Advances in Animal and Veterinary Sciences
Research Article
Efficacy of the Cruciferous Vegetable on the Thyroid Gland and the Gonads in Rabbits
Ali Abdul-Aziz, Khalid Kamil Kadhim*
Department of Anatomy and Histology, Faculty of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | The aim of this study was to examine the changes in the thyroid glands and the gonads of rabbits after eating the cruciferous vegetable. The animals divided into three equal groups (eight mature female, mature male and eight of their kits for each group). The first group was fed normal vegetable, the second group was fed cruciferous vegetable for two days followed by normal food at third day and persisted for one month, while were estimated. The triiodothyronine (T3) and thyroxine (T4) were determined in the serum. Results show the effects of the cruciferous vegetable were increased the thyrocytes height and number, vacuolation of the sytoplasm and decreases of the follicular colloid and the follicular size, in addition to decreases in T3 and T4 hormones in the serum. The activities of thyroid gland and in turn adversely affects the effectiveness of the male and female gonads in addition to its impact on young.
Keywords | Cruciferous vegetable, Thyroid gland, Gonands, Colloid
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 14, 2015; Revised | February 14, 2015; Accepted | February 15, 2015; Published | February 21, 2015
*Correspondence | Khalid Kamil Kadhim, University of Baghdad, Iraq; Email: [email protected]
Citation | Abdul-Aziz A, Kadhim KK (2015). Efficacy of the cruciferous vegetable on the thyroid gland and the gonads in rabbits. Adv. Anim. Vet. Sci. 3(3): 183-191.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.3.183.191
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Abdul-Aziz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The most widely used cruciferous vegetables belong to the genus Brassica and include broccoli, Brussels sprouts, cabbage, cauliflower, collard greens, kale, kohlrabi, mustard, rutabaga and turnip (Latte et al., 2011). When these cruciferous are digested by intestinal bacteria, they release the goitrogens that increase the need for iodine when consumed in small amounts and can damage the thyroid gland when consumed in large amounts (Parchami and Fatahian, 2012). These goitrogens inhibit the transfer of iodine into mother’s milk and can cross maternal milk during lactation and also cross the placenta into the fetal bloodstream during pregnancy (Vidinov et al., 2013).
The thyroid gland is the largest endocrine gland in the body, its synthesis hormones: T3 & T4 which are important in body metabolism (shoonover, et al., 2004).
Thyroid tissue is composed of microscopic spheres called follicles, these are lined by columnar to cuboidal cells, centre of these follicle is filled with secretion of thyroid which is called colloid (Ali, 2011).
Increase the thyroid function is represented by smaller follicles, higher follicular epithelium, decreased quantity of the colloid and by increased proliferative activity (Jelinek, et al., 2003). The follicular size is depends on the number of cells and the amount of colloid and these are changes according to biological activity (Nielsen et al., 2005).
Hypothyroidism can interrupt the menstrual cycle or cause infertility in female (Lincoln et al., 1999). Thyroid hormones play important roles in proliferation and apoptosis of various kinds of cells, such as granulosa cell accordingly, limited numbers of follicles are reaches the ovulation stage, the other follicles undergo atresia (Asahara et al., 2003).
The effect of hypothyroidism on gonadotrophin secretion is at the level of the hypothalamus-pituitary. The thyroid hormone plays an important role in testicular function. The T3 controlling Sertoli cell and Leydig cell proliferation and differentiation during testicular development in rats and other mammal species (Holsberger and Cooke, 2005). This study was designed to evaluate the histomorphological changes and hormonal secretion of thyroid gland in mature rabbits and their kits eating cruciferous vegetables.
Materials and methods
Animals and Dietary Manipulation
Twenty four adult female rabbits (1.72 – 1.77 kg body weight), fifteen adult male (1.42 - 1.79 kg body weight) and twenty four kits (484 - 470 gm body weight) were used in this study. The animals were assigned to three groups:
1- Group (A): Eight mature female rabbits with five male were fed green forage (grass and alfalfa) as a control group. The food and water were available ad libitum. After parturition of these female, eight of their kits were taken as a subgroup. The adult rabbits and their kits are used before wean from their mothers.
2- Group (B): Eight mature female rabbits with five male were fed cruciferous vegetable for two days followed with normal vegetables at third day interval this persisted for one month as a second group, water were available ad libitum. After parturition of these female, eight of their kits were taken as a subgroup. The adult rabbits and their kits are used before wean from their mothers.
3- Group (C): Eight mature female rabbits with five male were fed cruciferous vegetable only for one month.
Sampling Procedure
Collection of Serum for Hormonal Assessment
Cardiocentesis (cardiac puncture) were used as a procedure to collect blood under anesthesia. Large-bore (21G) 1.5 inch needles were used. 3 – 5 ml were collected in a vacuum tube with gel clot activator. The blood were centrifuged 3000 rpm for 5 minute for isolation of serum, the collected serum were putted in eppendorf tube and preserved in refrigerator till used for hormone assessment.
Collection of Thyroid Gland
The animals were euthanized by intravenous injection of Sodium pentobarbital overdose 100 mg/kg. The total body weight of each rabbit was recorded. The whole thyroid glands were isolated via midline skin incision at the ventral surface of the neck, and the weight was recorded.
Histological Samples
The whole thyroid gland (both right and left lobes with their isthmus) and the gonads were immersed immediately in 10% buffered formalin for histological examination. The collected samples were processed by routine paraffin technique. The sections were stained with hematoxylin and eosin (H&E), Masson’s trichrome stain, and Periodic acid schiff (Humason, 1972).
Histomorphometry
The measurements was performed on histological slides from thyroid glands after photographed through a light microscopy (Olympus-Japan) provided by digital camera (MEM 1300) using image J (Java-based image processing program developed at the National Institutes of Health). The histological parameters were measured in this study as follows:
Thyroid gland: Follicular cells height, Follicular cells number. The diameter of the follicle was determined by the mean of its two longest perpendicularly situated lengths of the follicle. These follicles were classified in accordance to their diameter in three size categories: large (175.1–615.0 μm), medium (80.1–174.0 μm), small (15.0–79.0 μm) (Jelinek, et al., 2003). Finally the percentage of follicles in the thyroid tissue was calculated.
Ovaries: the numbers of the primary, secondary and mature follicles were calculated per histological sections.
Testis: the arrangement of the spermatogenic cells per cross section of the siminefrous tubules were examined.
Table 1: Total body weight and the relative weight of the thyroid gland (adult and kit) and the relative weight of male and female gonads
|
Categories |
Group A |
Group B |
Group C |
|
|
Mature female |
Body weight (gm) |
1725±354 a |
1768±264 a |
1772±121 a |
|
Thyroid weight (mg/100gm B.W) |
9.2±2.5 a |
9.2±1.5 a |
9.6±3 a |
|
|
Ovary weight (mg/100gm B.W) |
35.8±6.7 a |
12.2±4.2 b |
15.9±5.2 b |
|
|
Mature male |
Body weight (gm) |
1422±76 a |
1612±28 b |
1792±65 c |
|
Thyroid weight(mg/100gm B.W) |
9.8±1.9 a |
9.3±0.7 a |
10±1.2 |
|
|
Testes weight (mg/100gm B.W) |
59.9±5.7 a |
55.2±4.9 a |
53.9±4.6 a |
|
|
kit |
Body weight (gm) |
484±72 a |
470±28 a |
---- |
|
Thyroid weight (mg/100gm B.W) |
13.4±3.5 a |
14.4±0.8 a |
---- |
Values are means ± SD, N= 8. Different letter between groups indicating significant differences (P<0.05).
Table 2: The per cent ratio of the thyroid follicles (small, medium and large) number in the mature and their kits
|
Categories |
Group A |
Group B |
Group C |
|
|
Mature |
Small follicle (%) |
51.9±1.2 a |
63.5±1.4 b |
65.5± 0.9 c |
|
Medium follicle (%) |
32.6±1.1 a |
27±0.7 b |
16±1 c |
|
|
Large follicle (%) |
17±2.1 a |
15.7±1 a |
8.4±0.6 b |
|
|
kit |
Small follicle (%) |
51.1±0.9 a |
90.9±1.1 b |
---- |
|
Medium follicle (%) |
41.8±1.3 a |
8.3±0.7 b |
---- |
|
|
Large follicle (%) |
7.8±0.7 a |
2.1±0.5 b |
---- |
Values are means ± SD, N= 8. Different letter between groups indicating significant differences (P<0.05).
Thyroid Hormone Level
Serum concentration of triiodthyronine (T3), tyroxine (T4), and their free fraction (FT3, FT4) were measured by radioimmunoassay (Eriksson et al., 1983).
Statistical Analysis
All data presented as mean ± standard deviation. The comparisons of data were done between the three groups. The significance of the differences between the data from three groups was estimated with one way ANOVA using SPSS version 20.
Results and discussion
The results showed no significantly (P< 0.05) difference between the three groups (A, B and C) in this experiment regarding the relative weight of the thyroid and testes. Despite there were light thyroid hypertrophy, but this may be due to the short duration of the experiment. However, the relative ovarian weight of the group (B) and (C) showed significantly (P<0.05) lower than the control group (A) (Table 1).
It is worth mentioning here, in relation to the group (C), which was limited only with cruciferous fed, the female did not get pregnant or births during the period of the experiment, therefore there were no kits for this group. Lincoln et al. (1999) showed that hypothyroidism can interrupt the menstrual cycle or cause infertility in female.
The histological sections of the thyroid in control group (A) showed different sizes of follicles that filled by homogenous colloid (Figure 1A), the large follicles showed decrease in numbers in group (B and C) as shown in (Figure 1B, 1C). Table 2 showed the percentage of the follicular numbers according to their size. The small thyroid follicles were significantly (P<0.05) increased after eating the cruciferous vegetables. In contrast to the medium and large follicles that showed significantly (P<0.05) decreases in both mature rabbit and their kits. However, Nielsen et al. (2005) and Abeer and Safaa (2012) had suggested that the follicular size is depends on the number of cells and the amount of colloid, and these are interchangeable and vary according to biological activity.
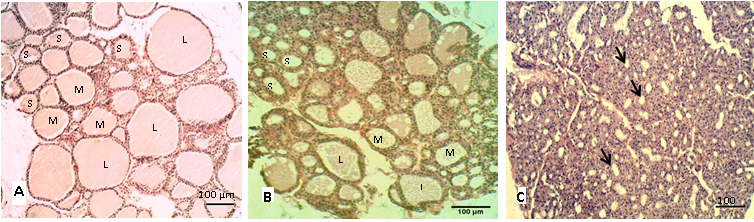
Figure 1: Sections of thyroid gland in adult
(A) Control group; (B) group eating mixed feed with cruciferous vegetable; (C) group eating only cruciferous vegetable. Showing the thyroid follicles in different size, (L) Large size, (M) Medium size, (S) Small size. H & E stain
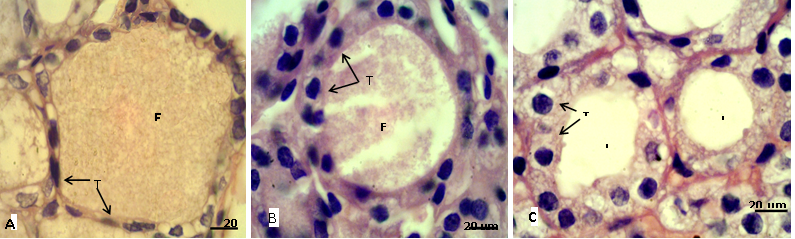
Figure 2: Sections of thyroid gland in adult
(A) Control group, showing flattened thyrocytes (T) with homogenous cytoplasm and the follicular colloid (FC) filled the follicular lumen; (B) group eating mixed feed with cruciferous vegetable, showing cuboidal thyrocytes (T) and the follicular colloid (FC) partially filled the follicular lumen; (C) group eating only cruciferous vegetable, showing cuboidal to columnar thyrocytes (T) with vacuolated cytoplasm and the follicular lumen (L) empty from colloid. H & E stain.
In adult rabbit, the epithelial cells (thyrocytes) of the thyroid follicles were flattened to low cuboidal with homogenous cytoplasm in the control group (A), whereas, these epithelial cells became tall cuboidal or more columnar in group (B) and (C) respectively, the cytoplasm of these later groups showed abundant vacuolation or foamy appearance particularly in the group (C) (Figure 2). This may be due to increase reabsorption rate of thyroglobulin from the follicular lumen (Jelinek et al., 2003). In kits, the thyrocytes were flattened to cuboidal in control group, and it became high cuboidal in the group that their mothers belong to group (B) (Figure 3, 4). Optiz et al. (2006), Elbakry and Twafik (2014) had reported that hyperthyroidism is represented by increase in the epithelium height, cellular vesicles, and vacuolated Colloid.
The results showed significantly (P<0.05) increased in follicular cells height for group (B) and greater for group (C) compared with group (A) (Figure 5). These were synchronizing with significant increases in the thyrocytes numbers in the kits groups but not significant in adult (Figure 6). Jelinek et al. (2003); Beyzai and Adibmoradi (2010) had reported that an increase in the thyroid function is represented by tall and columnar follicular epithelium and by increase proliferative activity. However, Garrido et al. (2013) showed that the follicular cell hypertrophy is eventually followed by hyperplasia.
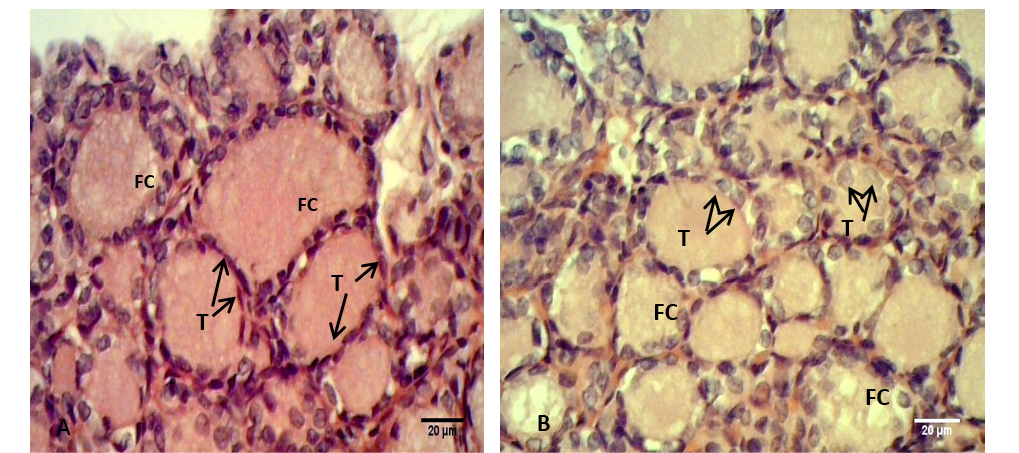
Figure 3: Sections of thyroid gland in Kit
(A) Control group, showing flattened to cuboidal thyrocytes (T) with homogenous cytoplasm and the follicular colloid (FC) filled the follicular lumen; (B) group their mother’s eating mixed feed with cruciferous vegetable, showing cuboidal thyrocytes (T) and the follicular colloid (FC) had foamy appearance. H & E stain.
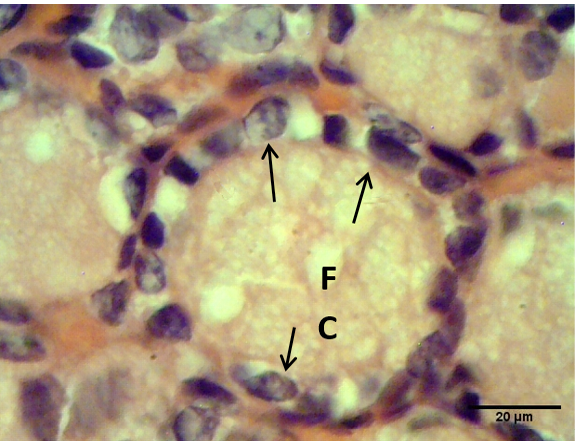
Figure 4: Sections of thyroid gland in Kit from group (B), showing cuboidal thyrocytes (arrows) and the follicular colloid (FC) that filled the follicular lumen had foamy appearance. H & E stain
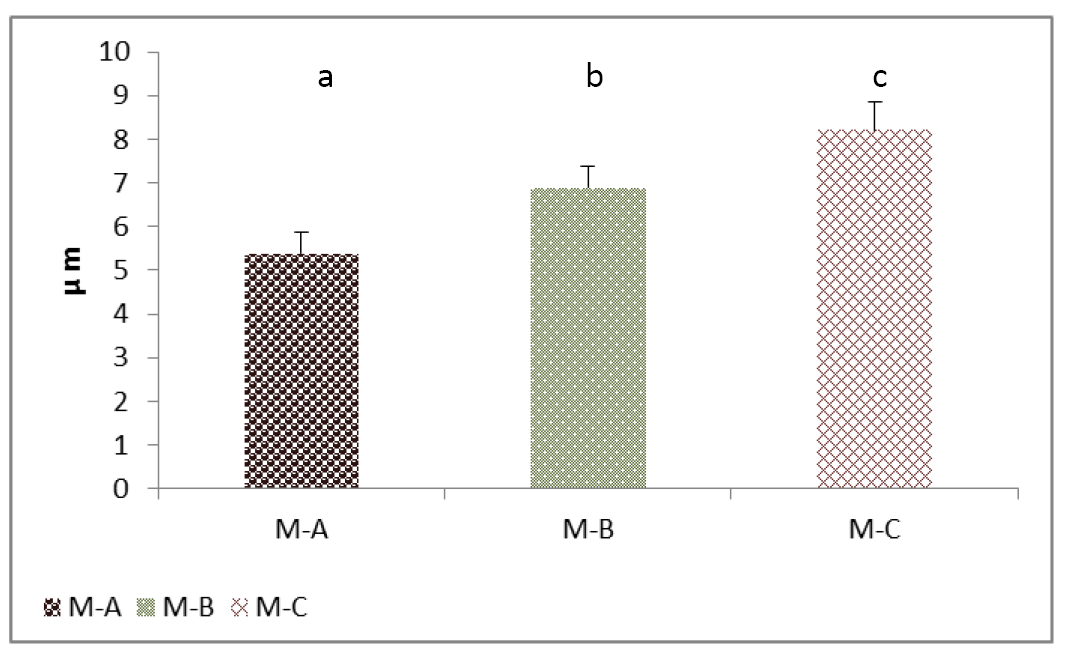
Figure 5: Cell height (Thyrocytes) of thyroid gland of the mature rabbit. Where the (M.A) control group, (M.B) group eating mixed feed with cruciferous vegetable, (M.C) group eating only cruciferous vegetable. Values are means ± SD, N= 8. Different superscript letter indicating significant (P<0.05) differences.
The thyroid follicles of the control group (A) were filled with homogenous colloid. While in the group (B) the colloid showed peripheral vacuolation and moderately filled the follicles. Meanwhile the follicles contain little or no colloid in group (C) (Figure 3). In kits that belong to group (B) the follicular colloid had abundant foamy or vacuolated appearance (Figure 4). These results were explained by Jelinek et al. (2003) that when the TSH stimulates, the follicular cell hypertrophy and hyperplasia will occur and the reabsorption rate of thyroglobulin from the follicular lumen is increase also. However, Garrido et al. (2013) had suggested that the follicular cell and colloid changes did not have a strong correlation with circulating T4 and T3 hormone levels, which may be consistent with the dynamic levels of these hormones in circulation.
The decreases in the level of T3 in adult females were obtained in the group (B) and (C), compared with control group (A) (Table 3), while, it was significant (P>0.05) lowered in group (C) only. Meanwhile the decreases of T4 in group (B) and (C) were not significant (P<0.05). Similar results were observed for mature male, however, the level of T4 in the group (C) showed significantly (P>0.05) lower than the control group. These results were suspected for groups feeding with cruciferous vegetable. Parchami and Fatahian (2012) have suggested that these crucifers release the goitrogens when digested by intestinal bacteria, and this increase the need for iodine when consumed in small amounts and can damage the thyroid gland when consumed in large amounts.
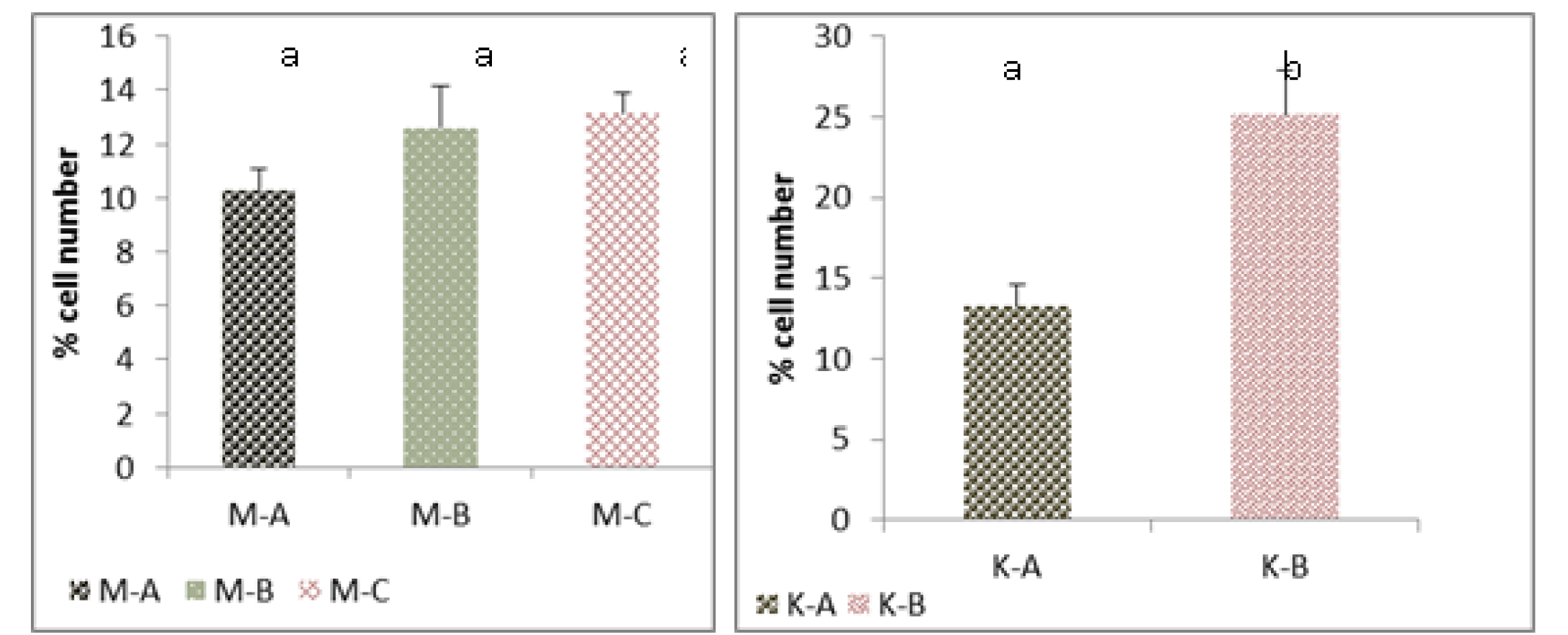
Figure 6: Cell number (Thyrocytes) of thyroid gland of the mature rabbit (Left) and their kit (Right)
Where the (M.A) mature control group, (M.B) mature group eating mixed feed with cruciferous vegetable, (M.C) mature group eating only cruciferous vegetable. (K.A) Kit from control group, (K.B) kit from group (B). Values are means ± SD, N= 8. Different superscript letter indicating significant (P<0.05) differences
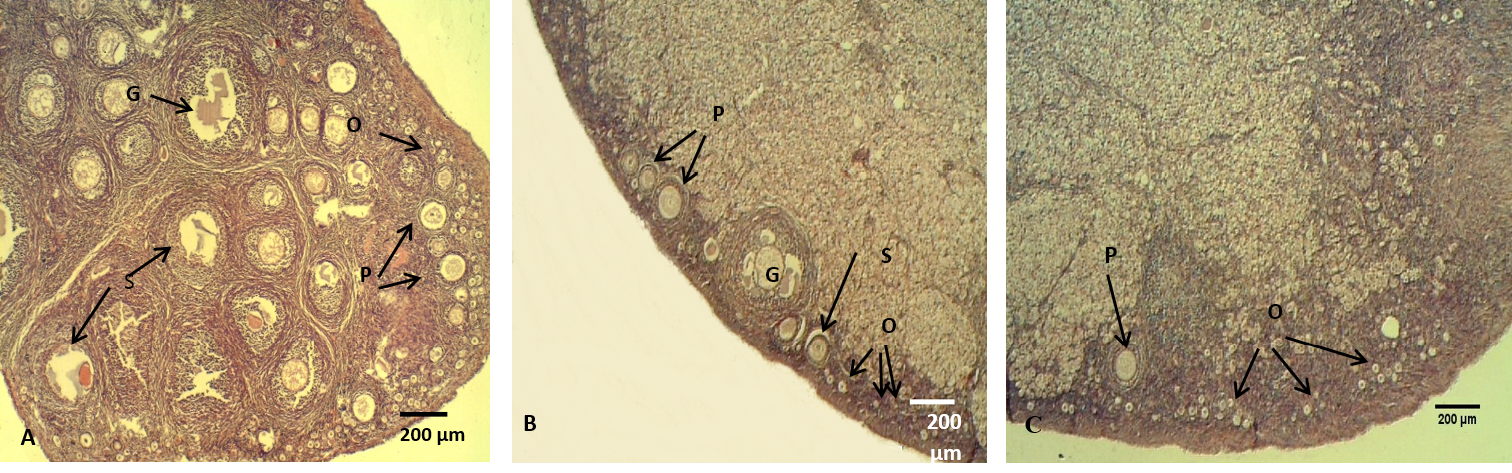
Figure 7: The ovary of adult rabbit
(A) Control group, showing high ovarian activity; (B) group eating mixed feed with cruciferous vegetable, showing low in ovarian activity; (C) group eating only cruciferous vegetable, showing lower ovarian activity. Where (O) Oocytes (P) Primary ovarian follicle, (S) Secondary ovarian follicles, (G) Mature ovarian follicles. H & E stain
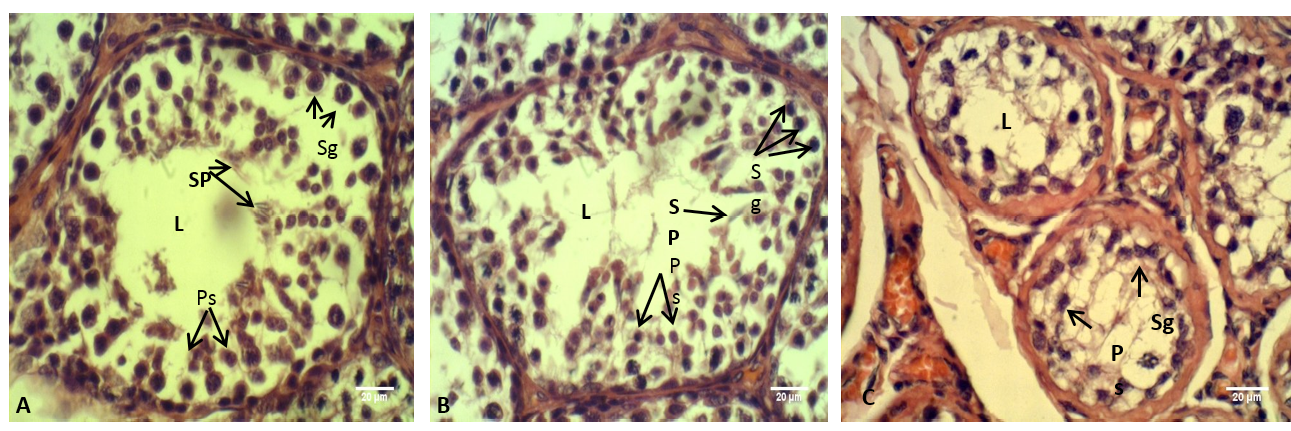
Figure 8: The testis of adult rabbit
(A) Control group, showing the epithelium of the seminiferous tubules with various stages of spermatogenesis; (B) group eating mixed feed with cruciferous vegetable, showing not typical arrangement of the spermatogenic cells; (C) group eating only cruciferous vegetable, showing spermatogenic cells were clustered together. Where (L) Lumen of seminiferous tubules (Sp) Spermatid, (Ps) Primary spermatozoa, (Sg) Spermatogonia. H & E stain
Table 3: The level of thyroid hormones (T3 and T4) in the serum of the adult rabbits and their kits in this experiment
|
Categories |
Group A |
Group B |
Group C |
|
|
Mature female |
T3 |
1.3±0.4 a |
0.9±0.1 ab |
0.6±0.1 b |
|
T4 |
5.4±2.3 a |
4.6±1 a |
3.9±0.6 a |
|
|
Mature male |
T3 |
1.14±0.3 a |
0.3±0.05 b |
0.1±0.06 b |
|
T4 |
4±0.4 a |
3.9±0.1 a |
3.2±0.5 b |
|
|
kit |
T3 |
2.04±0.4 a |
0.3±0.03 b |
---- |
|
T4 |
5.7±0.4 a |
4.04±0.6 b |
---- |
T3 (ng/ml), T4 (ug/dl). Values are means ± SD, N= 8. Different letter indicating significant (P<0.05) differences between groups.
The levels of these hormones in the serum of group (B) kits showed significantly (P>0.05) lower than the control group (Table 3). Kalin et al. (2013) had reported that the goitrogenes can cross into the fetus during lactation and the transfer of iodine cross ma ternal milk and placenta into the fetal bloodstream is inhibit by these goitrogenes.
The histological sections of the female ovaries represented significant (P<0.05) decreases in the numbers of the follicles (primary, secondary and graafian) in group (B) compared with control group (A), meanwhile, group (C) showed significantly lowest follicles number than the other groups (Figure 7, Table 4). These results is explained by Asahara et al. (2003) that of the follicles (primary, secondary and graafian) in group (B) compared with control group (A), meanwhile, group (C) showed significantly lowest follicles number than the other groups (Figure 7, Table 4). These results are explained by Asahara et al. (2003) that the thyroid hormones can effects the proliferation and apoptosis of such kinds of cells like granulosa cell, Accordingly, limited numbers of ovarian follicles are reaches the mature stage, the other follicles undergo atresia. In addition, T3 provide target steroidogenesis to promote ovarian function (Falzacappa et al., 2012; Mutinati et al., 2013).
Table 4: The numbers of ovarian follicles (Primary, Secondary and Graafian) in the three experimental groups of adult rabbits
|
Categories |
Group A |
Group B |
Group C |
|
Primary follicles |
14.5±1 a |
8.7±1.3 b |
7.7±1.7 b |
|
Secondary follicles |
11.6±1.3 a |
5.5±1.1 b |
3.2±0.7 c |
|
Graafian follicles |
9.6±1.6 a |
3.8±1 b |
1±0.8 c |
Values are means ± SD, N= 8. Different letter indicating significant (P<0.05) differences between groups.
The histological sections through the male testes illustrated in Figure 8. It is appeared that the lumen of the seminiferous tubules in control group (A) showed normal stages of spermatogenesis, normal arrangement of the Sertoli cells and the spermatogenic cells with the presence of spermatozoa in the tubules lumen. These normal histological observations of rabbit testis were agreed with the previous report of Hussen and Arrack (2014). While in the current study, little or no spermatozoa were found in the group (C). The stage of spermatogenesis could not be identified because the supporting cells of Sertoli and spermatogenic cells were clustered together. Whereas in the testes of the group (B) few numbers of the spermatozoa were identified in the lumens of the seminiferous tubules, furthermore the Sertoli cells and the spermatogenic cells were numerous but they not had typical arrangement compared with that in control group (A). According to the reports of Buzzard et al. (2000) that thyroid hormone plays a key role in testicular development. Further confirm by Jannini et al. (2000); Holsberger and Cooke (2005) that T3 plays an important role in testicular function by controlling Sertoli cell and Leydig cell proliferation and differentiation.
In conclusion, the cruciferous vegetable can make hypothyroidism in rabbit which resulting hypertrophy and in kits hyperplasia of the thyroid gland, in addition to the effects the male and female gonads function.
References





