Advances in Animal and Veterinary Sciences
Review Article
Bluetongue in India: A Review
Karam Chand1*, Sanchay Kumar Biswas1, Awadh Bihari Pandey1, Dhanavelu Muthuchelvan1, Bimalendu Mondal2
1Division of Virology, Indian Veterinary Research Institute, Mukteswar Campus, Dist. Nainital 263 138, Uttarakhand, India; 2Eastern Regional Station, Indian Veterinary Research Institute, 37, Belgachia Road, Kolkata 700 037, West Bengal, India.
Abstract | Bluetongue (BT) is a vector-borne viral disease of ruminant and camelid species which is transmitted by Culicoides spp. The causative agent of disease is bluetongue virus (BTV), which belongs to genus Orbivirus of the family Reoviridae. The clinical disease is seen mainly in sheep and subclinical infections of BT are seen in domestic and wild ruminants. Currently, a total of 27 serotypes of BTV are recognized worldwide, and there is evidence for circulation of at least 22 serotypes in India. BT is endemic in India. Till date, at least 13 different serotypes (BTV-1-4, 6, 9, 10, 12 16-18, 21 and 23) have been isolated under All-India Network Program on Bluetongue (AINP-BT) and by other research laboratories in the country. Diagnosis of BT requires isolation of virus, standard serological methods and several other antigen and nucleic acid detection assays. BTV serotypes are identified by virus neutralization test and sequencing of VP2 gene. Because of a large number of susceptible hosts, BTV serotypes and Culicoides vector, control of BT is not an easy task. Recently, in India, an inactivated pentavalent vaccine (BTV-1, 2, 10, 16 and 23) is developed and commercialized.
Keywords | Bluetongue, India, Serotype, Diagnosis, Control
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 27, 2015; Revised | September 09, 2015; Accepted | September 11, 2015; Published | October 21, 2015
*Correspondence | Indian Veterinary Research Institute, Mukteswar Campus, Uttarakhand, India; Email: [email protected]
Citation | Chand K, Biswas SK, Pandey AB, Muthuchelvan D, Mondal B (2015). Bluetongue in India: A review. Adv. Anim. Vet. Sci. 3(11): 605-612.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.11.605.612
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Chand et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bluetongue (BT) is a vector-borne disease of ruminants which affects many species of domestic and wild ruminants such as sheep, goat, cattle, buffalo, white-tailed deer, antelope and sambar, and camelid species such as camels and llamas (Spreull, 1905; MacLachlan, 1994; Meyer et al., 2009). The manifestation of the disease varies from asymptomatic to lethal outcome which depends upon the BTV serotype, species, breed and age of animal (Elbers et al., 2008). In domestic animals, clinical signs are seen mainly in sheep, however, in Western and Central Europe, cattle infected with BTV-8 also showed clinical disease (Darpel et al., 2007; Elbers et al., 2008). The BT disease is caused by bluetongue virus (BTV) which is transmitted between vertebrate hosts by Culicoides species (Mellor, 1990). BTV belongs to the Orbivirus genus in the family Reoviridae (Pringle, 1999). Presently, worldwide at least 27 distinct BTV serotypes have been recognized with the possible addition of two more serotypes (Hofman et al., 2008; Maan et al., 2011; Zientara et al., 2014). In India, at least 22 serotypes have been recognized based on serology and/or virus isolation. Till date, 13 serotypes viz., BTV-1, 2, 3, 4, 6, 9, 10, 12 16, 17,18, 21 and 23 have been isolated by researchers involved in the All India Network Program on Bluetongue (AINP-BT) and other research laboratories (AINP-BT, 2012; Rao et al., 2014). A preliminary diagnosis of BT disease is usually done by epidemiology, clinical sign and post-mortem finding (Afshar, 1994). Confirmatory diagnosis requires isolation of virus, standard serological methods and several other antigen and nucleic acid detection assays. BTV can be isolated in the embryonated chicken egg (ECE), cell culture and sheep (Afshar, 1994; Clavijo et al., 2000). Competitive ELISA (c-ELISA), indirect ELISA (i-ELISA) and agar gel immunodiffusion (AGID) assay can be used for detection of serogroup-specific antibodies (Afshar et al., 1989; Pathak et al., 2008). The BTV serotypes are routinely identified by virus neutralization test (VNT). Sequence analysis of VP2 gene also provides important information related to serotype of the virus, virus evolution and its relatedness to other viruses (Maan et al., 2007; Biswas et al., 2010). Full length sequencing of VP2 gene and VNT was carried out to study the neutralization behavior among Indian BTV-1 isolate to understand the antigenic similarity and relationship with other Indian BTV-1 isolates (Biswas et al., 2015). Serogroup-specific sandwich ELISA (s-ELISA) is used for the detection of BTV antigen from blood and tissue samples (Thevasagayam et al., 1996; Chand et al., 2009). Reverse transcription-polymerase chain reaction (RT-PCR) is also used for detection of BTV nucleic acid from blood or tissue samples (Dangler et al., 1990; Wade-Evans et al., 1990). A quantitative detection and quantification of viral RNA in a clinical sample is carried out by real-time RT-PCR (Shaw et al., 2007). Due to a large number of susceptible hosts, BTV serotypes and Culicoides vector, control of BT is very difficult. For effective control of BT in our country, it is important to commence sentinel and vector trap systems and proper diagnosis of disease. An inactivated pentavalent vaccine (BTV-1, 2, 10, 16 and 23) was developed under AINP-BT and is available in the country (Reddy et al., 2010).
HISTORY AND EPIDEMIOLOGY
Bluetongue was first reported in the18th century in South Africa when fine-wool sheep were imported from Europe (Spreull, 1905). In 1880, Hutcheon reported the clinical aspect of disease in his annual report. However, it was not until 1902, the disease “Malarial catarrhal fever” or “Epizootic catarrh” was first reported in scientific literature (Hutcheon, 1902). “Bluetongue”, the term is derived from the African “bloutong”, which was coined by South-African farmers after observing the cyanosis of tongue in clinically infected animals (MacLachlan et al., 2009).
In India, first outbreak of BT in sheep and goats from Maharashtra state was reported by Sapre (1964). During 1981, in Southern India disease was widely spread and many outbreaks were reported. In Tamil Nadu, a total of 258 outbreaks were reported between 1986 and 1995 (Sreenivasulu et al., 2004). In Northern India, an outbreak of BT was reported from Dehradun, Uttarakhand (that time Uttar Pradesh) (Mehrotra et al., 1995), in that more than 60 goats died.
In India, wide serological surveys have been carried out in different parts of the country. Mehrotra and Shukla (1984) observed seroprevalence in sheep ranges from 16.4-61.1% in different states viz. Maharashtra, Andhra Pradesh, Karnataka, Jammu and Kashmir and Himachal Pradesh. In studied conducted during 1991 revealed the prevalence of BTV antibodies in sheep, goats, cattle and buffalo was 45.71%, 43.56%,33.4% and 20% respectively (Walton, 2004). This higher prevalence in small ruminants may reflect their association in the basic ecology of the virus. Similarly high prevalence of antibody in small ruminants was also observed by Sharma et al. (1985) in Rajasthan. Harbola et al. (1982) reported BTV antibodies in 37.5% of sheep serum samples collected from Maharashtra State. Others workers have also reported the seroprevalence of BTV antibodies from Gujarat, Maharashtra, Madhya Pradesh, West Bengal and Tamil Nadu (Mehrotra et al., 1990; Janakiraman, et al., 1991). Sodhi et al. (1981) reported a seroprevalence of 6.64% in Punjab with a higher prevalence in exotic breeds than in indigenous sheep. Bandopadhyay and Mullick (1983) made similar observations. In Gujarat, serological survey was conducted in sheep, goat, cattle, buffaloes and camels and seroprevalence was 24.66, 29.15, 24, 34.72 and 9.33% respectively (Chandel et al., 2004). An overall seroprevalence of 5.1% was observed in sheep and goats in Kerala State (Ravisankar et al., 2005). A seroprevalnce rate of 47% was observed in the goats of costal saline (Sunderban) areas of West Bengal by De et al. (2007). Joardar et al. (2014) observed prevalence of BTV antibody in sheep, goat and cattle in different district ranges from 34.78-48.71%.
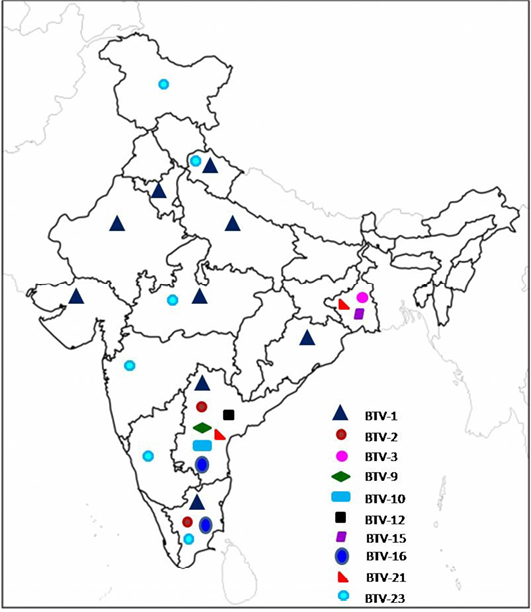
Till 2000, serotyping was carried out at one or the other BT reference laboratory (Prasad et al., 2009). Since 2001, serotyping has been carried out in the country under AINP-BT in addition to other laboratories. Till date, at least 13 different BTV serotypes viz., BTV-1,2,3,4, 6, 9, 10, 12,15 16,17,18, 21 and 23) were isolated mostly from southern states of India (Mehrotra et al.,1995; Sreenivasulu et al., 1999b; Bommineni et al., 2008; Prasad et al., 2009; Biswas et.al., 2010; Bisht et al., 2011; Dadawala et al., 2012; Gollapalli et al., 2012; Maan et al., 2012 a, b; Minakshi et al., 2012; Mondal et al., 2012; Rao et al., 2012, 2013; Susmitha et al., 2012; Chauhan et al., 2014). Prevalence of different BTV serotypes based on isolation of virus in India is shown in Figure 1.
Bluetongue is endemic between 40° north and 53° south latitude where Culicoides midges are present (Cornil et al., 2008). The outbreak of BT depends on the diverse climatic condition, population and breeds of sheep. There are few reports on susceptibility of the breed to BT in India. Initially, the BT outbreaks were observed in exotic and cross sheep breeds but recent outbreaks were observed in native sheep breeds (Deshmukh and Gujar, 1999; Sreenivasulu and Rao, 1999a; Bommineni et al., 2008; Prasad et al., 2009; Joardar et al., 2009; Rao et al., 2012a; Susmitha et al., 2012).
BLUETONGUE VIRUS
Bluetongue virus belongs to the genus Orbivirus of the family Reoviridae (Pringle, 1999). BTV particle is ~ 65-80 nm diameters, non-enveloped with icosahedral symmetry. Viral genome consist of ten discrete double-stranded (ds) RNA segments which codes for seven structural (VP1-VP7) and at least four non-structural (NS1-NS3/NS3A and NS4) proteins (Mertens et al., 1984; Belhouchet et al., 2011). The complete genome of BTV is ~ 19.2 kbp in length where the length of dsRNA segments varies from 3954 to 822 bp. The outer capsid layer is consisted of VP2 and VP5 proteins (Verwoerd et al., 1972; Roy and Noad, 2006). VP2 is the serotype-specific antigen and also major neutralizing antigen (Huisamans and Erasmus, 1981; Roy, 2008). The core of BTV consists of VP7 protein and it is a major group-specific antigen determinant. The innermost layer consists of VP1, VP3, VP4 and VP6 proteins which is involved in viral replication and transcription (Verwoerd et al., 1972; Nason et al., 2004). NS1 - a non-structural protein is highly conserved tubule forming protein (Roy, 1989). NS2 can recruit protein required for core assembly to the virus inclusion bodies (Fukusho et al., 1989; Roy, 2008). BTV encodes a third non-structural protein, which occurs in two isoforms, full-length NS3, and N-terminally truncated NS3A arising from an alternative translation start site. The NS3 protein exhibits viroporin-like properties and it is important for proficient virus release from infected cells (Hyatt et al., 1989; Roy, 2008). An additional non- structural protein, NS4 has been also identified that is produced from a different reading frame in segment 9.
The data for BTV-4, 6, 17 and 18 (not shown in figure) are not available/not known.
This protein has nucleolar localization and associates with the cell lipid droplet (Belhouchet et al., 2011).
TRANSMISSION
Bluetongue is naturally transmitted by Culicoides species and consequently outbreaks depend on the presence of efficient midge vector and susceptible animals. There are 1300-1400 species of Culicodes (Mellor et al., 2000); however, only 30 of them are identified as a vector for transmission of BTV (Meiswinkel et al., 2008). Culicoides are often present in warm, humid and muddy areas and they are mostly vigorous one hour before sunset and one hour after sunrise (Mellor et al., 2000). The global warming has permitted the longer activity of Culicodes and, therefore, capable of BTV transmission for longer periods (Tweedle and Mellor, 2002). In India, BTV has been isolated from Culicoides vector (Jain et al., 1988; Dadawala et al., 2012).
The virus is conventionally believed to be transmitted only through the bite of infected vector and not by contact or through infected products (Mellor et al., 1984). Some serotypes of BTV have been reported to be transmitted via oral and by the vertical route (Darpel et al., 2009). Isolation of BTV from aborted fetus of dogs has also been reported without any history of contaminated vaccine (Dubovi et al., 2013).
BLUETONGUE DISEASE
Bluetongue virus infects all ruminants, but clinical disease is observed mainly in sheep; a fatal hemorrhagic disease also seen in white-tailed deer (Odocoileus virginianus) (Howerth et al., 1988; Johnson et al., 2006). However, in Western and Central Europe cattle infected with BTV-8 also showed clinical disease (Darpel et al., 2009; Elbers et al., 2008).
In sheep, the disease is manifested as an acute, chronic and subclinical infection. The incubation period of the disease is 4-8 days. The clinical signs observed are fever 105-106°F, depression, anorexia, tachypnea, hyperemia of the lips and nostrils, salivation, nasal discharge, edema of the face and tongue, conjunctivitis and ulcers on oral mucosa. In rare cases, there is cyanosis of the tongue. After pyretic phase, animal have coronitis, laminitis, muscle weakness, and lameness, consequently, animal stand with an arched back. Dermatitis, torticollis and wool break may also occur (Brewer and MacLachlan, 1994; Tweedle and Mellor, 2002; Darpel et al., 2007; Elbers et al., 2008).
DIAGNOSIS
Preliminary diagnosis of BT is based on by clinical signs, post-mortem findings, and epidemiology and it is further confirmed by laboratory examination (Afshar, 1994). For the diagnosis of BT, isolation of BTV is the most reliable technique. Samples of choice for laboratory examination include blood in anticoagulant, serum, post-mortem samples like spleen, lymph node, bone marrow, lung, liver, heart and brain from aborted fetuses (Afshar, 1994; Tweedle and Mellor, 2002).
Virus Isolation
The most sensitive method for isolation of virus is inoculation of 9-12 days old embryonated chicken eggs (ECE) through intravenous (IV) or yolk sac (YS) route. IV route of inoculation is 100-1000 fold more sensitive and more rapid than YS route (Goldsmit and Barzilai, 1985; Clavijo et al., 2000). In case of blood sample with low virus titre, inoculation of sheep may also be a good approach (Afshar, 1994). Many mammalian (BHK-21, Vero and CPAE cell line) and insect cell line such as KC - derived from Culicoides sonorensis and C6/36 from Aedes albopictus can also be used for the isolation of virus (Girard et al., 1967; McPhee et al., 1982; Wechsler and McHolland, 1988; Wechsler et al.,1989; Mecham, 2006).
Antigen and Nucleic Acid Detection
Serogroup-specific s-ELISA has been developed and routinely used for detection of BTV antigen in blood and tissue sample (Thevasagayam et al., 1996; Chand et al., 2009). The BTV serotypes are routinely identified by VNT (Reddington et al., 1991). The majority of Indian isolates were typed by VNT; however since 2001 serotyping was carried out by sequencing of VP2 gene (AINP-BT, 2012).
For detection of BTV nucleic acid in clinical samples and infected cell culture materials, RT-PCR is a sensitive and rapid assay. Diagnostic PCR based on the NS1 gene specific primers and primer pairs targeting highly conserved region of S7 gene were reported to detect viral nucleic acid in blood and tissue samples (Wade-Evans et al., 1990; Dangler et al., 1990). Wilson and Chase (1993) developed a highly sensitive ‘Nested PCR’ test for detection of BTV dsRNA from Culicoides variipennis based on NS1 gene sequence. OIE (2012) recommended NS1 gene based PCR assay as one of the official tests for detection of BTV in animals for international movement and transport. A number of real-time RT-PCR assays have also been developed for detection of viral RNA and serotyping of BTV (Wilson et al., 2009; Vanbinst et al., 2010; Leblanc et al., 2010). Loop-mediated isothermal amplification (LAMP) assay for detection of BTV nucleic acid from the blood sample has been developed using NS1 gene (Mohandas et al., 2015). Genomic dsRNA segments of BTV can be resolved discretely in agarose gel electrophoresis as well as polyacrylamide gel electrophoresis of RNA (RNA-PAGE). The electropherogram can be used as a diagnostic tool for identification of BTV in from clinical samples (Squire et al., 1983). By using RNA-PAGE, Ramakrishnan et al. (2005) demonstrated up to 13 segments in cell culture adopted BTV isolates.
Antibody Detection
Antibody (serogroup-specific) against BTV is detected c-ELISA, i- ELISA and AGID (Afshar et al., 1989; Pathak et al., 2008). The serum neutralization test (SNT) is most sensitive and specific, but it is most tedious and time consuming (Reddington et al., 1991; Hamblin, 2004). Other available diagnostic methods include, immunoflurorescence test (IFT), complement-fixation test (CFT), haemagglutination-inhibition test, immunospot, and immunoperoxidase, but they are not routinely used (OIE, 2000).
CONTROL
The control strategy of bluetongue is mainly through vaccination of animals, management practices as well as the control of vector. Due to a large number of susceptible hosts, BTV serotypes and, control of BT is very difficult. Control of BT is intended with keeping susceptible animals away from Culicoides vector but all time this is not possible. Control of vector can be tried with pouring insecticides, but it is expensive and does not attain complete freedom from the vector.
Live attenuated vaccines, as well as inactivated vaccines, have been successfully used in China, South Africa, Europe and other countries (Stott et al., 1985; Murray and Eaton, 1996; Odeon et al., 1997; Bhanuprakash et al., 2009). A genetically engineered virus-like particles (VLPs) has also been projected as a next generation vaccine. However, prevalence of multiple serotypes within a limited geographical area and incidence of genetic and phenotypic drift during natural infection in vectors and hosts, ensuing in development of neutralization-resistant phenotypic variants within a serotype which make the circumstances further difficult (DeMaula et al., 2000; Bonneau et al., 2001; White et al., 2004; Tembhurne et al., 2010).
In India, few attempts have been made to develop inactivated BTV vaccines using BEI and Hydroxylamine. BEI inactivated BTV vaccine elicited both neutralizing and group specific antibodies (Ramakrishnan et al., 2006) whereas Hydroxylamine inactivated vaccine elicited group-specific antibodies (Ramakrishnan et al., 2005). Both the inactivated vaccine showed reduction in clinical signs and reduction in duration of viremia. A pentavalent (BTV-1, 2, 10, 16 and 23) inactivated vaccine is developed under AINP-BT and commercialized (Reddy et al., 2010). Modified live vaccines (MLVs) produce a viremia in animals which lead to further spread of the virus. If viremic animals are vaccinated with MLVs, troubles may occur with viral reassortment (Gollapalli et al., 2012). In India, MLVs are not a preferred option as animals (mainly sheep and goats) under nomadic people which can leads to spread of virus. Hence, for control of disease use of multivalent inactivated vaccines is a good option. To monitor the serotypes circulating in the different parts of the country, it is essential to set up a system. Based on which particular serotype of the virus can be identified and included in the multivalent vaccine formulation for successful control of disease in India.
ACKNOWLEDGEMENT
The authors wish to thank the Director of ICAR-IVRI for necessary facilities and the work is supported by AINP-BT and DBT -NER-BT projects.
CONFLICT OF INTREST
There exists no conflict of interest.
Authors’ contribution
Karam Chand and Sanchay Kumar Biswas collected literature adn wrote teh paper. Karam Chand, Sanchay Kumar Biswas, Awadh Bihari Pandey, Dhanavelu Muthuchelvan and Bimalendu Mondal performed the final check.
REFERENCES