Comparative Study of the Phenolic Profile and Antibacterial Activity of Honeybee Propolis from Different Regions of South Punjab, Pakistan
Comparative Study of the Phenolic Profile and Antibacterial Activity of Honeybee Propolis from Different Regions of South Punjab, Pakistan
Shabana Islam1, Shahida Shujaat1*, Erum Akbar Hussain1, Mateen Abbas2
1Department of Chemistry, Lahore College for Women University, Lahore, Pakistan
2Department of Toxicology, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Abstract | There are numerous adverse effects of the COVID-19 pandemic on both public health and the global economy. The population of the third world has been struck particularly severely. The objective of this research is to identify affordable medical resources that can be utilized to treat prevalent bacterial infections that impact the local population. Ten propolis samples from South Punjab were analyzed. Phenolic acids (Sinapic acid, Caffeic acid, and gallic acid) and flavonols (kaempeferol, Quercetin, and myricetin) were quantified by means of UV detection in reverse phase high-performance liquid chromatography (RP-HPLC). The propolis samples exhibited substantial variation (P < 0.05) in the total flavonol and phenolic acid contents, which span a range of 52 to 183 mg/kg dried matter respectively. The agar well diffusion method was employed to assess the additional in vitro antibacterial activity of the samples against two Gram positive bacteria (Staphylococcus aureus, Bacillus cereus) and three Gram negative bacteria (Pseudomonas aeruginosa, Escherichia coli, and Salmonella typhimurium). The effectiveness of the samples was greater against Gram positive bacteria (MIC = 0.3 mg/mL) compared to Gram negative bacteria (MIC = 0.9 mg/mL). Upon testing the synergy between Ciprofloxacin and one of the samples, it was found to be exceptionally potent.
Novelty Statement | As the use of indigenous materials is of global interest, so we chose propolis- a bio-waste from honeybee hives to investigate its therapeutic potential. The present work is the first report on the phenolic profile and antibacterial activity of Propolis from different regions of South Punjab, Pakistan.
Article History
Received: January 17, 2024
Revised: April 20, 2024
Accepted: May 13, 2024
Published: May 29, 2024
Authors’ Contributions
SI conducted the research. SS supervised the study. EAH co-supervised the study. SS and EAH provided la-boratory facilities and guidance for research work. MA provided laboratory facilities and guidance for HPLC run.
Keywords
Ciprofloxacin, RP-HPLC, propolis, and both Gram-positive and Gram-negative microorganisms
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding Author: Shahida Shujaat
shahidashujaat@lcwu.edu.pk
To cite this article: Islam, S., Shujaat, S., Hussain, E.A. and Abbas, M., 2024. Comparative study of the phenolic profile and antibacterial activity of honeybee propolis from different regions of south Punjab, Pakistan. Punjab Univ. J. Zool., 39(1): 127-134. https://dx.doi.org/10.17582/journal.pujz/2024/39.1.127.134
Introduction
An increasing global concern is the development of antibiotic resistance against pathogenic microorganisms (Davies and Davies, 2010). Moreover, as the adverse effects of contemporary synthetic pharmaceuticals continue to escalate, individuals are reverting to traditional herbal remedies on the grounds that they are more secure (Khan et al., 2018). Asia is a continent whose climate is optimal for supporting its distinct flora and fauna. The vast majority of nations in this area are underdeveloped. Numerous individuals are unable to pay for costly prescriptions due to poverty. As a result, an investigation into the antibacterial medications from resources available in these nations is required (Shahbaz et al., 2015). Throughout history, honey bee products have been utilized by humans for medicinal intentions (Bankova et al., 2018). According to the literature (Eteraf-Oskouei et al., 2013; Ghisalberti, 1979; Kuropatnicki et al., 2018), ancient Greeks, Romans, Arabs, Europeans, and even Asians utilized these products to treat a wide range of ailments. A variety of health benefits are associated with propolis, a chemical produced by honey bees (Kuropatnicki et al., 2013). Honey bees (A. mellifera) use propolis for protection of their hives (Bankova et al., 2020). Combining bee secretions and beeswax with exudate from tree flowers, fluid flows, or other botanical sources, produces resinous propolis (Simone-Finstrom et al., 2017). This construction protects the hive against dampness, insects, reptiles, birds, and snakes by enclosing any unwanted openings (Figure 1). Ribeiro et al. (2020) say propolis’ volatile components give it a pleasant sweat smell and boost its bioactivity. Propolis may be red, dark brown, yellow, or green depending on its botanical origin and season (Gur et al., 2020; Santos, 2020).
Because of its acceptance in folk medicines, it has now become the subject of study. The chemical makeup of propolis has been studied since the early 1900s (Oroian et al., 2020). Over three hundred propolis components have been separated and extracted so far, but new ones are always being discovered. Propolis has a complicated chemical composition and structure (Al-Ghamdi et al., 2017; Thirugnanasampandan et al., 2012). According to Nada a and Alaa (2015), raw propolis contains 10% essential oils, 5% pollen, 50% resin, and 30% wax. Propolis contains flavonoids, phenolic acids, esters, terpenes, caffeic acid phenethyl ester (CAPE), and anthraquinones as its main bioactive components. These active components combine in different proportions to make it an effective antibacterial agent (Rivero-Cruz et al., 2020). According to Pujirahayu et al. (2014), flavonoids and phenolic acids are used to evaluate propolis quality. Their concentration depends on the plant source and extraction methods used (Paviani et al., 2013). These chemicals are produced by microbial infections and environmental stress and have several biological properties.
Mašek et al. (2018) and Ghisalberti et al. (1977) identified bactericidal effects in propolis. By preventing bacterial cell division, it may harm the cytoplasm or cell wall and limit protein synthesis (Al-Fahdawi, 2015; Rufatto et al., 2011). Propolis boosts the body immune system and give us a natural defence against harmful pathogens (Morsy et al., 2021). The chemical composition and biological activity of propolis depend on its botanical and geographical origin (Cunha et al., 2004; Markham et al., 1996), therefore discovering novel bioactive compounds in unknown propolis variations is vital. Numerous researches showed its antibacterial, hepatoprotective, antiviral, anti-inflammatory, and antifungal effects. Presently the antimicrobial potential of propolis is a subject of interest both in animal and plant research (Ali et al., 2017). Previous investigations on animal and human models have shown that propolis is non-toxic and has pharmacological capabilities (Jalali et al., 2020; Zampini et al., 2021). Punjab, being an agricultural land has several plant species and propolis as gifts.
Limited research has been undertaken thus far regarding this significant bee product originating from this area (Khan et al., 2018). The purpose of this study is to determine whether ten propolis samples contain flavonols (kaempeferol, quercetin and myricetin) and phenolic acids (sinapic acid, caffeic acid, and gallic acid) (Figure 2). Additionally, two Gram positive bacteria (Staphylococcus aureus and Bacillus cereus) and three gram negative bacteria (Pseudomonas aeruginosa, Escherichia coli, and Salmonella typhimurium) were subjected to in vitro assessments of their antibacterial activity (Cibanal et al., 2020; Hochheim et al., 2020; Surek et al., 2020). The selection of these bacteria was based on the frequency with which they caused food poisoning, diarrhea, cutaneous infections, urinary tract infections, and respiratory infections among the local population. There have been reports of fever and vomiting due to these microbes (Miryan et al., 2021; Ghoshal et al., 2021; Lind et al., 2013). Ciprofloxacin, a fluoroquinolone antibiotic with a broad spectrum of activity (Hamdan et al., 2021), was also examined for potential synergy with propolis in one of the samples, given that propolis has been shown to synergize with a variety of antibiotics in the literature (Fernandes Jr et al., 2005). It halts bacterial cell division by eliminating essential enzymes required for bacterial DNA separation, including topoisomerase II and topoisomerase IV (also known as DNA gyrase) (Khondker et al., 2021).
Materials and Methods
Sample collection
Ten propolis samples were collected from beekeepers during 2018 to 2020, from South Punjab (Dera Ghazi Khan (Latitude 30.0489 ⁰N, Longitude 70.3301 ⁰E), Bahawalnagar (Latitude 30.0025 ⁰N, Longitude 73.2412 ⁰E), Layyah (Latitude 30.9693 ⁰N, Longitude 70.9428 ⁰E), Vehari (Latitude 29.9719 ⁰N, Longitude 72.4258 ⁰E), and Multan (Latitude 30.1575 ⁰N, Longitude 71.5249 ⁰E) in summer as well as in winter (P1 to P10). Samples were carefully transported, and stored in refrigerator at -20°C until further use.
Chemicals
Standards of phenolic acids (sinapic acid, caffeic acid, gallic acid) and flavonoids (myricetin, quercetin, kaempeferol) were provided by Sigma Chemicals Co. (St. Louis, MO, USA). The additional compounds employed in this investigation, namely hydrochloric acid, ethanol, acetonitrile, and methanol, were supplied by Merck (Darmstadt, Germany). Stock solutions of flavonoids and phenolic acids were prepared in methanol at respective concentrations of 100 µg/ml and 10 µg/ml. Working solutions and stock were stored in the dark within a refrigerator at 4 °C. Calibration curves were constructed by comparing peak area to concentration.
Test microorganisms
Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 14579), Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922), Salmonella typhimurium (ATCC 700931).
Propolis extraction
Each individual sample weighed 30g. The material was subsequently filtered through Wattman filter paper after being submerged in a 1:10 solution of 95% ethanol and agitated for 24 hours at 30 degrees Celsius in an Irmeco swaying incubator (Germany). The aforementioned procedure was iterated triple. The filtrates were combined and desiccated at 70-75 degrees Celsius in a Heilhoff Laborta 4000 effective rotary evaporator. Table 1 presents the % yield of all extracts (P 1-10) calculated as.
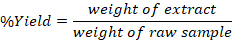
HPLC analysis
Chromatographic analysis was performed utilizing an Agilent 1100 series HPLC system equipped with the following components: A quaternary pump (G1311A Quat pump), a DAD detector (G1315B DAD), an auto-sampler/autoinjector (G1313A ALS), and a column compartment (G1316A Colcom). At a flow rate of 1.0 mL/min, the mobile phase was utilized; for flavonoids, it comprised 50% tri-fluoroacetic acid (0.3%), 30% acetonitrile, and 20% methanol; for phenolic acids, it was 40% tri-fluoroacetic acid (0.3%), 40% acetonitrile, and 20% methanol. Before elution, the mobile phase underwent degassing and filtration via a 0.45 m Nylon membrane filter. The isolation and detection of flavonoids and phenolic acids were accomplished by employing isocratic elution and detection at 360 nm and 280 nm, respectively. Through a comparison of their retention periods with those of authentic standards (Table 1), the compounds were identified. The software
Table 1: % Yield and HPLC analysis of propolis samples from South Punjab.
|
Sample code |
% yield |
Flavonoids (µg/mg) |
Phenolic acids (µg/mg) |
||||
|
Myricetin |
Quercetin |
Kaempeferol |
Sinapic acid |
Caffeic acid |
Gallic acid |
||
|
P 1 |
12.27 |
0.16(16)±0.030 |
0.31(31)±0.022 |
0.05(5) ±0.004 |
0.25(25)±0.012 |
0.05(5)±0.021 |
1.10(110)±0.010 |
|
P 2 |
8.05 |
0.26(26)±0.006 |
ND٭ |
ND٭ |
0.20(20) ±0.001 |
0.08(8)±0.011 |
0.36(36) ±0.001 |
|
P 3 |
13.33 |
0.23(23)±0.020 |
0.08(8) ±0.020 |
0.1(10) ±0.004 |
0.30(30) ±0.001 |
0.32(32)±0.001 |
1.21(121)±0.011 |
|
P 4 |
9.34 |
0.20(20)±0.040 |
ND٭ |
0.05(5) ±0.004 |
0.35(35) ±0.001 |
0.05(5) ±0.001 |
0.85(85) ±0.011 |
|
P 5 |
13.34 |
0.15(15)±0.004 |
ND٭ |
0.27(27)±0.002 |
0.28(28) ±0.001 |
0.19(19)±0.001 |
1.10(110)±0.013 |
|
P 6 |
9.02 |
0.05(5) ±0.004 |
0.12(12)±0.001 |
0.20(20)±0.001 |
ND٭ |
0.20(20)±0.001 |
0.50(50) ±0.023 |
|
P 7 |
13.86 |
0.27(27)±0.010 |
0.08(8) ±0.003 |
0.02(2) ±0.001 |
0.42(42) ±0.011 |
0.05(5) ±0.001 |
0.33(33) ±0.002 |
|
P 8 |
9.34 |
0.10(10)±0.009 |
0.05(5) ±0.001 |
ND٭ |
0.05(5) ±0.001 |
ND٭ |
1.10(110)±0.022 |
|
P 9 |
10.22 |
0.17(17)±0.012 |
0.01(1) ±0.032 |
0.20(20)±0.001 |
ND٭ |
0.21(21)±0.001 |
0.60(60) ±0.111 |
|
P 10 |
7.02 |
0.07(7) ±0.008 |
ND٭ |
0.05(5) ±0.001 |
ND٭ |
ND٭ |
0.87(87) ±0.002 |
٭ND, not detected.
Agilent Chem Station was utilized to analyze the chromatographic outcomes.
Antibacterial activity
Antibacterial activity was assessed utilizing the Agar well diffusion method (Perez, 1990). A fresh 100 μl bacterial culture containing 108 CFU/ml was added to each Petri plate along with 25 ml of sterile selective medium and allowed to solidify at room temperature. Wells were created and three known concentrations (5 mg/mL, 10 mg/mL, and 20mg/ mL) of each propolis extract were added. Plates were incubated at 37°C for an entire day and zone of inhibition was measured in millimeters. Macro broth dilution method was used to measure the minimum inhibitory concentration (MIC) for each bacterial strain using 48-well plates (Wiegand et al., 2008). Because propolis extracts were ineffective against Salmonella typhimurium, the minimum inhibitory concentration (MIC) could not be determined.
Statistical analysis
The results obtained from each sample were analyzed in triplicate, and the average (n= 3 SD) is presented. A variance analysis (ANOVA) was conducted utilizing Minitab 2000 Version 13.2 (Minitab Inc., PA, USA), a statistical programme. The threshold for a statistically significant difference was set at p < 0.05.
Results and Discussion
Propolis samples obtained from various regions of South Punjab were purified via maceration (Oroian et al., 2020). Diverse plant origins and collection locations might account for the fluctuating yield percentages observed in the samples (Table 1) (de Lima et al., 2016). Propolis’s diverse bioactive properties are attributed to its polyphenolics, which consist of phenolic acids and flavonoids (Bankova et al., 2020; Hindi, 2015). Gargouri and Fernández-Muiño (2019) reported a new type of propolis rich in flavonoids that have a good antibacterial activity. Nevertheless, variations in the concentration of polyphenolics in propolis can be attributed to geographical dissimilarities and botanical provenance (Bankova et al., 2020). The investigation assessed the concentrations of total phenolics and flavonols, including Sinapic acid, Caffeic acid, Gallic acid, kaempeferol, Myricetin, and Quercetin, in the various propolis samples (Table 1). Not with standing the fact that Gallic acid was detected in the maximum concentration in all ten propolis samples, Quercetin was only detectable in six of them. Similar inconsistencies were observed in the quantity and composition of total phenolics and flavonols examined in this research as were observed in China, Brazil, and Taiwan. The antibacterial effectiveness of each extract was found to be promising in comparison to ciprofloxacin against a variety of bacterial isolates (Table 2). A reference medication, ciprofloxacin has been selected on account of its broad-spectrum efficacy against Gram positive and Gram negative microorganisms. At a concentration of 5 mg/ml, the extracts exhibited no or very little antibacterial activity against Pseudomonas aeruginosa (Pa), Escherichia coli (Ec) and Salmonella typhimurium (St) while activity enhanced by increasing the concentration to 20 mg/mL.
The MIC for P4’s antibacterial activity against Staphylococcus aureus is 0.3 mg/mL, which is lowest among all the tested samples while values increases for rest of the strains. A comparable pattern was observed in other samples of propolis (Figure 3). Augmenting an ethanolic propolis extract in conjunction with antibiotics may reduce the risk of drug resistance, treatment doses, and adverse effects associated with pharmaceuticals, according to a prior study. When compared to all bacterial isolates, P4 in combination with Ciprofloxacin demonstrated a greater zone of inhibition against G+ve bacteria but no discernible alteration against G-ve bacteria (Figure 4). Consequently, propolis might have enhanced the effectiveness of the medication (Noori, 2012; Wojtyczka et al., 2013). Further in vivo studies are required to see the effectiveness of present research.
Table 2: Zone of inhibition (mm) of propolis samples from south Punjab.
|
EEP/antibiotic |
Conc. (mg/ml) |
Microbes and their zone of inhibition (mm) |
||||
|
Bs |
Sa |
Pa |
Ec |
St |
||
|
Ciprofloxacin (Ab) P 1 |
20 |
26.0±0.4 |
24.0±0.5 |
22.0±0.4 |
24.0±0.6 |
20.0±0.4 |
|
20 |
18.0±0.4 |
17.0±0.5 |
9.0±0.4 |
9.5±0.6 |
NA٭ |
|
|
10 |
17.0±0.4 |
15.0±0.7 |
8.5±0.6 |
8.5±0.4 |
NA٭ |
|
|
5 |
15.5±0.3 |
13.0±0.6 |
NA٭ |
NA٭ |
NA٭ |
|
|
P 2 |
20 |
16.0±0.6 |
18.0±0.4 |
14.0±0.3 |
10.0±0.3 |
9.3±0.3 |
|
10 |
15.0±0.4 |
17.5±0.3 |
12.0±0.4 |
8.5±0.3 |
NA٭ |
|
|
5 |
13.0±0.3 |
16.0±0.2 |
11.0±0.6 |
NA٭ |
NA٭ |
|
|
P 3 |
20 |
20.0±0.6 |
18.0±0.5 |
12.0±0.5 |
11.5±0.6 |
11.0±0.9 |
|
10 |
18.0±0.4 |
16.0±0.3 |
9.5±0.4 |
10.0±0.6 |
8.5±0.3 |
|
|
5 |
14.0±0.3 |
12.0±0.4 |
8.5.0±0.4 |
8.0±0.3 |
NA٭ |
|
|
P 4 |
20 |
20.0±0.5 |
15.5±0.4 |
10.0±0.2 |
10.0±0.6 |
9.00±0.3 |
|
10 |
18.5±0.3 |
13.5±0.4 |
8.5±0.3 |
8.5±0.2 |
NA٭ |
|
|
5 |
16±0.2 |
11.0±0.3 |
NA٭ |
NA٭ |
NA٭ |
|
|
P 5 |
20 |
16.0±0.5 |
15.0±1.4 |
10.5±0.5 |
10.0±1.3 |
NA٭ |
|
10 |
14.0±0.7 |
12.5±0.3 |
9.00±0.4 |
8.5±0.4 |
NA٭ |
|
|
5 |
12.0±0.3 |
12.0±1.0 |
8.5±0.3 |
NA٭ |
NA٭ |
|
|
P 6 |
20 |
14.0±0.3 |
17.0±0.3 |
10.0±0.4 |
NA٭ |
NA٭ |
|
10 |
10.0±0.4 |
15.0±0.5 |
8.5±0.4 |
NA٭ |
NA٭ |
|
|
5 |
8.0±0.8 |
10.0±0.3 |
NA٭ |
NA٭ |
NA٭ |
|
|
P 7 |
20 |
15.0±1.4 |
15.0±0.3 |
10.0±0.4 |
10.0±0.4 |
8.5±0.6 |
|
10 |
13.5±0.4 |
12.0±0.3 |
8.5±0.3 |
8.7±0.3 |
NA٭ |
|
|
5 |
12.0±0.3 |
11.0±0.5 |
NA٭ |
NA٭ |
NA٭ |
|
|
P 8 |
20 |
17.5±0.5 |
18.0±0.3 |
11.0±0.3 |
12.0±0.4 |
11.0±0.7 |
|
10 |
16.0±0.4 |
16.0±0.2 |
9.5±0.5 |
10.0±0.6 |
9.0±0.3 |
|
|
5 |
14.5±0.3 |
14.5±0.2 |
NA٭ |
8.5.0±0.7 |
NA٭ |
|
|
P 9 |
20 |
14.5±0.4 |
16.0±0.1 |
NA٭ |
8.5±0.9 |
NA٭ |
|
10 |
10.0±0.7 |
10.0±1.1 |
NA٭ |
NA٭ |
NA٭ |
|
|
5 |
8.5±0.5 |
8.5±0.4 |
NA٭ |
NA٭ |
NA٭ |
|
|
P 10 |
20 |
20.0±0.7 |
17.0±0.5 |
12.0±0.6 |
9.0±0.5 |
NA٭ |
|
10 |
16.5±0.4 |
15.5±0.7 |
9.5±0.6 |
NA٭ |
NA٭ |
|
|
5 |
14.0±0.5 |
14.0±0.5 |
8.5±0.4 |
NA٭ |
NA٭ |
|
Ab, Antibiotic; Bs, Bacillus cereus; Sa, Staphylococcus aureus; Pa, Pseudomonas aeruginosa; Ec, Escherichia coli; St, Salmonella typhimurium; ٭NA, not active.
Conclusions and Recommendations
In conclusion, this research has established that propolis samples procured from South Punjab, Pakistan, comprise phenolics, which consist of flavonoids and phenolic acids. The concentration of propolis samples influences their antimicrobial activity. Salmonella typhimurium exhibited the highest resistance, while Bacillus cereus and Staphylococcus aureus were the most susceptible microorganism. In addition, propolis also synergistically enhanced the efficacy of antibiotic, ciprofloxacin. The possibility exists that this synergistic effect could inspire the development of novel pharmaceutical combinations intended to combat bacterial infections.
Acknowledgments
We are thankful to Department of Botany and Department of Biotechnology, Lahore College for Women University Lahore, for providing us Bacterial Strains. We are also thankful to University of Veterinary and Animal Sciences, Lahore, Pakistan for providing us HPLC facility.
Conflict of interest
The authors have declared no conflict of interest.
References
Al-Fahdawi, I.H., 2015. Potential indication of propolis in treatment of oral infection for denture wearers. Mouth, 18: 19. https://doi.org/10.15226/jdodt.2015.00146
Al-Ghamdi, A.A., Bayaqoob, N.I., Rushdi, A.I., Alattal,Cécere, B.G., da Silva, A.S., Molosse, V.L., Alba, D.F., Leal, K.W., da Rosa, G., Pereira, W.A., da Silva, A.D., Schetinger, M.R.C., Kempka, A.P. and Nunes, A., 2021. Addition of propolis to milk improves lactating lamb’s growth: Effect on antimicrobial, antioxidant and immune responses in animals. Small Rumin. Res., 194: 106265. https://doi.org/10.1016/j.smallrumres.2020.106265
Ali, A., Wei, Y.Z. and Mustafa, M.A. 2017. Exploiting propolis as an antimicrobial edible coating to control post-harvest anthracnose of bell pepper. Packag.Technol. Sci., 28: 173-179.
Bankova, V.S., de Castro, S.L. and Marcucci, M.C., 2000. Propolis: recent advances in chemistry and plant origin. Apidologie, 31: 3-15.
Bankova, V., Popova, M. and Trusheva, B., 2018. The phytochemistry of the honeybee. Phytochemistry, 155: 1-11.
Cibanal, I.L., Fernández, L.A., Positano, G.G., Chebataroff, L.B., Garayalde, A.F., Gallez, L.M. and Pérez, E.S., 2020. Chemical characterization and in vitro antimicrobial activity of honeybee propolis and Scaptotrigona jujuyensis geopropolis against tomato pathogenic bacteria. Semin Ciên. Agrár., 41: 1799-1808. https://doi.org/10.5433/1679-0359.2020v41n5p1799
Cunha, I., Sawaya, A.C., Caetano, F.M., Shimizu, M.T., Marcucci, M.C., Drezza, F.T., Povia, G.S. and Carvalho, P.D.O., 2004. Factors that influence the yield and composition of Brazilian propolis extracts. J. Braz. Chem. Soc., 15: 964-970. https://doi.org/10.1590/S0103-50532004000600026
Davies, J. and Davies, D., 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev., 74: 417-433. https://doi.org/10.1128/MMBR.00016-10
de Lima, G.G., de Souza, R.O., Bozzi, A.D., Poplawska, M.A., Devine, D.M. and Nugent, M.J., 2016. Extraction method plays critical role in antibacterial activity of propolis-loaded hydrogels. J. Pharma. Sci., 105: 1248-1257. https://doi.org/10.1016/j.xphs.2015.12.027
Eteraf-Oskouei, T. and Najafi, M., 2013. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci., 16: 731.
Fernandes Júnior, A., Balestrin, E.C., Betoni, J.E.C., Orsi, R.D.O., Cunha, M.D.L.R.D.S.D. and Montelli, A.C., 2005. Propolis: Anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Memór. Inst. Oswaldo Cruz, 100: 563-566. https://doi.org/10.1590/S0074-02762005000500018
Gargouri, W., Osés, S.M., Fernández-Muiño, M.A., Sancho, M.T. and Kechaou, N., 2019. Evaluation of bioactive compounds and biological activities of Tunisian propolis. Lwt, 111: 328-336. https://doi.org/10.1016/j.lwt.2019.05.044
Ghisalberti, E.L., 1979. Propolis: A review. Bee World, 60: 59-84. https://doi.org/10.1080/0005772X.1979.11097738
Ghisalberti, E.L., Jefferies, P.R. and Lanteri, R., 1977. Potential drugs from propolis. In: Mass spectrometry in drug metabolism. Boston, MA: Springer US. pp. 111-130. https://doi.org/10.1007/978-1-4613-4151-2_9
Ghoshal, U.C., Singh, R. and Rai, S., 2021. Prevalence and risk factors of gastroesophageal reflux disease in a rural Indian population. Indian J. Gastroenterol., 40: 56-64. https://doi.org/10.1007/s12664-020-01135-7
Gur, N., Bayrak, N. and Topdemir, A., 2020. Determination of antimicrobial activity and some biochemical properties of honey and propolis in Turkish markets. Prog. Nutr., 22.
Hamdan, I.I., El-Sabawi, D., Darwish, R. and Dahabiyeh, L.A., 2021. Preparation, characterization and antimicrobial assessment of selected ciprofloxacin salts. Acta Pharma., 71: 365-382. https://doi.org/10.2478/acph-2021-0028
Hindi, N., Al-Charrakh, A., Naher, H.S. and Abbas, A.S., 2015. Study of chemical analysis of Iraqi propolis and active component of Proplis, Iraq. J. Sci. Microbiol., pp. 2277-3290.
Hochheim, S., Pacassa Borges, P., Boeder, A.M., Scharf, D.R., Simionatto, E.L., Yamanaka, C.N., Alberton, M.D., Guedes, A. and de Cordova, C.M.M., 2020. A bioguided approach for the screening of antibacterial compounds isolated from the hydroalcoholic extract of the native Brazilian Bee’s Propolis using mollicutes as a model. Front. Microbiol., 11: 558. https://doi.org/10.3389/fmicb.2020.00558
Jalali, M., Ranjbar, T., Mosallanezhad, Z., Mahmoodi, M., Moosavian, S.P., Ferns, G.A., Jalali, R. and Sohrabi, Z., 2020. Effect of propolis intake on serum C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) levels in adults: A systematic review and meta-analysis of clinical trials. Complement. Therapies Med., 50: 102380. https://doi.org/10.1016/j.ctim.2020.102380
Khan, M.F., Tang, H., Lyles, J.T., Pineau, R., Mashwani, Z.U.R. and Quave, C.L., 2018. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front. Pharmacol., 9: 815. https://doi.org/10.3389/fphar.2018.00815
Khondker, A., Bider, R.C., Passos-Gastaldo, I., Wright, G.D. and Rheinstädter, M.C., 2021. Membrane interactions of non-membrane targeting antibiotics: The case of aminoglycosides, macrolides, and fluoroquinolones. Biochim. Biophys. Acta (BBA)-Biomembranes, 1863: 183448. https://doi.org/10.1016/j.bbamem.2020.183448
Kuropatnicki, A.K., Kłósek, M. and Kucharzewski, M., 2018. Honey as medicine: Historical perspectives. J. Apicult. Res., 57: 113-118. https://doi.org/10.1080/00218839.2017.1411182
Kuropatnicki, A.K., Szliszka, E. and Krol, W., 2013. Historical aspects of propolis research in modern times. Evid. Based Complement. Altern. Med., https://doi.org/10.1155/2013/964149
Lind, L., Elmståhl, S., Bergman, E., Englund, M., Lindberg, E., Michaelsson, K., Nilsson, P.M. and Sundström, J., 2013. Epi Health: A large population-based cohort study for investigation of gene–lifestyle interactions in the pathogenesis of common diseases. Eur. J. Epidemiol., 28: 189-197. https://doi.org/10.1007/s10654-013-9787-x
Markham, K.R., Mitchell, K.A., Wilkins, A.L., Daldy, J.A. and Lu, Y., 1996. HPLC and GC-MS identification of the major organic constituents in New Zeland propolis. Phytochemistry, 42: 205-211. https://doi.org/10.1016/0031-9422(96)83286-9
Mašek, T., Perin, N., Racané, L., Cindrić, M., Čipčić Paljetak, H., Perić, M., Matijašić, M., Verbanac, D., Radić, B., Šuran, J. and Starčević, K., 2018. Chemical Composition, antioxidant and antibacterial activity of different extracts of poplar type propolis. Croatica Chem. Acta, 91: 81-88. https://doi.org/10.5562/cca3298
Miryan, M., Alavinejad, P., Abbaspour, M., Soleimani, D. and Ostadrahimi, A., 2021. Effects of propolis supplementation on the severity of disease in irritable bowel syndrome subjects: A randomized, double-blind clinical trial. https://doi.org/10.21203/rs.3.rs-144646/v1
Morsy, A.S., Soltan, Y.A., El-Zaiat, H.M., Alencar, S.M.D. and Abdalla, A.L., 2021. Bee propolis extract as a phytogenic feed additive to enhance diet digestibility, rumen microbial biosynthesis, mitigating methane formation and health status of late pregnant ewes. Anim. Feed Sci. Technol., 273: 114834. https://doi.org/10.1016/j.anifeedsci.2021.114834
Nada K. and Alaa H., 2015. Study of chemical analysis of Iraqi propolis and active component of propolis, Iraq. J. Sci., 5: 1095-1103.
Noori, A.L., Al-Ghamdi, A., Ansari, M.J., Al-Attal, Y. and Salom, K., 2012. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci., 9: 793. https://doi.org/10.7150/ijms.4722
Oroian, M., Dranca, F. and Ursachi, F., 2020. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. J. Fd. Sci. Technol., 57: 70-78. https://doi.org/10.1007/s13197-019-04031-x
Paviani, L.C., Fiorito, G., Sacoda, P. and Cabral, F.A., 2013. Different solvents for extraction of Brazilian green propolis: composition and extraction yield of phenolic compounds. In: III iberoamerican conference on supercritical fluid. pp. 1-5.
Perez, C., 1990. Antibiotic assay by agar-well diffusion method. Acta Biol. Med. Exp., 15: 113-115.
Pujirahayu, N., Ritonga, H. and Uslinawaty, Z., 2014. Properties and flavonoids content in propolis of some extraction method of raw propolis. Int. J. Pharm. Pharm. Sci., 6: 338–340.
Ribeiro, V.P., Arruda, C., Mejía, J.A.A., Candido, A.C.B.B., Dos Santos, R.A., Magalhães, L.G. and Bastos, J.K., 2021. Brazilian southeast brown propolis: Gas chromatography method development for its volatile oil analysis, its antimicrobial and leishmanicidal activities evaluation. Phytochem. Anal., 32: 404-411. https://doi.org/10.1002/pca.2988
Rivero-Cruz, J.F., Granados-Pineda, J., Pedraza-Chaverri, J., Pérez-Rojas, J.M., Kumar-Passari, A., Diaz-Ruiz, G. and Rivero-Cruz, B.E., 2020. Phytochemical constituents, antioxidant, cytotoxic, and antimicrobial activities of the ethanolic extract of Mexican brown propolis. Antioxidants, 9: 70. https://doi.org/10.3390/antiox9010070
Rufatto, L.C., Luchtenberg, P., Garcia, C., Thomassigny, C., Bouttier, S., Henriques, J.A.P., Roesch-Ely, M., Dumas, F. and Moura, S., 2018. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res., 214: 74-82. https://doi.org/10.1016/j.micres.2018.05.003
Santos, L.M., Fonseca, M.S., Sokolonski, A.R., Deegan, K.R., Araújo, R.P., Umsza-Guez, M.A., Barbosa, J.D., Portela, R.D. and Machado, B.A., 2020. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Fd. Agric., 100: 1369-1382. https://doi.org/10.1002/jsfa.10024
Shahbaz, M., Zahoor, T., Randhawa, M.A. and Nawaz, H., 2015. In vitro antibacterial activity of hydroalcoholic extract of propolis against pathogenic bacteria. Pak. J. Life Soc. Sci., 13: 132-136.
Simone-Finstrom, M., Borba, R.S., Wilson, M. and Spivak, M., 2017. Propolis counteracts some threats to honey bee health. Insects, 8: 46. https://doi.org/10.3390/insects8020046
Surek, M., Fachi, M.M., de Fátima Cobre, A., de Oliveira, F.F., Pontarolo, R., Crisma, A.R., de Souza, W.M. and Felipe, K.B., 2021. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol., 269: 113662. https://doi.org/10.1016/j.jep.2020.113662
Thirugnanasampandan, R., Raveendran, S.B. and Jayakumar, R., 2012. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pac. J. Trop. Biomed., 2: 651-654. https://doi.org/10.1016/S2221-1691(12)60114-2
Wiegand, I., Hilpert, K. and Hancock, R.E., 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc., 3: 163-175. https://doi.org/10.1038/nprot.2007.521
Wojtyczka, R.D., Dziedzic, A., Idzik, D., Kępa, M., Kubina, R., Kabała-Dzik, A., Smoleń-Dzirba, J., Stojko, J., Sajewicz, M. and Wąsik, T.J., 2013. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules, 18: 9623-9640. https://doi.org/10.3390/molecules18089623
Zampini, I.C., Salas, A.L., Maldonado, L.M., Simirgiotis, M.J. and Isla, M.I., 2021. Propolis from the Monte region in Argentina: A potential phytotherapic and food functional ingredient. Metabolites, 11: 76. https://doi.org/10.3390/metabo11020076
To share on other social networks, click on any share button. What are these?








