Effect of Commercially Available Probiotics Lactobacillus rhamnosus GG on the Body Composition of Grass Carp Fingerlings
Effect of Commercially Available Probiotics Lactobacillus rhamnosus GG on the Body Composition of Grass Carp Fingerlings
Maqsood Jan1, Muhammad Zubair Anjum1*, Zahir Muhammad1, Misbah Farooq1, Muhammad Qayash Khan2 and Shamim Akhter1
1Department of Zoology, Wildlife and Fisheries, PMAS-Arid Agriculture University Rawalpindi, 46300, Pakistan
2Department of Zoology, Abdul Wali Khan University, Mardan, Khyber Pakhtunkhwa, Pakistan
ABSTRACT
The objective of current study was to evaluate the effect of commercially prepared probiotics (PREPRO) containing Lactobacillus rhamnosus GG on body composition of grass carp (fingerlings). Grass carp is a fresh water fish of edible test and having multiple feed sources. The study was aimed to enhance the production and improve the meat quality of a grass carp in controlled environment. A total of 120 grass carp (fingerlings) were selected and stocked in 12 glass aquaria (n=10 fish in each) in a triplicate manner with four different diet groups D1 (control), D2 (2g of L. rhamnosus/kg), D3 (4g of L. rhamnosus/kg), D4 (6g of L. rhamnosus/kg) as feed additive. Statistical analysis was done by using one-way analysis of variance and was applied the Duncan’s multiple range test for the identification of significant differences within the treated groups by using statistical package for the social sciences (SPSS) computer software. After the 90 days of experimental trial the results revealed that probiotics treated groups had significantly (P < 0.05) better meat quality as compared to control. The best meat quality was observed in D4 in terms of crude protein, crude fat, ash content and total moisture (45.86±0.44, 33.59±0.28, 8.80±0.34, 63.98±0.01) compared to control and other tasted groups. The L. rhamnosus improves the meat quality of grass carp.
Article Information
Received 24 January 2023
Revised 05 May 2023
Accepted 25 May 2023
Available online 26 July 2023
(early access)
Published 19 July 2024
Authors’ Contribution
SA and MZA designed the study. MJ and MF performed the research work and compile the data. MZA supervised the experiment. ZM and MQK analyzed the data. MJ processed the data and wrote manuscript.
Key words
Grass carp, Probiotics, Lactobacillus rhamnosus GG
DOI: https://dx.doi.org/10.17582/journal.pjz/20230124170136
* Corresponding author: [email protected]
0030-9923/2024/0005-2189 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Grass carp (Ctenopharyngodon idella) is a herbivore fresh water fish species belonging to the family Cyprinidae. It is considered to be one of the largest members of the family. It is native to East Asian lakes and large rivers from temperate to sub-tropical region in the world and its native countries are Vietnam, China and southern Russia (Shireman and Smith, 1983). In Pakistan it has been introduced from china to control over the aquatic weeds in lakes and other aquatic bodies (Khan et al., 2004).
C. idella is considered to be an important fish because of rapid growth, taste, nutritional value and aquatic weeds control (Cai and Curtis, 1989). C. idella in 2019 contributed 5.7 million tons which is 10.5% of the total freshwater aquaculture global production and was the highest fish yield among all economical fish species (FAO, 2020).
Aquatic diseases, and low fish production were the profound challenges faced by the aquaculture industry (Kuebutornye et al., 2020). The percentage infections in fishes are 54.9% bacterial, 22.6% viral, 3.1% fungal and 19.4% are from other parasitic agents (Dhar et al., 2014). To overtake this problem vaccines and antibiotics were used but commercially available vaccines were expensive and were not effective against multiple pathogenic diseases. Besides that, by using antibiotics more harmful mutated strains of bacteria were produced (Dadar et al., 2016; Kawser et al., 2019). Therefore, antibiotics and vaccines were replaced by the probiotics (Hoseinifar et al., 2017). Probiotics are living microorganisms that when administered in an adequate amount improve host health (Kuebutornye et al., 2019). Probiotics can also be defined as live microorganism that are added to food or water to be ingested in the gastrointestinal tract (GIT), where they work to balance out the body’s microbial population and promote health (Gatesoupe, 1999). Probiotics can be provided as supplement even during early stages of fish (Muroga et al., 1987). Major taxa of probiotics that have been used as a probiotics included Pediococcus, Lactobacillus, Bacillus, Enterococcus, Micrococcus, Lactococcus and yeasts (such as Aspergillus oryzae and Saccharomyces cerevisiae) (Kuebutornye et al., 2019). Probiotics improve growth rate, survival rate, gut-microbiota, and inhibit pathogen growth (Grimoud et al., 2010). Probiotics also enhance innate immunity, maintain water quality, improve meat quality, and broadly utilize in dairy food products (Duan et al., 2019; Buffie and Pamer, 2013; Perales-Puchalt et al., 2018; Yi et al., 2019; Yih et al., 2019; Xia et al., 2020; Grande et al., 2017). Probiotics also increasing villus length promoting feed utilization by increasing digestibility through production of digestive enzymes and also absorption (Thiam et al., 2015; Xia et al., 2018).
Lactobacillus are non sporolating, anaerobic, fermentative, rod shaped bacteria (El-Shall et al., 2020; Alagawany et al., 2018). They are found in variety of habitat but prefer carbohydrate rich substrate such as spoiled food, fermented milk, human mucosal membrane, plants and their derived materials (Verse and Schrezenmeir, 2008). Most of lactobacillus species produce antimicrobial substances such as (hydrogen peroxides, antimicrobial peptides, and organic acids) which constrain pathogen growth and enhanced the performance of beneficial gastrointestinal microbiota of the host (Fooks and Gibson, 2002; Vieira et al., 2008; Abdel-Latif et al., 2020).
Lactobacillus rhamnosus GG has been reported to improve body weight, meat quality, RBCs count, hemoglobin level, WBCs and the significant number of neutrophils in rabbit (Kadja et al., 2021; Simonova et al., 2008). In Pakistan varieties of probiotics have been used in aquaculture with different combinations and concentrations but there is lack of data on the effect of probiotics on grass carp. Thus the present study was undertaken to evaluate the effect of commercially available probiotics L. rhamnosus GG on meat quality of grass carp.
MATERIALS AND METHODS
Study site and fingerlings collection
The experimental trial was done at the Aquaculture and Fisheries laboratory, Department of Zoology, Wildlife and Fisheries, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi. The grass carp fingerlings were obtained from the Mahseer Fish Hatchery, Garyala Attock.
Acclimatization
Before the start of experiment, fish were acclimatized in glass aquaria of (1×1×1.5 ft3) size for 7 days. The aquaria were well equipped with continuous aeration system and commercially available floating fish feed was used of (2mm) (Marine Grow Fish Feed; Hi-Tech Feeds Private Limited, Pakistan) containing 30% of crude proteins. The commercially prepared probiotic (PREPRO having ≥ 5×109 CFU of Lactobacillus rhamnosus) in powder form was purchased from the local pharmacy.
Experimental setup and diet
Fish were divided in twelve glass aquaria with 10 fingerlings per aquarium in a triplicate manner. The initial mean body weight 3.48±0.51 g. Fish were fed with four different experimental diet i.e., D1 (only supplementary fish feed), D2 (L. rhamnosus GG 2g/kg as feed additive), D3 (L. rhamnosus GG 4g/kg as feed additive) and D4 (L. rhamnosus GG 6g/kg as feed additive). Fish were fed @ 5% body weight for 90 days. On daily basis aquaria were siphoned to remove waste material of fish and the remaining feed material and was replaced with 50% of fresh water.
Proximate composition
At the end of feeding trial, fish were randomly selected from each treatment group for meat quality assessment. The viscera, fins, scales and heads were removed. Meat quality of fish were observed by proximate analysis. Crude protein, crude fat, crude ash and total moisture percentage were determined according to the standard procedures of AOAC. The moisture content was determined by drying the samples in oven at 60 ºC for one hour in dry dishes.
The moisture content were calculated as follows.
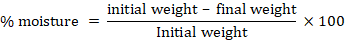
For determining crude protein Kjeldahl technique was used. For the determination of crude fat Soxhlet technique was used for 8 h in petroleum ether while total ash was done at 550 oC in a muffle furnace from the 5 g of sample for 12 h.
Statistical analysis
The obtained data was analyzed by using one-way analysis of variance (ANOVA) and the Duncan’s multiple range test (DMRT) was applied for the identification of significant differences within the treated groups by using statistical package for the social sciences (SPSS) software. The proposed results were presented as means ± standard deviation and the level of significant was set at p ≤ 0.05.
Table I. Effect of commercial probiotics on proximate composition (mean±standard deviation) of grass carp.
|
Treatments |
D1 |
D2 |
D3 |
D4 |
|
Crude protein |
40.50±0.52d |
41.86±0.57c |
43.33±0.70b |
45.86±0.44a |
|
Crude fats |
37.08±0.71a |
35.93±0.05b |
35.33±0.30b |
33.59±0.28c |
|
Total ash |
11.10±0.10a |
10.10±0.10b |
9.46±0.05c |
8.80±0.34d |
|
Moisture |
75.81±0.05a |
65.39±0.05c |
65.99±0.00b |
63.98±0.01d |
Means ± standard deviation in the same row with different superscripts are significantly different (P < 0.05). D1, control group; D2, 2g L. rhamnosus/kg; D3, 4g L. rhamnosus/kg; D4, 6g L. rhamnosus/kg.
RESULTS and Discussion
Proximate composition
After 90 days of experimental trial proximate composition of crude proteins, crude fat, total ash and moisture of C. idella was analyzed (Table I). Significantly higher moisture content was found in D1 (75.81±0.05) followed by D2, D3 and the lowest was found in D4. Crude protein was found significantly increased (P<0.05) in D4 (45.86±0.44) followed by D3, D2 and D1. The significantly higher (P<0.05) percentage of crude fat was found in control group D1 (37.08±0.71) followed by D2, D3 and the lowest crud fat was found in D4. Ash content was found as highest in control group D1 (11.10±0.10) followed by D2, D3 and significantly lower (P<0.05) recorded in D4.
Probiotics are live microorganisms that can be given to fish as a food supplement to improve their health and growth performance. Probiotics provide various advantages to fish, including increased feed efficiency, improved immunity, and flourishing intestinal microbiota (Akhter et al., 2015; Huyssnh et al., 2017; Wang et al., 2018). Probiotics (L. rhamnosus GG, Bifidobacterium animalis subsp. Lactis BB-12) used as a feed additive have been found to have significantly positive effect on the proximate composition of rabbit just as we noted in this study (Kadja et al., 2021). Other studies have also reported significantly higher (17.51%) protein content and lower (4.82%) fat content after supplementing rainbow trout feed with probiotics Lactobacillus rhamnosus GG (Hooshyar et al., 2020). Vitamin A has also been used as feed additive for grass carp. The results indicated that vitamins A supplemented at 1798 IU/kg showed significant increase in crude proteins free amino acids (methionine, glutamic acids, lysine, threonine and arginine) in the muscles. Crude fat, ash and total moisture of grass carp were significantly decreased compare to that of control group (Wu et al., 2022). In our study the same fish showed significant increase in crude protein and decrease in the other parameters when its feed was supplemented with Lactobacillus rhamnosus GG.
The current result of protein and crude fat were likewise with the previously reported by Hassaan et al. (2014). The crude protein value showed significantly higher values is probably due to (1) increased intake of fish feed, (2) improved digestion and (3) higher nutritional absorption in the body (Abdel-Tawwab et al., 2008). Along with this, synthesis of protein and its deposition in muscle tissues is also increased. This could help researchers figure out the best conditions for raising Nile tilapia fingerlings (Oreochromis niloticus), having best chemical composition of fish meat in terms of higher protein contents (Jim et al., 2017).
Bisht et al. (2012) have reported that C. carpio fed at 4×108 cells 100/g B. subtilis supplemented rice bran-based diet had lower moisture content (6.75%), while protein and lipid contents (30% and 8%) were higher as compared to control group. Similarly, Suprayudi et al. (2017) recorded higher protein and lipids absorption in the body of Nile tilapia upon feeding of diet supplemented with 0.25 and 0.5 g kg-1 of dietary probiotics.
Hoyoux et al. (2009) reported that the diet containing 1×107 CFUg-1 probiotics for fish showed highest protein, fat and gross energy contents in Nile tilapia. Sahandi et al. (2019) observed improved apparent digestibility coefficient of crude protein (68.33%), crude fat (9.55%) while using 1 × 107 of two probiotics strains of Bifidobacterium. In contrast Ayoola et al. (2013) found that the control diet has the highest moisture (8.20%), fat (12.7%), and ash (4.34%) values, whereas the Clarias gariepinus group fed 1g commercially manufactured probiotics (Lactobacillus and Bifidobacterium) had the highest crude protein (66%).
CONCLUSION
In the current study it has been concluded that the use of commercial probiotics (L. rhamnosus GG) in high amount/concentration having better meat quality (high level of crude protein, low level of ash, moisture and crude fat) results compared to that of control and other groups.
Funding
No funds were provided for this project.
IRB approval
The study was approved by the institutional review board of PMAS Arid Agriculture University, Rawalpindi.
Ethical statement
We have adopted all the rules and regulations approved by the ethical committee of Pir Mehr Ali Shah Arid Agricultural University Rawalpindi in the research work.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdel-Latif, H.M., Soliman, A.A., Sewilam, H., Almeer, R., Van Doan, H., Alagawany, M. and Dawood, M.A., 2020. The influence of raffinose on the growth performance, oxidative status, and immunity in Nile tilapia (Oreochromis niloticus). Aquacult. Res., 18: 100-457. https://doi.org/10.1016/j.aqrep.2020.100457
Abdel-Tawwab, M., Abdel-Rahman, A.M. and Ismael, N.E., 2008. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture, 280: 185–189. https://doi.org/10.1016/j.aquaculture.2008.03.055
Akhter, N., Wu, B., Memon, A.M. and Mohsin, M., 2015. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol., 45: 733–741. https://doi.org/10.1016/j.fsi.2015.05.038
Alagawany, M., El-Hack, A., Mohamed, E., Farag, M.R., Sachan, S., Karthik, K. and Dhama, K., 2018. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res., 25: 10611-10618. https://doi.org/10.1007/s11356-018-1687-x
Ayoola, S.O., Ajani, E.K. and Fashae, O.F., 2013. Effect of probiotics (Lactobacillus and Bifidobacterium) on growth performance and hematological profile of Clarias gariepinus juveniles. World J. Fish Mar. Sci., 5: 01–08.
Bisht, A., Singh, U.P. and Pandey, N.N., 2012. Bacillus subtilis as a potent probiotic for enhancing growth in fingerlings of common carp (Cyprinus carpio Linnaeus). Indian J. Fish, 59: 103–107.
Buffie, C.G. and Pamer, E.G., 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol., 13: 790-801. https://doi.org/10.1038/nri3535
Cai, Z., and Curtis, L.R., 1989. Effects of diet on consumption, growth and fatty acid composition in young grass carp. Aquaculture, 81: 47-60. https://doi.org/10.1016/0044-8486(89)90229-9
Dadar, M., Dhama, K., Vakharia, V.N., Hoseinifar, S.H., Karthik, K., Tiwari, R. and Joshi, S.K., 2017. Advances in aquaculture vaccines against fish pathogens: Global status and current trends. Rev. Fish. Sci. Aquacult., 25: 184-217. https://doi.org/10.1080/23308249.2016.1261277
Dhar, A.K., Manna, S.K. and Thomas, A.F.C., 2014. Viral vaccines for farmed finfish. Virus Dis., 25: 1-17. https://doi.org/10.1007/s13337-013-0186-4
Duan, Y., Wang, Y., Liu, Q., Dong, H., Li, H., Xiong, D. and Zhang, J., 2019. Changes in the intestine microbial, digestion and immunity of Litopenaeus vannamei in response to dietary resistant starch. Sci. Rep., 9: 1-10. https://doi.org/10.1038/s41598-019-42939-8
El-Shall, N.A., Awad, A.M., El-Hack, M.E.A., Naiel, M.A., Othman, S.I., Allam, A.A. and Sedeik, M.E., 2019. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. J. Anim., 10: 70. https://doi.org/10.3390/ani10010070
FAO, 2020. The state of world fisheries and aquaculture 2020. Sustainability in action, Rome.
Fooks, L.J. and Gibson, G.R., 2002. Probiotics as modulators of the gut flora. Br. J. Nutr., 88(S1): 39-49. https://doi.org/10.1079/BJN2002628
Gatesoupe, F.J., 1999. The use of probiotics in aquaculture. Aquaculture, 180: 147-165. https://doi.org/10.1016/S0044-8486(99)00187-8
Grande, R., Celia, C., Mincione, G., Stringaro, A., Di Marzio, L., Colone, M. and Stoodley, P., 2017. Detection and physicochemical characterization of membrane vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol., 8: 1040. https://doi.org/10.3389/fmicb.2017.01040
Grimoud, J., Durand, H., Courtin, C., Monsan, P., Ouarné, F., Theodorou, V., and Roques, C., 2010. In vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe, 16: 493-500. https://doi.org/10.1016/j.anaerobe.2010.07.005
Hassaan, M.S., Soltan, M.A. and Ghonemy, M.M.R., 2014. Effect of synbiotics between Bacillus licheniformis and yeast extract on growth, hematological and biochemical indices of the Nile tilapia (Oreochromis niloticus). Egypt. J. aquat. Res., 40: 199–208. https://doi.org/10.1016/j.ejar.2014.04.001
Hooshyar, Y., Abedian Kenari, A., Paknejad, H. and Gandomi, H., 2020. Effects of Lactobacillus rhamnosus ATCC 7469 on different parameters related to health status of rainbow trout (Oncorhynchus mykiss) and the protection against Yersinia ruckeri. Probiotics Antimicrob. Proteins, 12: 1370-1384. https://doi.org/10.1007/s12602-020-09645-8
Hoseinifar, S.H., Dadar, M. and Ringø, E., 2017. Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: The functional feed additives scenario. Aquacult. Res., 48: 3987-4000. https://doi.org/10.1111/are.13368
Hoyoux, C., Zbinden, M., Samadi, S., Gaill, F. and Compère, P., 2009. Wood-based diet and gut microflora of a galatheid crab associated with Pacific deep-sea wood falls. Mar. Biol., 156: 2421–2439. https://doi.org/10.1007/s00227-009-1266-2
Huynh, T.G., Shiu, Y.L., Nguyen, T.P., Truong, Q.P., Chen, J.C. and Liu, C.H., 2017. Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish Immunol., 64: 367–382. https://doi.org/10.1016/j.fsi.2017.03.035
Jim, F., Garamumhango, P. and Musara, C., 2017. Comparative analysis of nutritional value of Oreochromis niloticus (Linnaeus), Nile Tilapia, meat from three different ecosystems. J. Fd. Qual., 2017: e6714347. https://doi.org/10.1155/2017/6714347
Kadja, L., Dib, A.L., Lakhdara, N., Bouaziz, A., Espigares, E. and Gagaoua, M., 2021. Influence of three probiotics strains, Lactobacillus rhamnosus GG, Bifidobacterium animalis subsp. Lactis BB-12 and Saccharomyces boulardii CNCM I-745 on the biochemical and haematological profiles and body weight of healthy rabbits. Biology, 10: 1194. https://doi.org/10.3390/biology10111194
Kawsar, M.A., Alam, M.T., Ahamed, S. and Mou, M.H., 2019. Aqua drugs and antibiotics used in freshwater aquaculture of North Chittagong, Bangladesh. Int. J. Fish. aquat. Stud., 7: 28-34.
Khan, M.S., Khan, S.A., Chaudhary, Z.I., Khan, M.N., Aslam, A., Ashraf, K. and Rai, M.F., 2004. Mercury intoxication in grass carp (Ctenopharyngodon idella). Pak. Vet. J., 24: 33-38.
Kuebutornye, F.K., Abarike, E.D. and Lu, Y., 2019. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol., 87: 820-828. https://doi.org/10.1016/j.fsi.2019.02.010
Kuebutornye, F.K., Abarike, E.D., Sakyi, M.E., Lu, Y., and Wang, Z., 2020. Modulation of nutrient utilization, growth, and immunity of Nile tilapia, Oreochromis niloticus: The role of probiotics. Aquacult. Res., 28: 277-291. https://doi.org/10.1007/s10499-019-00463-6
Muroga, K., Higashi, M., and Keitoku, H., 1987. The isolation of intestinal microflora of farmed red seabream (Pagrus major) and black seabream (Acanthopagrus schlegeli) at larval and juvenile stages. Aquaculture, 65: 79-88. https://doi.org/10.1016/0044-8486(87)90272-9
Perales-Puchalt, A., Perez-Sanz, J., Payne, K.K., Svoronos, N., Allegrezza, M.J., Chaurio, R.A. and Conejo-Garcia, J.R., 2018. Frontlinse Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol., 103: 799-805. https://doi.org/10.1002/JLB.5HI1117-446RR
Sahandi, J., Jafaryan, H., Soltani, M. and Ebrahimi, P., 2019. The use of two Bifidobacterium strains enhanced growth performance and nutrient utilization of Rainbow Trout (Oncorhynchus mykiss) fry. Probiotics Antimicrob. Proteins., 11: 966–972. https://doi.org/10.1007/s12602-018-9455-2
Shireman, J.V. and Smith, C.R., 1983. Synopsis of biological data on the grass carp, Ctenopharyngodon idella (Cuvier and Valenciennes, 1844) (No. 135). Fd. Agric. Org., page number 86.
Simonová, M., Marcináková, M., Strompfová, V., Cobanová, K., Gancarcíková, S., Vasilková, Z. and Lauková, A., 2008. Effect of probiotics Lactobacillus rhamnosus GG and new isolate Enterococcus faecium EF2019 (CCM 7420) on growth, blood parameters, microbiota and coccidia oocysts excretion in rabbits. Int. J. Probiotics Prebiotics, 3: 7.
Suprayudi, M.A., Maeda, M., Hidayatullah, H., Widanarni, W., Setiawati, M. and Ekasari, J., 2017. The positive contributions of PowerLacTM supplementation to the production performance, feed utilization and disease resistance of Nile tilapia Oreochromis niloticus (L.). Aquacult. Res., 48: 2145–2156. https://doi.org/10.1111/are.13052
Thiam, S., Fall, J., Loum, A., Sagne, M. and Diouf, M., 2015. Use of effective microorganisms (Em) in tilapia diets: Effects of growth performance and carcass composition. Int. J. Curr. Microbiol. appl. Sci., 4: 536-549.
Vieira, F.D.N., Buglione Neto, C.C., Mouriño, J.L.P., Jatobá, A., Ramirez, C., Martins, M.L. and Vinatea, L.A., 2008. Time-related action of Lactobacillus plantarum in the bacterial microbiota of shrimp digestive tract and its action as immunostimulant. Pesqui. Agropecu. Bras., 43: 763-769. https://doi.org/10.1590/S0100-204X2008000600013
Vrese, M.D. and Schrezenmeir, A.J., 2008. Probiotics, prebiotics, and synbiotics. Fd. Technol. Biotechnol., 111: 1-66. https://doi.org/10.1007/10_2008_097
Wang, A.R., Ran, C., Ringø, E. and Zhou, Z.G., 2018. Progress in fish gastrointestinal microbiota research. Rev. Aquacult., 10: 626–640. https://doi.org/10.1111/raq.12191
Wu, P., Zhang, L., Jiang, W., Liu, Y., Jiang, J., Kuang, S. and Feng, L., 2022. Dietary vitamin A improved the flesh quality of grass carp (Ctenopharyngodon idella) in relation to the enhanced antioxidant capacity through Nrf2/Keap 1a signaling pathway. Antioxidants, 11: 148. https://doi.org/10.3390/antiox11010148
Xia, Y., Lu, M., Chen, G., Cao, J., Gao, F., Wang, M. and Yi, M., 2018. Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol., 76: 368-379. https://doi.org/10.1016/j.fsi.2018.03.020
Xia, Y., Yu, E., Lu, M. and Xie, J., 2020. Effects of probiotic supplementation on gut microbiota as well as metabolite profiles within Nile tilapia, Oreochromis niloticus. Aquaculture, 527: 735428. https://doi.org/10.1016/j.aquaculture.2020.735428
Yi, C.C., Liu, C.H., Chuang, K.P., Chang, Y.T. and Hu, S.Y., 2019. A potential probiotic Chromobacterium aquaticum with bacteriocin-like activity enhances the expression of indicator genes associated with nutrient metabolism, growth performance and innate immunity against pathogen infections in zebrafish (Danio rerio). Fish Shellfish Immunol., 93: 124-134. https://doi.org/10.1016/j.fsi.2019.07.042
Yu, D., Xu, Y., Regenstein, J. M., Xia, W., Yang, F., Jiang, Q. and Wang, B., 2018. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Fd. Chem., 242: 412-420. https://doi.org/10.1016/j.foodchem.2017.09.037
To share on other social networks, click on any share button. What are these?









