<0.05) between months. One way ANOVA was used following Duncan multiple range test in SPSS-22.0
The findings on motility duration in the present study corroborates with the results reported by Verma et al. (2009) for silver carp (Hypopthalmichthys molitrix), who had reported 75±3.5 s as the highest mean motility duration during the month of June when induced with Ovaprim. The variation in motility duration throughout the study period can be attributed to the changing season, temperature, pH, osmolality (Lahnsteiner et al., 1998; Cosson et al., 2008) and are found to fluctuate between different males depending on the ripeness during the spermiation period (Billard, 1986). The motility duration in several Cyprinids were reported to be in the range of 90-120 s (Suzuki, 1959).
Sperm density
The sperm density fluctuated throughout the course of study period. The highest spermatozoa density (2.5x109 Cells /ml) was obtained during the month of January 2021 (Table I). Almost similar spermatozoa density of 1.89 x109 Cells /ml and 1.8 x109 Cells /ml was obtained in the milt samples collected during April 2021 and May 2021 respectively. However, during November 2020 the density of spermatozoa was recorded to be least with 1x109 Cells /ml (Table I).
This is in accordance with the work of Nahiduzzaman et al. (2014) who reported the highest mean sperm density of 2.28 x109 Cells /ml in C. carpio during the month of December and the lowest density of 1.08 x109 Cells /ml during March when induced with carp pituitary. Alavi et al. (2008) has demonstrated a similar seasonal change of sperm concentration (18.81 ×109 Cells/ml in March to 12.45×109 Cells/ml in May) in barbel (Barbus barbus). The difference in the concentration of sperm density across the seasons might be related to gonadal development and maturation, which is regulated by change in climate, day length and food supply, hormonal stimulation methods, stress, environmental conditions and age of the brood fish (Piros et al., 2002).
Effect of dilution ratios on the milt quality
Motility duration
The initial motility duration of spermatozoa after dilution was 79.6±1.63 s, 77.3±3.74 s and 75.6±2.16 s at 1:10, 1:20 and 1:40 dilution, respectively. When the milt was evaluated after 24 h of short-term preservation, the highest motility duration of 74.3±2.16 s was obtained at 1:10 dilution ratio. The second highest motility duration (72.0±1.63 s) was observed in 1:20 dilution, whereas the lowest motility duration of 70.3±2.82 s was obtained at 1:40 dilution. The values obtained were statistically significant (p<0.001) when analysed using paired sample t test.
This result is in accordance with the reports of Basavaraja and Hegde (2004) who mentioned highest post-thaw motility duration of 77 s during the cryopreservation of deccan mahseer (Tor Khudree) milt at 1:10 dilution ratios. Muchlisin et al. (2004) reported decreased motility duration with increase in dilution ratio (1:20, 1:30 and 1:40) and highest post-thaw motility duration of 71 s was observed at lower dilution of 1:20 in tropical bagrid fish (Mystus nemurus) when the milt was stored for 24 h in freezer. Alawi et al. (1995) reported a decreasing trend in motility duration as the dilution ratio increased. This may be because, at higher dilution ratios, the swimming velocity of spermatozoa and the percentage of linearly motile spermatozoa were decreased (Suquet et al., 2000; Lahnsteiner, 2007) due to removal of protective components of seminal plasma. Chauvaud et al. (1995) reported the protective action of proteins in the diluent on sperm motility and prevention of sperm aggregation in turbot spermatozoa. The motility and fertility of deep frozen spermatozoa of C. carpio were reported to be significantly improved when the dilution ratio was reduced from 1:100 to 1:2 (Cognie et al., 1989).
The other explanations regarding decreased motility duration of spermatozoa with increased dilution involves ionic composition of the diluent used as it affects the sperm motility (Erdahl and Graham, 1987; Erdahl et al., 1987). K+ ions are major constituents of carp seminal plasma and were found to increase the viability and speed of spermatozoa (Morisawa et al., 1983). Various authors reported increase in sperm motility in carp, goldfish and crucian carp when the level of K+ ions were higher in seminal plasma and concluded that it may help maintain the spermatozoa viability as the milt is diluted (Clemens and Grant, 1965; Grant et al., 1969).
Furthermore, Bozkurt et al. (2005), reported higher motility duration of 360.16±177 s when the milt of C. carpio was stored for short period of time at 1:3 dilution ratio. According to Saad et al. (1988), when the C. carpio milt was stored for short period of time at 4 ⁰C, they obtained higher percentage of motile spermatozoa up to 2 days which rapidly decreased after 6-8 days. Contrary to this, Ani and Jayaprakas (2013) obtained very low motility duration of 18.6 ± 0.51 s and 45 ± 0.31 s for slender rasbora (Rasbora daniconius) and filament barb (Puntius filamentosus) respectively, when the milt was stored at 4⁰C for 24 h.
Percentage of live and dead cells
When the C. carpio milt was analysed after short term preservation, the highest percentage of live cells observed was 85% at 1:10 dilution ratio, while at dilution ratio of 1:20 and 1:40, the live cells recorded was 79% and 76%, respectively which falls in line with the reports of Betsy and Kumar (2016), who reported 80% live cells at lowest dilution ratio of 1:40 (1:40, 1:80, 1:120) during cryopreservation of C. carpio.
Effect of freezing rate on the milt quality
Motility duration
The highest initial mean post-thaw motility duration of 77.14± 4.38 s was observed when FP-I was used which decreased to 62.28± 2.12 s on 30th day (Table II). The reduction in motility duration was 15%. The lowest post-thaw motility duration was obtained when FP-III was used with initial value of 68.33 ± 1.63 s that declined drastically to 44.9±0.87 s (Table II) at the end of cryopreservation with reduction in the motility duration by 23%. The values obtained were statistically significant (p<0.001) when analysed using paired sample t test.
Table II. Motility duration and fertilizing ability of cryopreserved milt with three different freezing protocol at the end of experiment.
|
Freezing protocol |
Motility duration (s) |
Fertilization rate (%) |
Hatching rate (%) |
|
|
Initial |
Final |
|||
|
I |
77.14±4.38a |
62.28±2.12a |
77.3±1.63 a |
61.6±2.44a |
|
II |
70.66±1.69b |
59.33±1.28b |
74.33±0.81b |
59.6±2.16b |
|
III |
68.33±1.63c |
44.9±0.87c |
70.6±2.16 c |
53.3±2.94c |
Data expressed as Mean ± SE (n=5, r=3); Mean values in same column with different subscript differ significantly (p<0.0001). Paired sample t test was used in SPSS-22.0
The freezing programme followed in FP-I had the slow rate of freezing (4⁰C/min) that had the best protective effects for cells during freezing when followed by rapid thawing as compared to the other two freezing rates (5⁰C/min and 10⁰C/min). The rate of cooling largely influences the success of cryopreservation as described by several authors (Lahnsteiner et al., 2003; Irawan et al., 2010) as it affects the post-thaw motility of spermatozoa by influencing the formation of ice crystals and osmotic pressure during the phase of freezing. However, highest post-thaw motility may be obtained from using a combination of two-step cooling rates (Conget et al.,1996; Suquet et al., 2000).
The present work falls in line with the work of Urbanyi et al. (1999), who reported successful use of slow freezing rate (4⁰C/min) during the cryopreservation of African catfish (Clarias gariepinus). Linhart et al. (2000) followed a similar cooling program following slower cooling rate (4⁰C/min) during the cryopreservation of Bohemian common carp, which showed high post-thaw motility duration (60 s) as compared to other cooling rates. The results obtained from the present study also correspond with the works carried out by Cognie et al. (1989), Margary et al. (1996) and Sultana et al. (2009).
The possible explanation for better post-thaw motility at slow freezing rate may be that, the slow cooling leads to increased water permeability leading to equilibration by transfer of internal water to external ice thus maintaining the osmotic balance. However, due to decreased water permeability, rapid cooling produces intracellular crystals which gets enlarged during warming because of their high surface energies. Rapid cooling is more damaging than slow cooling for various other cells too (Mazur, 1970).
Different types of cells may require different cooling rates, which also depend on the nature of extender and concentrations of cryoprotectants used (Suquet et al., 2000). In contrast to the present work, various researchers (Bernath et al., 2015; Boryshpolets et al., 2017) obtained high post thaw motility duration at higher cooling rates. Irawan et al. (2010) obtained highest post thaw motility duration (99.7±12.8 s) when the milt of C. carpio was cryopreserved at the rate of 10⁰C/min from an initial temperature of 25⁰C to −40⁰C and then free fall to −180⁰C.
Percentage of live and dead spermatozoa
The highest percentage of live cells of 84% was observed with FP-I which decreased to 62% on the last day of cryopreservation. When FP-II was followed, percentage of live cells recorded was 81% which dropped to 63% at the end of experiment. The lowest live cells of 77% was recorded when FP-III was employed which declined to 53% on 30th day.
The present work was in accordance with Cognie et al. (1989), who reported intact spermatozoa (66%) after thawing. Horokhovatsky et al. (2018) reported decline in live cells from 96% to 70% after cryopreservation of sterlet (Acipenser ruthenus). Conget et al. (1996) showed higher percentage (63%) of live spermatozoa and progressive motility during cryopreservation of O. mykiss. Linhart et al. (2005) reported 57.6% of intact spermatozoa after cryopreservation of Wels catfish (Silurus glanis).
Fertilizing ability
From Table II, it can be seen that the highest fertilisation and hatching rate of 77.3±1.63% and 61.6±2.44% was observed when milt frozen with FP-I was used. The milt samples frozen using FP-II yielded 74.33±0.81% of fertilisation rate and 59.6±2.16% of hatching rate (Table II). The lowest fertilisation and hatching rate of 70.6±2.16% and 53.3±2.94%, respectively was obtained when the milt was cryopreserved following FP-III (Table II). The values obtained were statistically significant (p<0.001) when analysed using paired sample t test.
The results comply with the reports of Irawan et al. (2010), who reported fertilisation and hatching rate of about 73.6±6.5 % and 62.8±5.9 %, respectively in C. carpio when slow rate of freezing was followed. Additionally, Warnecke and Pluta (2003) observed no difference in hatching rates (80 ±2 %) and swim up (78±2%) between cryopreserved milt and fresh milt of C. carpio when slow cooling was employed during cryopreservation. Furthermore, Vuthiphandchai et al. (2015) reported fertilisation and hatching rates of 64.5± 4.6% and 45.4±5.2%, respectively with cryopreserved milt of Silver barb (Barbodes gonionotus) which was almost similar to that of fresh milt.
Contrary to our results, Linhart et al. (2000) obtained lower fertilisation (56±10 %) and hatching rates (52±9%) in C. carpio though they yielded very high post-thaw motility duration of spermatozoa. The inconsistency among the studies may be attributed to the quality of spermatozoa post thawing and also the number of thawed spermatozoa used for fertilisation of the eggs (Lahnsteiner et al., 1996). Billard and Cosson (1992) stated that diminished post-thaw spermatozoa quality may be due to irregular sensitivity to the duration of storage. Correspondingly, the variability occurred in frozen-thawed spermatozoa due to individual and seasonal fluctuation of gamete quality may also influence the duration of motility and fertilisation success after cryopreservation (Lubzens et al., 1997; Linhart et al., 2000).
CONCLUSION
The present study demonstrated the superiority of non-induced fish as regards to spermatological properties over the induced fish. The study also concluded successful cryopreservation of C. carpio milt at 1:10 dilution ratio following slow freezing rate. However, the level of cryoinjuries the spermatozoa undergoes during various freezing rates to be studied to develop a standard freezing protocol.
ACKNOWLEDGEMENTS
The ICAR merit fellowship is greatly acknowledged for the funding support throughout the PG programme.
Funding
There was no funding received for this study.
IRB approval and ethical statement
The experiment was conducted following the procedures of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment and Forests (Animal Welfare Division), Government of India on care and use of animals in scientific research.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Agarwal, N.K., 2011. Cryopreservation of fish semen. In: Himalayan aquatic biodiv conservation and new tools in biotechnology. Transmedia Publication, Srinagar (Garhawal) Uttarakhand, India.
Akcay, E., Bukan, N., Bozkurt, Y., Tekin, N. and Secer, S., 2004. Correlation between biochemical and spermatological parameters in rainbow trout (Oncorhynchus mykiss) semen. Israeli J. Aquacult.-Bamidgeh, 56:20390.
Alavi, S.M.H., Psenicka, M., Rodina, M., Policar, T., and Linhart, O., 2008. Changes of sperm morphology, volume, density and motility and seminal plasma composition in Barbus barbus (Teleostei: Cyprinidae) during the reproductive season. Aquaculture, 21: 75-80. https://doi.org/10.1051/alr:2008011
Alawi, H., Nuraini, A. N., and Hutapea, S., 1995. Fish Breeding. Fakultas Perikanan Univ. Riau. Pekanbaru., 11-17.
Aliniya, M., Khara, H., Noveiri, S.B., and Dadras, H., 2013. Influence of age of common carp (Cyprinus carpio) broodstock on reproductive traits and fertilization. Turk. J. Fish. aquat. Sci., 13: 19-25. https://doi.org/10.4194/1303-2712-v13_1_03
Ani J. and Jayaprakas, V., 2014. Factors affecting the motility and short-term preservation of spermatozoa of two species of indigenous ornamental fishes, Rasbora daniconius and Puntius filamentosus. J. aquat. Biol. Fish., 2: 52-61.
Aramli, M.S., Kalbassi, M.R., and Nazari, R.M., 2013. Study of sperm concentration, seminal plasma composition and their physiological correlation in the Persian sturgeon, Acipenser persicus. Reprod. Domest. Anim., 48: 1013-1018. https://doi.org/10.1111/rda.12207
Babiak, I., Glogowski, J., Luczynski, M.J., Kucharczyk, D., and Luczynski, M., 1995. Cryopreservation of the milt of the northern pike. J. Fish Biol., 46: 819-828. https://doi.org/10.1111/j.1095-8649.1995.tb01604.x
Basavaraja, N., and Hegde, S.N., 2004. Cryopreservation of the endangered mahseer (Tor khudree) spermatozoa: I. Effect of extender composition, cryoprotectants, dilution ratio, and storage period on post-thaw viability. Cryobiology, 49: 149-156. https://doi.org/10.1016/j.cryobiol.2004.05.007
Basudha, C.H., Singh, N.G., Devi, N.S., and Sinthoileima, C.H., 2017. Induced breeding and embryonic development of an indigenous fish Bangana dero (Hamilton) in captivity using wova FH. Int. J. Fish. Aquat. Stud., 5: 428-432.
Belova, N.V., 1981. The ecological and physiological peculiarities of sperm in pond cyprinids. Communication I. Production and ecological and physiological peculiarities of the sperm of cyprinids. J. Ichthyol., 21: 90-102.
Bernáth, G., Żarski, D., Kása, E., Staszny, Á., Várkonyi, L., Kollár, T., and Horváth, Á., 2016. Improvement of common carp (Cyprinus carpio) sperm cryopreservation using a programable freezer. Gen. comp. Endocrinol., 237: 78-88. https://doi.org/10.1016/j.ygcen.2016.08.013
Betsy, J. and Kumar, S., 2014. New classification of motility score in fishes to determine the quality of spermatozoa. Int. J. Fish. Aquat. Stud., 1: 20-23.
Betsy, C.J., and Kumar, J.S.S., 2016. Effect of three different extenders on the spermatological parameters of Cyprinus carpio spermatozoa. J. Aquatcult. Trop., 31: 25.
Betsy, J., Kumar, S. and Thilak, J., 2017. Effect of age on the spermatological and fertilisation parameters of common carp Cyprinus carpio (Linnaeus 1758) brooders cryopreserved at three dilution ratios. Asian Fish. Sci., 30: 195-205.
Betsy, C.J., Kumar, J.S.S., and Jawahar, K.T.P., 2019. Effect of different extenders and dilution ratios on the spermatological and fertilization parameters of Cyprinus carpio during cryopreservation. Biopreserv. Biobank., 17: 157-162. https://doi.org/10.1089/bio.2018.0110
Billard, R., 1986. Spermatogenesis and spermatology of some teleost fish species. Reprod. Nutr. Dev., 26: 877-920. https://doi.org/10.1051/rnd:19860601
Billard, R., and Cosson, M.P., 1992. Some problems related to the assessment of sperm motility in freshwater fish. J. exp. Zool., 261: 122-131. https://doi.org/10.1002/jez.1402610203
Boryshpolets, S., Sochorová, D., Rodina, M., Linhart, O., and Dzyuba, B., 2017. Cryopreservation of carp (Cyprinus carpio L.) sperm: Impact of seeding and freezing rates on post–thaw outputs. Biopreserv. Biobank., 15: 234-240. https://doi.org/10.1089/bio.2016.0065
Bozkurt, Y., Öğretmen, F., and Erçin, U., 2008. Seminal plasma composition and its relationship with physical spermatological parameters of grass carp (Ctenopharyngodon idella) semen: with emphasis on sperm motility. Aquacult. Res., 39: 1666-1672. https://doi.org/10.1111/j.1365-2109.2008.02041.x
Bozkurt, Y., Seçer, S., Tekin, N., and Akçay, E., 2005. Cryopreservation of rainbow trout (Oncorhynchus mykiss) and mirror carp (Cyprinus carpio) sperm with glucose based extender. Turk. J. Fish. aquat. Sci., 13: 19-25.
Bromage, N., and Cumaranatunga, R., 1998. Egg production in the rainbow trout. In: Recent advances in aquaculture. Springer, Dordrecht.
Büyükhatipoglu, S., and Holtz, W., 1984. Sperm output in rainbow trout (Salmo gairdneri). Effect of age, timing and frequency of stripping and presence of females. Aquaculture, 37: 63-71. https://doi.org/10.1016/0044-8486(84)90044-9
Chauvaud, L., Cosson, J., Suquet, M., and Billard, R., 1995. Sperm motility in turbot, Scophthalmus marimus: initiation of movement and changes with time of swimming characteristics. Environ. Biol. Fish.., 43: 341-349. https://doi.org/10.1007/BF00001167
Christ, S.A., Toth, G.P., McCarthy, H.W., Torsella, J.A., and Smith, M.K., 1996. Monthly variation in sperm motility in common carp assessed using computer-assisted sperm analysis (CASA). J. Fish Biol., 48: 1210-1222. https://doi.org/10.1111/j.1095-8649.1996.tb01815.x
Chutia, I.P., Krishna, G.A., and Chaudhary, A., 1998. Biochemical and biometrical analysis of carp milt. In: Fish genetics and biodiversity conservation. Nature Conservators, Muzaffarnager, India. pp. 205-213.
Ciereszko, A., Glogowski, J., and Dabrowski, K., 2000. Biochemical characteristics of seminal plasma and spermatozoa of freshwater fish and the relation to semen biology, quality and cryopreservation. Cryopreserv. aquat. Spec., 46-79.
Clemens, H.P., and Grant, F.B., 1965. The seminal thinning response of carp (Cyprinus carpio) and rainbow trout (Salmo gairdnerii) after injections of pituitary extracts. Copeia, pp. 174-177. https://doi.org/10.2307/1440720
Cloud, J., and Patton, S., 2008. Basic principles of fish spermatozoa cryopreservation. In: Methods in reproductive aquaculture. CRC Press. pp. 259-272. https://doi.org/10.1201/9780849380549-16
Cognie, P.F., Billard, R., and Chao, N.H., 1989. La cryoconservation de la laitance de la carpe, Cyprinus carpio. J. appl. Ichthyol., 5: 165-176. https://doi.org/10.1111/j.1439-0426.1989.tb00489.x
Conget, P., Fernández, M., Herrera, G., and Minguell, J., 1996. Cryopreservation of rainbow trout (Oncorhynchus mykiss) spermatozoa using programmable freezing. Aquaculture, 143: 319-329. https://doi.org/10.1016/0044-8486(96)01275-6
Cosson, J., Groison, A. L., Suquet, M., Fauvel, C., Dreanno, C., and Billard, R., 2008. Studying sperm motility in marine fish: an overview on the state of the art. J. appl. Ichthyol., 24: 460-486. https://doi.org/10.1111/j.1439-0426.2008.01151.x
Dziewulska, K., and Domagała, J., 2013. Spermatozoa concentration influences cryopreservation success in sea trout (Salmo trutta m. trutta L.). Theriogenology, 80: 659-664. https://doi.org/10.1016/j.theriogenology.2013.06.013
Erdahl, A.W., and Graham, E.F., 1987. Fertility of teleost semen as affected by dilution and storage in a seminal plasma-mimicking medium. Aquaculture, 60: 311-321. https://doi.org/10.1016/0044-8486(87)90296-1
Erdahl, A.W., Cloud, J.G., and Graham, E.F., 1987. Fertility of rainbow trout (Salmo gairdneri) gametes: gamete viability in artificial media. Aquaculture, 60: 323-332. https://doi.org/10.1016/0044-8486(87)90297-3
FAO, 2020. The state of world fisheries and aquaculture 2020. Sustainability in action. Rome.
Faruk, Aral., and Zafer, Dogu., 2005. Annual changes in sperm characteristics of young rainbow trout (Oncorhynchus mykiss W., 1792) during spawning season in Ataturk Dam Lake, Sanliurfa, Turkey. J. Anim. Vet. Adv., 7: 41-44.
Graham, J.K., 1996. Cryopreservation of stallion spermatozoa. Vet. Clin. North Am., 12: 131-147. https://doi.org/10.1016/S0749-0739(17)30300-0
Grant, F.B., Pang, P.K., and Griffith, R.W., 1969. The twenty-four-hour seminal hydration response in goldfish (Carassius auratus) I. sodium, potassium, calcium, magnesium, chloride and osmolality of serum and seminal fluid. Comp. Biochem. Physiol. B, 3: 273-280. https://doi.org/10.1016/0010-406X(69)90810-X
Gupta, S.D., Reddy, P.K. and Pani, K.C., 1990. Advancing maturity and spawning in Asiatic carps through brood stock management. In: Carp seed production technology, Proceedings of the workshop on carp seed production technology, 2-4 September, 1998, Special Publication 2, Asian Fisheries Society, Indian Branch. pp. 34-37.Mangalore, India.
Hanjavanit, C., Punchai, K., Kitancharoen, N., and Hatai, K., 2008. Histopathology of Nile Tilapia Oreochromis niloticus eggs with fungal infection. Aquacult. Sci., 56: 463-464.
Horokhovatskyi, Y., Dietrich, M. A., Lebeda, I., Fedorov, P., Rodina, M., and Dzyuba, B., 2018. Cryopreservation effects on a viable sperm sterlet (Acipenser ruthenus) subpopulation obtained by a Percoll density gradient method. PLoS One, 13: 202-514. https://doi.org/10.1371/journal.pone.0202514
Irawan, H., Vuthiphandchai, V., and Nimrat, S., 2010. The effect of extenders, cryoprotectants and cryopreservation methods on common carp (Cyprinus carpio) sperm. Anim. Reprod. Sci., 122: 236-243. https://doi.org/10.1016/j.anireprosci.2010.08.017
Judycka, S., Ciereszko, A., Dobosz, S., Zalewski, T., and Dietrich, G.J., 2017. Effect of dilution in sperm maturation media and time of storage on sperm motility and fertilizing capacity of cryopreserved semen of sex-reversed female rainbow trout. Gen. comp. Endocrinol., 245: 89-93. https://doi.org/10.1016/j.ygcen.2016.06.016
Khan, N.S., Sarder, M.R.I., Faroque, M.A.A., and Mollah, M.F.A., 2015. Standardization of sperm cryopreservation techniques of Indian major carp rohu (Labeo rohita, Hamilton 1822). Int. J. Fish. Aquat. Stud., 2: 175-181.
Lahnsteiner, F., 2007. Characterization of seminal plasma proteins stabilizing the sperm viability in rainbow trout (Oncorhynchus mykiss). Anim. Reprod. Sci., 97: 151-164. https://doi.org/10.1016/j.anireprosci.2006.01.003
Lahnsteiner, F., Berger, B., and Weismann, T., 2003. Effects of media, fertilization technique, extender, straw volume, and sperm to egg ratio on hatchability of cyprinid embryos, using cryopreserved semen. Theriogenology, 60: 829-841. https://doi.org/10.1016/S0093-691X(02)01300-6
Lahnsteiner, F., Berger, B., Horvath, A., Urbányi, B., and Weismann, T., 2000. Cryopreservation of spermatozoa in cyprinid fishes. Theriogenology, 54: 1477-1498. https://doi.org/10.1016/S0093-691X(00)00469-6
Lahnsteiner, F., Berger, B., Weismann, T., and Patzner, R.A., 1998. Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture, 163: 163-181. https://doi.org/10.1016/S0044-8486(98)00243-9
Lahnsteiner, F., Patzner, R.A., and Weismann, T., 1996. Semen cryopreservation of salmonid fishes: influence of handling parameters on the postthaw fertilization rate. Aquacult. Res., 27: 659-671. https://doi.org/10.1111/j.1365-2109.1996.tb01301.x
Linhart, O., Rodina, M., and Cosson, J., 2000. Cryopreservation of sperm in common carp Cyprinus carpio: sperm motility and hatching success of embryos. Cryobiology, 41: 241-250. https://doi.org/10.1006/cryo.2000.2284
Linhart, O., Rodina, M., Flajshans, M., Gela, D., and Kocour, M., 2005. Cryopreservation of European catfish, Silurus glanis sperm: Sperm motility, viability, and hatching success of embryos. Cryobiology, 51: 250-261. https://doi.org/10.1016/j.cryobiol.2005.07.005
Liu, Q.H., Li, J., Xiao, Z.Z., Ding, F.H., De Yu, D. and Xu, X.Z., 2007. Use of computer-assisted sperm analysis (CASA) to evaluate the quality of cryopreserved sperm in red seabream (Pagrus major). Aquaculture, 263:20-25. https://doi.org/10.1016/j.aquaculture.2006.11.017
Lubzens, E., Daube, N., Pekarsky, I., Magnus, Y., Cohen, A., Yusefovich, F., and Feigin, P., 1997. Carp (Cyprinus carpio L.) spermatozoa cryobanks strategies in research and application. Aquaculture, 155: 13-30. https://doi.org/10.1016/S0044-8486(97)00106-3
Magyary, I., Urbanyi, B., and Horvath, L., 1996. Cryopreservation of common carp (Cyprinus carpio L.) sperm II. Optimal conditions for fertilization. J. appl. Ichthyol., 12: 117-119. https://doi.org/10.1111/j.1439-0426.1996.tb00073.x
Mazur, P., 1970. Cryobiology: The freezing of biological systems. Science, 168: 939-949. https://doi.org/10.1126/science.168.3934.939
Moczarski, M., 1977. Deep freezing of carp Cyprinus carpio L. sperm. Bull. Polish Acad. Sci., 25:187–190.
Morisawa, M., Suzuki, K., Shimizu, H., Morisawa, S., and Yasuda, K., 1983. Effects of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J. exp. Biol., 107: 95-103. https://doi.org/10.1242/jeb.107.1.95
Muchlisin, Z.A., Hashim, R.A.S.C., and Chong, A.S.C., 2004. Preliminary study on the cryopreservation of tropical bagrid catfish (Mystus nemurus) spermatozoa; the effect of extender and cryoprotectant on the motility after short-term storage. Theriogenology, 62: 25-34. https://doi.org/10.1016/j.theriogenology.2003.05.006
Mylonas, C.C., Fostier, A., and Zanuy, S., 2010. Broodstock management and hormonal manipulations of fish reproduction. Gen. comp. Endocrinol., 165: 516-534. https://doi.org/10.1016/j.ygcen.2009.03.007
Nahiduzzaman, M., Akter, S., Hassan, M.M., Azad-Shah, A.K.M., and Hossain, M.A.R., 2014. Sperm biology of artificially induced common carp, Cyprinus carpio (Linnaeus, 1758). Int. J. Fish. aquat. Stud., 1: 27-31.
Piros, B., Glogowski, J., Kolman, R., Rzemieniecki, A., Domagala, J., Horvath, A., and Ciereszko, A., 2002. Biochemical characterization of Siberian sturgeon, Acipenser baeri and starlet, Acipenser ruthenus milt plasma and spermatozoa. Fish Physiol. Biochem., 26: 289-295. https://doi.org/10.1023/A:1026280218957
Rafiquzzaman, S.M., Sarder, M.R., Islam, M.S., and Sultana, R., 2007. Sperm cryopreservation of Indian major carp, Labeo rohita: Cryodiluents, sperm: Cryodiluent dilution ratio and cryoprotectant concentration. Bangladesh J. Fish. Res., 11: 141-152. http://aquaticcommons.org/id/eprint/17719
Rahman, M.M., Rahman, M.S., and Hasan, M., 2011. Changes in sperm quality of silver (Hypophthalmichthys molitrix) and bighead carps (Hypophthalmichthys nobilis) during the spawning season. Asian Fish. Sci., 24: 413-425. https://doi.org/10.33997/j.afs.2011.24.4.006
Saad, A., Billard, R., Theron, MC, and Hollebecq, M.G., 1998. Short-term preservation of carp (Cyprinus carpio) semen. Aquaculture,71: 133-150. https://doi.org/10.1016/0044-8486(88)90280-3
Sultana, M., Nahiduzzaman, M., Hassan, M.M., Khanam, M.U.H., and Hossain, M.A.R., 2009. Fertility of cryopreserved common carp (Cyprinus carpio) spermatozoa. Rajshahi Univ. J. Sci., 28: 51-55. https://doi.org/10.3329/ujzru.v28i0.5287
Suquet, M., Dreanno, C., Fauvel, C., Cosson, J., Billard, R., 2000. Cryopreservation of sperm in marine fish. Aquacult. Res., 31: 231-243. https://doi.org/10.1046/j.1365-2109.2000.00445.x
Suzuki, R., 1959. Sperm activation and aggregation during fertilization in some fishes III. non species-specificity of stimulating factor. J. Zool. Japan, 32:105-111.
Tsai, S. and Lin, C., 2012. Advantages and applications of cryopreservation in fisheries science. Brazil. Arch. Biol. Technol., 55:425-434. https://doi.org/10.1590/S1516-89132012000300014
Urbanyi, B., Horvath, A., Varga, Z., Horvath, L., Magyary, I., and Radics, F., 1999. Effect of extenders on sperm cryopreservation of African catfish, Clarias gariepinus (Burchell). Aquacult. Res., 30: 145-151. https://doi.org/10.1046/j.1365-2109.1999.00313.x
Verma, D.K., Routray, P., Dash, C., Dasgupta, S., and Jk, J.E.N.A., 2009. Physical and biochemical characteristics of semen and ultrastructure of spermatozoa in six carp species. Turk. J. Fish. aquat. Sci., 9:67-76.
Vuthiphandchai, V., Chomphuthawach, S., and Nimrat, S., 2009. Cryopreservation of red snapper (Lutjanus argentimaculatus) sperm: effect of cryoprotectants and cooling rates on sperm motility, sperm viability, and fertilization capacity. Theriogenology.,72: 129-138. https://doi.org/10.1016/j.theriogenology.2009.02.013
Vuthiphandchai, V., Wilairattanadilok, K., Chomphuthawach, S., Sooksawat, T., and Nimrat, S., 2015. Sperm cryopreservation of silver barb (Barbodes gonionotus): cryoprotectants, cooling rate and storage time on sperm quality. Aquacult. Res., 46: 2443-2451. https://doi.org/10.1111/are.12396
Warnecke, D., and Pluta, H.J., 2003. Motility and fertilizing capacity of frozen/thawed common carp (Cyprinus carpio L.) sperm using dimethyl-acetamide as the main cryoprotectant. Aquaculture, 215: 167-185. https://doi.org/10.1016/S0044-8486(02)00371-X
Yaron, Z., Bogomoinaya, A., Drori, S., Biton, I., Aizen, J., Kulikovsky, Z., and Levavi-Sivan, B., 2009. Spawning induction in the carp: Past experience and future prospects. A review. Israel. J. Aquacult. Bamidgeh., 61: 5-26. http://hdl.handle.net/10524/19275
Yoon, S.J., Kwon, W.S., Rahman, M.S., Lee, J.S., and Pang, M.G., 2015. A novel approach to identifying physical markers of cryo-damage in bull spermatozoa. PLoS One, 10: e0126232. https://doi.org/10.1371/journal.pone.0126232
To share on other social networks, click on any share button. What are these?









