Characterization of Stromal Vascular Fraction Harvested from Inguinal Fat of Rabbit for Healing of Full Thickness Burn Wound
Characterization of Stromal Vascular Fraction Harvested from Inguinal Fat of Rabbit for Healing of Full Thickness Burn Wound
Abdul Aziz1,2, Hamad Bin Rashid1*, Muhammad Arif Khan1, Asim Khalid Mahmood1, Ayesha Hassan1, Hamid Akbar1, Sadaf Imran1, Naveed Hussain1, Muhammad Umar1, Muhammad Asif1, Sajjad Javaid1 and Mamoona Chaudhry3
1Department of Veterinary Surgery and Pet Sciences, University of Veterinary and Animal Sciences 54000 Lahore, Pakistan.
2Department of Veterinary Clinical Sciences, University of Poonch 12350, Rawalakot, Pakistan.
3Department of Epidemiology and Public Health, University of Veterinary and Animal Sciences 54000 Lahore, Pakistan.
ABSTRACT
Stromal vascular fraction (SVF) has become a promising candidate for regenerative therapy. SVF is preferred over culture-expanded stem cells due to its quick availability for clinical use and easy preparation. Burn wounds are a serious problem faced by veterinary clinicians in various animals. In this regard, the study was planned for the preparation and characterization of SVF from the adipose tissue of rabbits. Twelve healthy rabbits having body weight of 1000-1500g and of the same age were included in this study. SVF was prepared from bilateral inguinal fat that was harvested under general anesthesia. The harvested fat was enzymatically digested with 0.1% collagenase-I. It was then centrifuged and the SVF pellet was collected. The pellet was characterized through total viable cell count, cell per gram and percentage of viability by using Neubauer’s chamber. The average cell yield per microliter was 2.992±1.527 x 104 cells/µl. SVF per gram of adipose tissues was 2.992±1.527 x 106 cells/g, percentage of viability was 97.98± 0.31%, while non-viable cell count was 2.02± 0.31%. It is concluded that enzymatic digestion is an easy technique requiring less time for SVF isolation and gives better cell yield per gram. SVF preparation process has advantages of minimum cell contamination and processing time. Hence, it is a cost effective and alternate procedure for developing countries like Pakistan.
Article Information
Received 26 April 2022
Revised 23 June 2022
Accepted 09 July 2022
Available online 14 November 2022
(early access)
Published 19 December 2023
Authors’ Contribution
HBR, MAK, AKM, MC, SI and AA conceptualized the idea of this manuscript. AA, MU and MA conducted the research, prepared the manuscript. AA and MC analyzed the data. SJ and NH edited the manuscript. HBR, AH, HA and MC reviewed the manuscript. All authors read and approved the final manuscript.
Key words
Adipose tissue, Cell viability, Burn wound, Characterization of SVF, Stem cell, Rabbit
DOI: https://dx.doi.org/10.17582/journal.pjz/20220426060423
* Corresponding author: [email protected]
0030-9923/2024/0001-0347 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Stromal vascular fraction (SVF) of adipose tissue is regarded as the treatment of choice for various abnormalities. It contains a heterogeneous population of progenitor cells, stem cells, and adult cells (Mehranfar et al., 2019). It also possesses blood cells, fibroblasts, pericytes, endothelial cells, and adipose-derived stem cells (ASCs) (Bashir et al., 2019). SVF has many factors which produces anti-inflammatory and analgesic effects. It stimulates immune-modulatory results which are beneficial for immune suppression of burn wounds (Ozturk and Karagoz, 2015). It also has the advantage of easy isolation, easy preparation and minimum lab work. Furthermore, harvesting of adipose tissue is less invasive as compared to bone marrow aspiration. The tissue of adipose origin contains 500 times greater stem cell count than bone marrow in one gram of tissue (Choudhery et al., 2013). Cell passage for mesenchymal stem cell (MSC) culture needs prolonged duration and extensive laboratory work. At the same time, processing for SVF may be performed in a shorter time and is readily available for clinical usage. Culture expanded MSC preparation mostly takes some days to weeks, while SVF preparation may be processed within hs. The process comprises harvesting of adipose tissue from an appropriate position by lipo-aspiration or lipectomy and then limited manipulation of tissues by manual mincing and washing with phosphate buffer saline (PBS). It is then digested by collagenase enzyme and neutralized by control media and finally centrifuged. The end pellet after centrifugation is called as SVF, which is ready to use in clinical conditions (Almeida et al., 2010; Kim et al., 2012; Ba et al., 2019).
Burn is the damaging of body tissue caused by elevated temperatures like steam or fire. Certain chemicals like alkali, acid, or heavy metals can also cause burn. It can also be caused by electric shock and radiations. At the same time, scalds are caused by severely low temperature like snow or ice. Burn injury causes a sudden release of toxins in the blood leading to dramatic elevation of prostaglandins, histamine, serotonin, and electrolytes. In response to these agents, tissue permeability increases, and plasma oozes out from vessels. This extravasation of plasma leads to blood thickening and dehydration resulting into hypovolemic shock and sometimes death (Efimova et al., 2019). Burns, diabetic ulcers, pressure ulcers, and venous necrosis are factors of delayed wound healing. In burn, a dynamic process of deepening in the injury occurs with passage of time which causes maximum tissue damage and hypertrophic scar formation (Liu et al., 2008).
Burn also causes the denaturation of proteins, resulting into delayed inflammatory stage which ultimately affect wound healing. Burn cases are the most challenging problems for medical and veterinary clinicians, as it leads to hemodynamic instability and hypovolemic shock. This difficulty is further aggravated by the limited availability of recommended treatment regimens in veterinary sciences. No burn registry program has been yet established in Pakistan in medical field to report burn incidence statistics (Riaz et al., 2020), same is the case in veterinary sciences. The scarcity of literature is the main problem in the veterinary field, hence, in most instances, information from human medicine is utilized. SVF is considered a novel treatment for burn wound management. No research has been conducted so far in Pakistan on pet and farm animals to characterize the SVF for viable cell count, percent viability. Therefore, this study was conducted to characterize the SVF harvested from adipose tissue of rabbits for various clinical applications, especially focusing on burn wound healing.
MATERIALS AND METHODS
Ethical approval
This study was approved in compliance with the Institutional Guidelines of Ethical Review Committee, Office of Research Innovation and Commercialization at University of Veterinary and Animal Sciences (UVAS), Lahore (vide No. DR/454 dated 06/10/2020).
Experimental animals and station
Twelve healthy rabbits of 1000-1500 g of same age were used in this study. Rabbits were purchased from local market of Lahore, Pakistan. They were acquired irrespective of breed, sex and color. They were housed in individual cages made of metallic wire at Laboratory Animal Room, Surgery Section, Department of Veterinary Surgery and Pet Sciences, Faculty of Veterinary Science, University of Veterinary and Animal Sciences, Lahore. All the rabbits were acclimatized for the period of one week. Normal physical status of all the rabbits were evaluated before the initiation of the trial. All surgical procedures were performed in Surgery Operation Theater, Department of Veterinary Surgery and Pet Sciences. Adipose tissues were harvested from inguinal fat pad and transported in phosphate buffer saline (PBS) for in-vitro procedures in laboratory.
Harvest of adipose tissue
For harvesting of adipose tissue, all rabbits were anesthetized by intramuscular injection of xylazine (Farvet Laboratories Netherlands) @ 5 mg/kg b.w. and followed by ketamine (Mylab Pakistan) @ 50 mg/kg b.w. (Abramov et al., 2007). Caudal midline incision was given starting from midway between umbilicus and extended up to pubis. Using a tissue forceps bilateral subcutaneous inguinal fat pad was elevated to exteriorize and resected. Harvested adipose tissue was collected in 50 ml falcon tube containing PBS (Gibco) which was treated with 1% penicillin and streptomycin antibiotics. Adipose tissue was weighed to obtain 5 g of tissue for further processing.
Isolation of SVF
Harvested adipose tissue was transferred in a plastic petri dish from the falcon tube and washed twice with phosphate buffer saline. Tissue debris and connective tissues were separated with the help of two fine tipped forceps. Similarly, all the vessels were separated and white fat tissues were transferred into another petri dish. Adipose tissue was then finely minced with scalpel blade no. 20. Minced fat was then transferred in another falcon tube and digested with 0.1% collagenase-I (Gibco) containing 1% penicillin and streptomycin. Digestion of adipose tissue was achieved by placing it into an incubator at temperature 37℃ for 1 h with intermittent agitation after 15 min. After 1 h, neutralization of collagenase-I was performed with control medium containing Dulbecco’s modified eagle’s medium (DMEM), (Gibco) 10% fetal bovine serum (FBS) (Gibco), penicillin and streptomycin. All the aliquot mixtures were filtrated through 100-μm cell strainer (Bilologix Group Limited). Mixture was then centrifuged for 10 min at 1200 rpm. Pellet accumulated at the bottom of centrifuge tube was collected as stromal vascular fraction. SVF pellet was resuspended in control medium (DMEM) to make the final volume of 0.5 ml for utilization in cell characterization and standardization.
Characterization of SVF
Isolated SVF was characterized for cell count, viability, cell yield per gram, and percent viability through manual cell counting method using Neubauer’s chamber (hemocytometer) (Germany). Equal volume (10 µL) of both sample and trypan blue stain was loaded in chamber of hemocytometer with the help of micropipette (Thermo Fisher). Neubauer’s slide was kept in undisturbed state for 5 min to allow the cells and stain to settle evenly in the chambers. Counting was done under microscope (40x) in four corner chambers and in the center chamber.
Total SVF count (cells/µL), number of non-viable cells, number of viable cells, SVF cell yields/ g of adipose tissue, percentage of viable and non-viable cells were calculated as follows.
Total cell count was calculated as cell population density for counting of viable and dead SVF cells. Total SVF count (cells/µL) was performed by the formula given below as described by (Sharun et al., 2021).
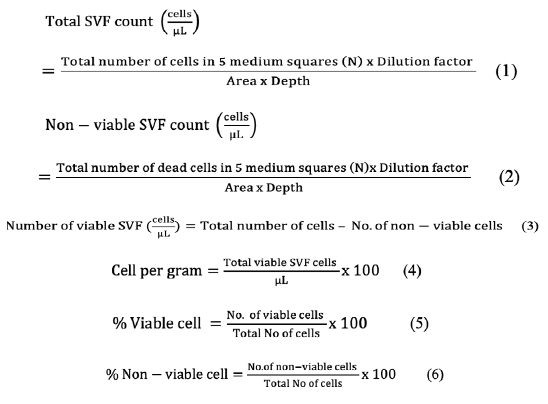
RESULTS
Average total cells of all rabbits counted in 5 designated squares were 610.667±31.137 cells (Mean±SD) (Table I). This number was utilized for calculation of the number of viable and nonviable cells. Mean total cells per microliter was 3.053±1.556 x 104 cells/µl. Average non-viable cells counted in 5 designated squares was 12.33 ±2.103 cells (Mean±SD) hence, calculated non-viable cells were 616.6 ±105.169 cells/ µL) (Table I).
Number of viable cells per µl became 2.992±1.527 x104 cells/µl. The mean viable SVF cell per gram was 2.992±1.527 x 106 cells/g (Fig. 1). While mean non-viable cell per gram was 6.166±1.052 x 104 cells/g.
It was observed that percent viability in current study showed 97.98± 0.31%, while non-viable cell present was 2.02± 0.31% (Table I).
DISCUSSION
Current study was conducted to characterize the harvested from inguinal fat of rabbits. Inguinal fat pad is an ideal site for harvesting of fat tissue. It provides ample
Table I. Total SVF cell count, SVF cell per µl, SVF cell yield per g and percentage of viable and non-viable cells in rabbits.
|
Mean±SD |
|
|
Total cells in 5 squares |
610.66±31.13 |
|
No of non-viable cells in 5 squares |
12.33±2.10 |
|
No of viable cells in 5 squares |
598.33±30.54 |
|
SVF per µl (104 cells/µl) |
2.99±1.52 |
|
SVF cell yield per g (106 cells/g) |
2.99±1.52 |
|
Percent viability |
97.98±0.31 |
|
Percent non-viability |
2.02±0.31 |
amount of tissue as compared to other sites. Inguinal site has been chosen by authors like Almeida et al. (2010), Kim et al. (2012), Behfar et al. (2014), Ni et al. (2015). Other anatomical positions preferred by researchers for SVF harvest are interscapular adipose tissue of rabbit (Sharun et al., 2021), from the suprascapular side (Kim et al., 2012), breast, abdomen, deep flank, right upper arm, or from the thigh (Choudhery et al., 2015; Jurgens et al., 2008), and from dorsal fat pad (Ba et al., 2019). SVF has been harvested from rabbits by surgical isolation with lipectomy at suprascapular site (Kim et al., 2012). One disadvantage of inguinal site is self-mutilation of the wound area. This problem may be prevented by applying neck color to the rabbits and pain management.
In the current study SVF cell characterization was conducted manually using Neubaur’s chamber hemocytometer. Number of viable cells per microliter was 2.99±1.52 x 104 cells/µl while the cell yield per gram of adipose tissue was 2.99±1.52 x 106 cells/g. Similar result was reported by Sharun et al. (2021) with total cell count 3.15±0.09 x104 cells/µL while SVF cell yield per gram was 3.15±0.09 x 106 cells/g. They harvested adipose tissue from interscapular fat tissue from New Zealand white rabbits. Although both of these studies used different harvest site and different collagenase enzyme but results are quite similar (Sharun et al., 2021). In a study SVF cell count was conducted by hemocytometer after extraction through manual and automatic methods. Manual extraction revealed 250,000±34,782 nucleated cells/ml, while automatic revealed 50,000±6,956 nucleated cells/ml from fat tissue. In automatic technique no digestion with collagenase was done, hence less cell count was obtained. Manual and automatic extraction methods may be the cause of difference in cell counts. Similarly, enzymatic and nonenzymatic digestion methods are also applied (Cervelli et al., 2011). In another study cell yield per gram was 2±0.5 x 106 cells/g (Behfar et al., 2012). In a study mean number of cells with enzymatic digestion was 3.38±3.63 x 106 cells/ml, while mechanical digestion was 1.34±1.69 x 106 cells/ml. According to their study the differences between two methods was 30 to 50%. These differences in cell yield were probably due to inappropriate release of stromal cells from adipose tissue matrix (Tiryaki et al., 2020).
Percentage of viability in current study showed 97.98± 0.31%, while non-viable cell present was 2.02± 0.31%. In a study cell viability percentage was 82% ranging from 62% to 97%. Cell viability was 597.75±338.4 x 105 cells/g. This disagreement in result may be due to lipoaspirates they used instead of resected fat tissue to enumerated cells. (Carstens et al., 2017). In another study cell viability was 82.86%±10.86 by enzymatic digestion while in mechanical digestion it was 85.82%±5.74 (Tiryaki et al., 2020).
In the current study SVF cell characterization was conducted by trypan blue exclusion technique. Trypan blue is vital stain and is consider as gold standard for cell density and cell viability. Nonviable cell gets stained with blue color while viable cell appeared transparent without stain. Cell membrane of the viable cells remain intact and does not take color while nonviable cell got stain due to nonfunctional and ruptured membrane hence appeared blue (Louis and Siegel, 2011). Chemical formula of trypan blue stain is C34H28N6O14S4. This stain binds intra cellular protein so, nonviable cells appeared blue irrespective of apoptotic cells and necrotic cells. Hence, some non-necrotic apoptotic cells appeared like normal cells. Different cell biology and stem cell culture labs are routinely using this technique for cell viability. It is also used for evaluation of cancer reduction and drug testing (Piccinini et al., 2017).
SVF has been extensively studied for last few decades and it is an approach that may be extendable to any species. Researchers studied effects SVF in domestic and laboratory animals such as in dog (Hendawy et al., 2021), and in sheep (Lv et al., 2018). Similarly, SVF was also used to cure neck wrinkle in human (Cai et al., 2020). In this study SVF was estimated per gram of adipose tissue and revealed 2.99±1.52 x 106 cells/g of SVF. As a result, the final dose obtained using this procedure is clinically useful. Required dose of SVF in rabbit for repaired of tendon was 4x106 cells of fresh SVF (Behfar et al., 2014), while dose in Wistar rats was 50,000 cells (Josh et al., 2021). Similarly, Bukowska et al. (2020) used 0.25 - 2.5 x 106 cells in mice. So, the number of generated cells in this study was ample to be used clinically. In Pakistan these techniques are limited to preclinical study level or in some human practices. No commercial distribution is reported to date for supply of fresh SVF. Furthermore, autologous SVF can be prepared at the point of care station (Andia et al., 2019). In veterinary practice SVF have been studied for the treatment of joint diseases, wound healing enhancement and other clinical situations (Kemilew et al., 2019). In our situations clinical use is not started in veterinary practices. It may be facilitated by collaboration between veterinary clinicians with academic institution. Harvest of adipose tissue sources may be facilitated by veterinarian and it may be processed in a laboratory setting by skilled individual for the preparation of SVF. The final SVF is ready to be injected and returned back to clinician for use.
CONCLUSION
It is concluded that inguinal fat pad is an ideal site for harvesting of appropriate amount of adipose tissue. In this study average cell yield was 2.99±1.52 x 104 cells/µl. This area and technique give better cell per gram count as compared to non-enzymatic techniques. SVF cell per gram obtained from adipose tissue was 2.992±1.527 x 106 cells/g, and viability percentage was 97.98± 0.31%, while non-viable cell was 2.02± 0.31%. It is also concluded that enzymatic digestion is an easy technique requiring less time for SVF isolation. It is a cost effective and alternative procedure for developing countries like Pakistan. It will open a new modality for treatment. It is recommended to use these approaches that, adipose tissue harvested from inguinal fat yield required dose of SVF per gram and percent viability.
ACKNOWLEDGEMENTS
We acknowledge Dr Azra Mehmood and Hafiz Ghufran from Center for Excellence in Molecular Biology (CEMB), University of the Punjab, Lahore for training and technical assistance. Abdul Aziz received faculty development scholarship from Higher Education Commission (HEC), Pakistan.
Statement of conflict of interest
The authors have declared no conflict of interest
REFERENCES
Abramov, Y., Golden, B., Sullivan, M., Botros, S.M., Miller, J.J.R., Alshahrour, A., Goldberg, R.P. and Sand, P.K., 2007. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen., 15: 80-86. https://doi.org/10.1111/j.1524-475X.2006.00188.x
Almeida, F.G., Nobre, Y.T.D., Leite, K.R. and Bruschini, H., 2010. Autologous transplantation of adult adipose derived stem cells into rabbit urethral wall. Int. Urogynecol. J., 21: 743-748. https://doi.org/10.1007/s00192-009-1090-8
Andia, I., Maffulli, N. and Burgos-Alonso, N., 2019. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther., 19: 1289-1305. https://doi.org/10.1080/14712598.2019.1671970
Ba, K., Wei, X., Ni, D., Li, N., Du, T., Wang, X. and Pan, W., 2019. Chondrocyte co-cultures with the stromal vascular fraction of adipose tissue in polyhydroxybutyrate/poly-(hydroxybutyrate-co-hydroxyhexanoate) scaffolds: Evaluation of cartilage repair in rabbit. Cell Transplant., 28: 1432-1438. https://doi.org/10.1177/0963689719861275
Bashir, M.M., Sohail, M., Ahmad, F.J. and Choudhery, M.S., 2019. Preenrichment with adipose tissue-derived stem cells improves fat graft retention in patients with contour deformities of the face. Stem Cells Int., 2019: 5146594. https://doi.org/10.1155/2019/5146594
Behfar, M., Javanmardi, S. and Sarrafzadeh-Rezaei, F., 2014. Comparative study on functional effects of allotransplantation of bone marrow stromal cells and adipose derived stromal vascular fraction on tendon repair: a biomechanical study in rabbits. Cell J. (Yakhteh), 16: 263.
Behfar, M., Sarrafzadeh-Rezaei, F., Hobbenaghi, R., Delirezh, N. and Dalir-Naghadeh, B., 2012. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Curr. Stem Cell Res. Ther., 7: 173-178. https://doi.org/10.2174/157488812799859874
Bukowska, J., Alarcon Uquillas, A., Wu, X., Frazier, T., Walendzik, K., Vanek, M. and Gimble, J.M., 2020. Safety of human adipose stromal vascular fraction cells isolated with a closed system device in an immunocompetent murine pressure ulcer model. Stem Cells Dev., 29: 452-461. https://doi.org/10.1089/scd.2019.0245
Cai, J., Wang, J., Hu, W. and Lu, F., 2020. Mechanical micronization of lipoaspirates for the treatment of horizontal neck lines. Plast. Reconstr. Surg., 145: 345-353. https://doi.org/10.1097/PRS.0000000000006456
Carstens, M., Pérez, M., Briceño, H., Valladares, S. and Correa, D., 2017. Treatment of late sequelae of burn scar fibrosis with adipose-derived stromal vascular fraction (SVF) cells: A case series. Cell R4, 5: e2404.
Cervelli, V., Gentile, P., De Angelis, B., Calabrese, C., Di Stefani, A., Scioli, M.G., Curcio, B.C., Felici, M. and Orlandi, A., 2011. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res., 6: 103-111. https://doi.org/10.1016/j.scr.2010.11.003
Choudhery, M.S., Badowski, M., Muise, A. and Harris, D.T., 2013. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy, 15: 330-343. https://doi.org/10.1016/j.jcyt.2012.11.010
Choudhery, M.S., Badowski, M., Muise, A., Pierce, J. and Harris, D.T., 2015. Subcutaneous adipose tissue–derived stem cell utility is independent of anatomical harvest site. Biol. Res. Open Access, 4: 131-145. https://doi.org/10.1089/biores.2014.0059
Efimova, O., Dimitrieva, A., Nesterova, O., Aldyakov, A., Obukhova, A. and Ivanova, T., 2019. Methods for the effective treatment of animal burns. IOP conference series: Environ. Earth Sci., 346: 012057. https://doi.org/10.1088/1755-1315/346/1/012057
Hendawy, H., Uemura, A., Ma, D., Namiki, R., Samir, H., Ahmed, M.F. and Tanaka, R., 2021. Tissue harvesting site effect on the canine adipose stromal vascular fraction quantity and quality. Animals, 11: 460. https://doi.org/10.3390/ani11020460
Josh, F., Soekamto, T.H., Adriani, J.R., Jonatan, B., Mizuno, H. and Faruk, M., 2021. The combination of stromal vascular fraction cells and platelet-rich plasma reduces malondialdehyde and nitric oxide levels in deep dermal burn injury. J. Inflamm. Res., 14: 3049. https://doi.org/10.2147/JIR.S318055
Jurgens, W.J., Oedayrajsingh-Varma, M.J., Helder, M.N., ZandiehDoulabi, B., Schouten, T.E., Kuik, D.J., Ritt, M.J. and Van Milligen, F.J., 2008. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res., 332: 415-426. https://doi.org/10.1007/s00441-007-0555-7
Kemilew, J., Sobczynska-Rak, A., Zylinska, B., Szponder, T., Nowicka, B. and Urban, B., 2019. The use of allogenic stromal vascular fraction (SVF) cells in degenerative joint disease of the spine in dogs. In Vivo, 33: 1109-1117. https://doi.org/10.21873/invivo.11580
Kim, A., Kim, D.H., Song, H.R., Kang, W.H., Kim, H.J., Lim, H.C., Cho, D.W. and Bae, J.H., 2012. Repair of rabbit ulna segmental bone defect using freshly isolated adipose-derived stromal vascular fraction. Cytotherapy, 14: 296-305. https://doi.org/10.3109/14653249.2011.627915
Liu, P., Deng, Z., Han, S., Liu, T., Wen, N., Lu, W., Geng, X., Huang, S. and Jin, Y., 2008. Tissue-engineered skin containing mesenchymal stem cells improves burn wounds. Artif. Organs, 32: 925-931. https://doi.org/10.1111/j.1525-1594.2008.00654.x
Louis, K.S. and Siegel, A.C., 2011. Cell viability analysis using trypan blue: Manual and automated methods. In: Mammalian cell viability (ed. M. Stodarrt). Methods Mol. Biol. (Methods and Protoc.) Springer, 740: 7-12. https://doi.org/10.1007/978-1-61779-108-6_2
Lv, X., He, J., Zhang, X., Luo, X., He, N., Sun, Z. and Wang, W., 2018. Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplant., 27: 1111-1125. https://doi.org/10.1177/0963689718773333
Mehranfar, S., Abdi Rad, I., Mostafavi, E. and Akbarzadeh, A., 2019. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: An overview of clinical trials. Artif. Cells Nanomed. Biotechnol., 47: 882-890. https://doi.org/10.1080/21691401.2019.1576710
Ni, Y., He, X., Yuan, Z., Liu, M., Du, H. and Zhong, X., 2015. Effect of fat particle-to-SVF ratio on graft survival rates in rabbits. Annls Plast. Surg., 74: 609-614. https://doi.org/10.1097/SAP.0b013e318298e6f5
Ozturk, S. and Karagoz, H., 2015. Experimental stem cell therapies on burn wound: Do source, dose, timing and method matter? Burns, 41: 1133-1139. https://doi.org/10.1016/j.burns.2015.01.005
Piccinini, F., Tesei, A., Arienti, C. and Bevilacqua, A., 2017. Cell counting and viability assessment of 2D and 3D cell cultures: Expected reliability of the trypan blue assay. Biol. Proced. Online, 19: 1-12. https://doi.org/10.1186/s12575-017-0056-3
Riaz, R., Riaz, L., Khan, J. and Baloch, M., 2020. Survey on knowledge of first aid management of burns amongst medical and non-medical students in Karachi, Pakistan: Need for an educational intervention? Cureus, 12: e6674. https://doi.org/10.7759/cureus.6674
Sharun, K., Pawde, A.M., Kumar, R., Kalaiselvan, E., Kinjavdekar, P., Dhama, K. and Pal, A., 2021. Standardization and characterization of adipose-derived stromal vascular fraction from New Zealand white rabbits for bone tissue engineering. Vet. World, 14: 508. https://doi.org/10.14202/vetworld.2021.508-514
Tiryaki, T., Condé-Green, A., Cohen, S.R., Canikyan, S. and Kocak, P., 2020. A 3-step mechanical digestion method to harvest adipose-derived stromal vascular fraction. Plast. Reconstr. Surg. Glob. Open, 8: e2652. https://doi.org/10.1097/GOX.0000000000002652
To share on other social networks, click on any share button. What are these?









