Advances in Animal and Veterinary Sciences
Research Article
Histopathological Alterations in Gills, Liver and Kidney of Common Carp, Cyprinus Carpio L. Exposed to Lead Acetate
Sanaa A. Mustafa, Jamal K. Al-Faragi, Noor M. Salman, Abdulmotalib J. Al-Rudainy
Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq.
Abstract | The current study was undertaken to investigate the histopathological alterations of gills, liver and kidney in common cap, Cyprinus carpio exposed to lead acetate [Pb(CH3COO)2] for a period of 60 days (LC50 4.24 mg l-1). For this rationale, a total of 100 fish were divided into five treatment groups: control group (untreated); T1 exposed to 0.21 mg l-1Pb; T2 were exposed to 0.21 mg l-1Pb; T3 were exposed to 0.42 mg l-1Pb; T4were exposed to 0.42 mg l-1. During the period of experimental exposure, water was changed regularly at 24 hr intervals with the same amount of the toxicant (stock solutions) except for T1 and T3 the water was replaced daily without adding toxicant. The main histopathological changes caused by Pb observed in gills of T4 were edema in the filamentary epithelium, lifting of lamellar epithelia, hyperplasia of the epithelium with fusion of adjacent lamellae and necrosis in primary and secondary lamellae. The changes are lesser in extent in gills of T1, T2 and T3. The hepatic alterations were more evident in fish exposed to higher concentrations (T4) were cytoplasmic vacuolation, hepatic necrosis with piknotic nucleus. Kidneys exhibited increasing degrees of damage in the tissues in association with Pb concentration, the main alterations were observed in kidney of T4 which including: hydrobic degeneration and necrosis of renal tubules with nuclear piknosis. It can be concluded that gills, kidney and hepatic alterations as a consequence of lead exposition of fish could be act as a sensitive bio-indicator for the toxicity of sub-lethal concentrations of heavy metals as well as other of anthropogenic origin.
Keywords | Cyprinus carpio, Heavy metal, Histopathology, Leadacetate
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 08, 2017; Accepted | August 28, 2017; Published | September 06, 2017
*Correspondence | Noor M Salman, Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq; Email: [email protected]
Citation | Mustafa SA, Al-Faragi JK, Salman NM, Al-Rudainy AJ (2017). Histopathological alterations in gills, liver and kidney of common carp, cyprinus carpio l. Exposed to lead Acetate. Adv. Anim. Vet. Sci. 5(9): 371-376.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.9.371.376
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Mustafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Water pollution is considered as one of the main problem of this century resulting from the addition of several contaminants in water systems via many ways and they changes their natural qualities of water (Elezaby et al., 2001). The contamination of environment with heavy metals has become a source of concern not only because their threats to aquatic organisms but also due to public health implications of such pollutants.
Lead (Pb) and its compounds are dangerous pollutants of the aquatic environment (Nordberg et al., 2007). Lead occurs naturally in the environment as well as being produced by many industries and enters aquatic environments with wastes from mines, chemical factories, paints, etc. (Health, 1987). Several researchers indicated that toxic and non-biodegradable metals such as lead accumulate in many fish species, causing various diseases such as renal lesion (Lliopoulou- Georgudaki and Kotsanis 2001; Gordon et al., 2002), hepatic lesions (Bano and Hasan, 1990) endocrine dysfunction, and effect of cell membrane lipids in cells of the central nervous system Veena et al. (1997), Park et al. (2006); Berrahal et al. (2007) and Mobarak and Sharaf (2011) reported that exposure to low level of lead during the early stage of development can cause neurological, gastrointestinal, reproductive, circulatory, physiological, histopathological and his to chemical disorders in the aquatic organisms.
Gills are considered the first target organ affected by contaminants in the water due to the persistent contact with the external environment (Perry and Laurent, 1993). The study of fish liver and kidney is also very important in the field of aquaculture induced by many problematic condition and aquatic pollution (Au, 2004). The examination of histopathology of various organs may therefore, is a highly sensitive and accurate tool to assess the effects of xenobiotic compounds in field and experimental studies which reflecting the health of entire aquatic environment (Thophon et al., 2003). Hence, this study was undertaken to investigate the effects of different levels of lead on histopathological aspects in selected organs (gills, liver and kidney) of C. carpio.
Materials and Methods
Fish Maintenance and Experimental Design
A total of 100 healthy C. carpio (30 ± 3 g) were obtained from a local fish farm (Babylon, Iraq). Fishes immediately were transported to the fish laboratory and reared with a continuous water and airflow system. The water quality parameters were checked daily and were within the values described for this species (Ivanova and Hadjinikolova, 2013): temperature (23.0 ± 0.39 °C), dissolved oxygen (6.7 ± 0.34 mgl–1) and pH (7.6 ± 0.04). Fish were kept in these conditions for two weeks for acclimatization before being used in the experimental study. Fish fed a commercial diet at a rate of 2% of average body mass twice daily. Before starting the experiment, a 96 h acute toxicity test was achieved for lead, and 96 h LC50 value was calculated using the probit analysis (4.24 mg l-1). The stock solution of lead as lead acetate [Pb(CH3COO)2] was prepared and kept in clean glass bottles and diluted to concentration of 0.21 mg l-1 and 0.42 mg l-1.
After the acclimatization period, fish were distributed randomly in 10 tanks (10 fish per tank) and were divided into 5 groups (2 tank per treatment), as the following: the first group set as control group (C) without exposed to lead; the second group (T1) exposed to 0.21 mg l-1Pb (CH3COO)2; the third group (T2) were exposed to 0.21 mg l-1Pb (CH3COO)2; the forth group (T3) were exposed to 0.42 mg l-1Pb (CH3COO)2; the fifth group (T4) were exposed to 0.42 mg l-1Pb (CH3COO)2. The treatment groups exposed to lead for 60 days. During the period of experimental exposure (i.e. 60 days) water was changed regularly at 24 hr intervals with the same amount of the toxicant (stock solutions) except for T1 and T3 the water was replaced daily without adding toxicant. No fish mortality was observed during the experiment. At the end of specified period of experimental exposure, tissues were dissected out for histopathlogical examination after sacrificing the fish.
Histopathological Study
Tissues (gills, liver and kidney) were collected from the exposed as well as from control fish and preserved in 10% formaldehyde solution as a fixatives for 48 hr. The technique of Bernet et al. (1999) was adopted for processing of tissues for histopathological studies. Briefly, tissues were dehydrated in different graded series of ethanol alcohol. Followed by clearing in xylene and then embedded in paraffin wax. After that, the sagittal sections (6 µm thickness) were cut using a rotary microtome and mounted on glass slides. Sections were deparaffinized in xylene, hydrated in alcohol and stained with haematoxylin and eosin (HE) for general histological examinations. Photomicrographs of stained sections were made under light microscope. Changes induced by treatment in the gills, kidney and liver tissues were photographed and analyzed using a digital camera (Optika camera) at a total magnification 400×.
Results and Discussion
Histopathological Study
A. Histopathological Alterations Of Gills
Histopathological study of the gills revealed a typical structural organization of the lamellae in the control group without pathological alterations (Figure 1 A). After 60 days exposure to different concentrations of Pb, T1 exhibited hyperplasia of the epithelium (Figure 1 B). The main changes observed in T2 were hyperplasia of the epithelial and accentuated lifting of the lamellar epithelium (Figure 1C). In addition, telangiectasis were also observed at gills secondary lamellae (Figure 1D). T3 group showed extensive epithelial lifting and hyperplasia of the epithelium (Figure 2E) The severe changes were found in T4 which including edema in the filamentary epithelium, dilation of the central venous with blood congestion, hyperplasia of the epithelium with fusion of adjacent lamellae in many areas as a result of filamentary epithelium proliferation. In addition, necrosis in primary and secondary lamellae were also observed (Figure 2 F, G and H). Histopathological studies have been used as essential tool in environmental monitoring that permits examining specific target organs. The histological results observed in all the tissues of C. carpio in the present study indicate that sub lethal concentrations of Pb caused moderate to severe alterations in gills structure, which are an important organs performing vital function like respiration, detoxification, osmoregulation, etc. Different concentrations of Pb used in this study showed various degree of histopathological changes in gills such as epithelial hyperplasia, epithelial lifting, ede-
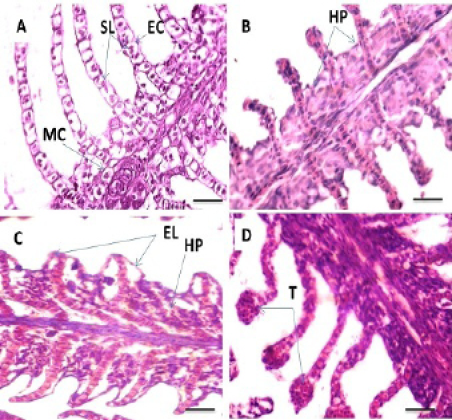
Figure 1: Photomicrograph showing histological structures through gillss of C.carpio exposed to different concentrations of Pb. (A): control gills showing secondary lamellae (SL), epithelial cells (EC) and mucous cells (MC). (B): T1 showing hyperplasia of the epithelial cells (HP). (C&D): T2 demonstrating hyperplasia of the epithelial cells (HP), epithelium lifting (EL) and telangiectasis (T). H&E stain; Thickness 6 μm. Scale bars 50μm.
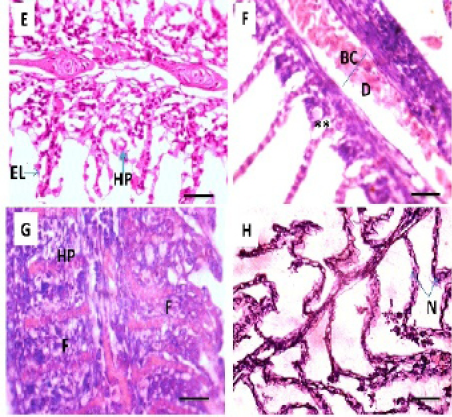
Figure 2: Photomicrograph showing histological structures through gillss of C.carpio exposed to different concentrations of Pb. (E): T3 showing epithelial lifting (EL), hyperplasia of the epithelium (HP). (F, G &H): T4 showing edema in the filamentary epithelium (**), dilation of the central venous (D) with blood congestion, hyperplasia of the epithelium (HP), fusion of the secondary lamellae (F) and necrosis (N) in primary and secondary lamellae. H&E stain; Thickness 6 μm. Scale bars 50μm.
ma, bloodcongestion of the central venous, telangiectasis, fusion of the secondary lamellae and necrosis. Epithelial lifting and hyperplasia of undifferentiated epithelial cells are known to be nonspecific alterations, which can be caused by heavy metals (Randy et al.,1996). Epithelial lifting, hyperplasia reactions could result in dysfunctional or even non- functional gills, and ultimately asphyxiate the fish. These pathological changes may be a reaction to toxicants intake or could be interpreted as a general defense mechanism to prevent the entry of the pollutants thorough the gills surface and probably due to increased capillary permeabilit y (Mallat, 1985).
These changes in the gills epithelia were observed by Thophon et al. (2003) in Lates calcarifer exposed to cadmium. These results are also in agreement with Hadi and Alwan (2012) who reported similar changes in gills of fresh water fish, Tilapia zillii, exposed to aluminum. Similar results are also observed by Figueiredo-Fernandes et al. (2007) in Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. These findings also, were found in fish exposed to other pollutants in the freshwater fish (Puntius gonionotus, Oreochromis niloticus), exposed to pesticides paraquat, and dimethoate (Cengiz and Unlu, 2006; Elezaby et al., 2001) respectively.
B. Histopathological alterations of liver
The current study reveals that the liver of control fish exhibits a normal architecture and there were no histopathological abnormalities. Thehepatocytes present a homogenous cytoplasm and a large central or sub-central spherical nucleus (Figure 3 A). Hepato-pancreatic alveoli of the exocrine pancreas were seen to be placed in the parenchyma of liver (Figure 3 B). The histopathological appearance of liver following exposure to Pb showed various histopathological changes; T1 and T2 exhibited patchy degeneration (Figure 3 C and D). T3 showed cytoplasmic vacuolation (Figure 3 E). The main changes were found in T4 including cytoplasmic vacuolation, hepatic necrosis and picnotic nucleus (Figure 3 F).
The observed hepatic vacuolations could be due to glycogen and/or lipid deposition which are related to the normal metabolic function of the liver (Myers et al., 1987). Pacheco and Santos (2002) described increased vacuolization of the hepatocytes as a signal of degenerative process that suggests metabolic damage, possibly related to exposure to contaminated water. While, vacuole formation was considered by Mollendroff (1973) as a cellular defense mechanism against materials injurious to hepatocytes. This finding is in line with Figueiredo-Fernandes et al. (2007) who observed that in Nile tilapia Oreochromis niloticus exposed to Cu 2.5mg l-1 of waterborne copper for a period of 21 days.
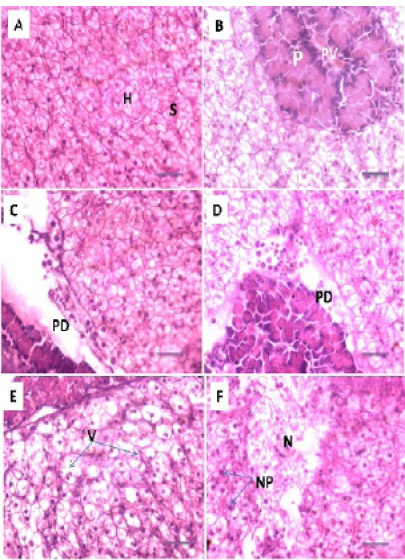
Figure 3: Photomicrograph showing histological structures through liver of C.carpio exposed to different concentrations of Pb. (A& B): control liver showing normal architecture of hepatic cells (H), sinusoids (S), portal vein (PV) with pancreatic tissue (P). (C&D): T1 & T3 showing patchy degeneration (PD). (E& F): T3 &T4 demonstrating cytoplasmic vocuolation (V), necrosis (N) with nuclear piknosis (NP). H&E stain; Thickness 6 μm. Scale bars 50μm.
While the hepatic necrosis and degenerative changes in the affected liver might be due to accumulative effect of metals in hepatic tissue exposed to higher concentration of Pb. Fish commonly accumulate greater levels of metals in liver than gills (Al-Yousuf et al., 2000; Mohamed, 2008). These hepatic lesions might probably be due to the major function of the liver in the metabolism and excretion of toxic agents where some morphological changes do occur in the process (Rocha and Monteiro, 1999). Similar results are observed by Udotong (2015) in Tilapia fish exposed to heavy metals (Fe, Pb and Cu). Also, Our results are in agreement with Abalaka (2015) who noted that Auchenoglanis occidentalis fish from Tiga dam, showed extensive necrosis of the liver. Similar changes were also observed by Hadi and Alwan (2012) in freshwater fish, Tilapia zillii, exposed to aluminum100 µg l-1) for a period 96 hour. Although liver histopsthological changes are not commonly specific to pollutants. However, there is a relationship between metal levels and fish hepatic lesions has been noted (Au, 2004). Hepatocytes could therefore be predictable the main targets of toxicants, providing an excellent bio-indicator of aquatic. The monitorization of histological changes in fish liver is a highly sensitive and accurate way to investigate the effects of xenobiotic compounds in aquaculture studies (Figueiredo-Fernandes et al., 2007).
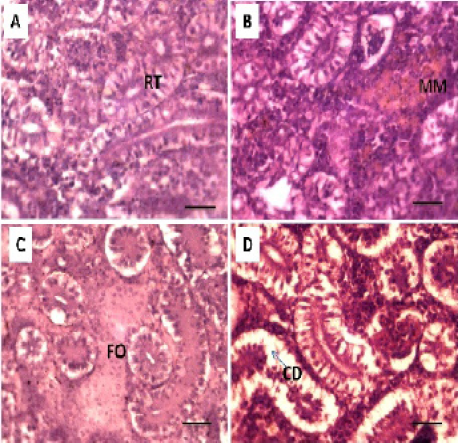
Figure 4: Photomicrograph showing histological structures through kidney of C.carpio exposed to different concentrations of Pb. (A): control kidney showing normal architecture of renal tubules (RT); (B & C): T1 showing hemosiderosis together with melanomacrophage aggregation (MM) with fibrous odematous (FO). (D): T2 showing cellular degeneration of renal epithelium (CD). H&E stain; Thickness 6 μm. Scale bars 50μm.
C. Histopathological alterations of kidney
Histopathological studies of the kidney and Hemopiotic tissue revealed a typical structural organization in the untreated group without histopathological alterations (Figure 4 A). The most important changes observed in T1 were hemosiderosis together with melanomacrophage aggregation and fibrous edematous (Figure 4 B and C). The main alterations in T2 and T3 were glomerular contraction, resulting in the dilation of Bowman’s space with cellular degeneration of renal tubules (Figure 4 D and Figure 5 E). While T3 and T4 revealed hydrobic degeneration in the form of cytoplasmic vacuolation with protein substances deposition in renal tubules, dilation of some renal tubules, necrosis of renal tubules with nuclear piknosis (Figure 5 F-H).
Alterations in the kidney of carp observed in the current study have been described in other species of the fishes exposed to heavy metals by Oliveira- Ribeiro et al. (2002), Jiraungkoorskul et al. (2003), Thophon et al. (2003), Gupta and Srivastava (2006), Kaoud and El-Dahshan (2010), Al-Balawi, et al. (2013), which were in agreement with the current study. Because the important role of kidney for the excretion and detoxification of harmful substances in the body (Vinodhini and Narayanan, 2008). The presence of tubule degeneration, with the necrosis in renal tubules in the present study could be indicated that the kidney suffered damage after exposed to Pb.
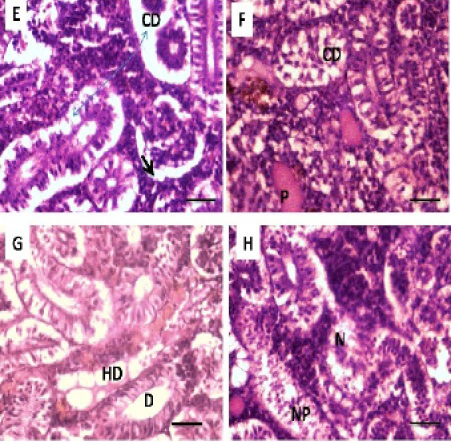
Figure 5: Photomicrograph showing histological structures through kidney of C.carpio exposed to different concentrations of Pb. (E & F): T3 demonstrating cellular degeneration in renal tubules (CD); dialtion of Bowmen’s space (black arrow) with deposition of protein substances in tubules (P); (G & H): T4 showing hydrobic degeneration on (HD) in the form of cytoplasmic vacuolation of the tubular epithelium, dialtion in some of renal tubules (D), necrosis (N) in the tubular epithelium with nuclear piknosis (NP) H&E stain; Thickness μm. Scale bars 50μm
Conclusion
The results obtained from this study indicated the importance of the histopathology as a useful biomarker for the assessment of environmental quality to investigate the toxicity of heavy metal as well as other pollutants that can compromise the quality of the aquatic environment. In the current study a variety of histopathological changes were observed in the gills, kidney and liver of C. carpio. The severity and frequency of organ lesions was found to be more pronounced in fish treated with higher level of Pb. Also, the results revealed that the degree of damage of the tissues was proportional to the concentration of the Pb and with water replacement.
Acknowledgments
Authors acknowledge the Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq.
Conflict Of Interest
There exists no conflict of interest.
Authors Contribution
All the authors have contributed equally in terms of giving their technical knowledge to frame the article.
References






