Advances in Animal and Veterinary Sciences
Research Article
Distribution of Cultivable Actinobacteria from the Marine Sediments along the Andaman Coast of Eastern Indian Ocean
Sumitha Gopalakrishnan1, Jai Sunder1, Sasidharan Venu2
1ICAR-Central Island Agriculture Research Institute, Port Blair, Andaman & Nicobar Islands, India; 2Department of Ocean Studies and Marine Biology, Pondicherry University, Port Blair, Andaman & Nicobar Islands, India.
Abstract | Andaman and Nicobar Islands is one of the important biodiversity hot spots in the world which is situated in the Eastern Indian Ocean. The combination of mangrove, rocky and coral reef habitats make it an interesting area for studying microbial population. The present study discusses the distribution status of 643 isolates of Actinobacteria isolated from the marine sediments along the coast of Andaman group of Islands at a depth from 0 to 10m. Spatially, the coast of South Andaman harbours most number of isolates (290) than the other zones and among these, the highest (7.5%) was isolated from Marina Park. Bathymetrically, the highest number of isolates (269) was recorded from 0 – 1 m depth zone and the most number was isolated from Burmanallah. Among these, Streptomyces spp. was found to be highly dominant (83.4%) spatially as well as bathymetrically. Other genera identified were Streptoverticillium spp., Streptosporangia spp., Nocardia spp., Micromonospora spp., Actinoplanes spp. and Actinomadura spp. Highest percentage of Streptomyces spp. was recorded from Burmanallah (7.3 %). Marina Park and Science Centre stations recorded highest number of Streptoverticillium spp. (11.1% each) and Nocardia spp. was found more in Carbyn’s Cove. Micromonospora spp., Streptosporangium spp. and Actinoplanes spp. were recorded only from few stations and Actinomadhura spp. was found only from two stations. In general, the number of isolates decreased from the shore to deeper areas. The mangrove and rocky habitats harbours had more number of Actinobacteria than the sandy and reef ecosystems. The present study have confirmed the potential of this region in terms of actinobacterial diversity and can form a baseline for further research in marine pharmacology.
Keywords | Marine Actinobacteria, Andaman, Indian Ocean, Streptomyces, Indian EEZ
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 15, 2014; Revised | November 28, 2014; Accepted | November 29, 2014; Published | December 10, 2014
*Correspondence | Sumitha Gopalakrishnan, Central Island Agriculture Research Institute, Port Blair, India; Email: [email protected]
Citation | Gopalakrishnan S, Sunder J, Venu S (2014). Distribution of cultivable actinobacteria from the marine sediments along the Andaman Coast of Eastern Indian Ocean. Adv. Anim. Vet. Sci. 2 (12): 668-682.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.12.668.682
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Gopalakrishnan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Andaman and Nicobar Islands contribute almost 26% (1960km) of 7500km coastline of India and the coast is gifted with rich biodiversity (Roy, 2003). The entire coastal region of Andaman group of islands is composed of an amalgamation of mangrove forests, coral reefs, rocky, sandy or muddy shores, which are mostly unpolluted (Dagar and Singh, 1999). The mangrove forests support a wide range of animals for their feeding and breeding due to the high productivity resulting from the high influx of nutrients by the activities like heavy leaf littering and tidal fluctuations (Mandal and Naskar, 2008). This high organic load in turn supports a variety of microbial population in the sediment and water (Maria et al., 2006; Farooqui et al., 2012). The coral reef habitats are furthermore diverse and extend to more than 30 m depth towards the sea from the shore. Habitat associations like mangrove and reef habitats with rocky, sandy and muddy coastal regions also support a higher diversity along the coast (Qasim and Wafar, 1990).
The disastrous earthquake of 26 December 2004 has caused submergence of some of the Islands and upheaval of some other Islands. Consequently, virgin land areas were exposed to wave attack (Roy et al., 2009). Andaman and Nicobar Islands, considered as the richest source for microbial community. The marine microbial taxa and actinomycetes diversity are not been fully explored (Abirami et al., 2013). So in the post tsunami scenario it is very imperative to evaluate the microbial wealth especially marine Actinobacteria in the modified coastal environment along the Bay Island. These microbes are considered to be terrestrial in origin and believed to occur in the ocean largely as dormant spores that were washed into the sea (Jensen et al., 2005; Goodfellow and Haynes, 1984). According to Takizawa et al. (1993) marine Actinomycetes are distributed throughout the marine environment from shallow to deep sediments. They are isolated from different depths, but it is found that littoral inshore zone is most favourable for their survival (Mincer et al., 2002).
Actinomycetes are well known as potent producers of a variety of secondary metabolites with distinct biological activities (Berdy, 2005; Ben et al., 2006), including AMSs active against both pathogenic (Sun et al., 2011; Xiong et al., 2012) and phytopathogenic microorganisms (Xiong et al., 2012; Jain and Jain, 2007). The exploration of soils and other habitats for microbes of biotechnological interest has led to the isolation of novel actinomycete strains (Ouhdouch et al., 2001). Secondary metabolites of Actinomycetes are therapeutically important compounds, especially antiviral, anti-cancerous, antibacterial compounds and around 70% of the antibiotics used in the world were identified and extracted from these microorganisms (Sivakumar et al., 2007). The isolation of actinomycetes from mixed micro flora present in nature is complicated by their characteristic slow growth (Kerkar, 1994). So the present study conducted with an aim to study the distribution of marine Actinobacteria species from the marine sediments of various habitats along the coasts of Andaman group of Islands.
MATERIALS AND METHODS
The entire Andaman coast (Figure 1) was divided into 3 zones viz. South Andaman (Chidiyatapu to Baratang), Middle Andaman (Baratang to Mayabunder) and North Andaman (Mayabunder to Diglipur). Sediment samples were collected aseptically from 0-1m, 5-6m and 10 -11m depth at each station by skin and scuba diving using PVC corer with 2.5cm diameter during the period January 2011-May 2013. Various physiochemical factors like Sea surface Temperature, Salinity, water pH and sediment pH were recorded from each station. Total Organic Carbon of the sediment was estimated following Walkley and Black (1934) and Udotong et al. (2008). Sediment texture was analysed using Pippet analysis (Jayaraj et al., 2007) to understand the habitat structure.
The sediment samples were pre-treated because some of the Actinobacteria may not appear by normal plating techniques. In order to isolate these Actinobacteria, three pre-treatment techniques were applied:
The isolates were inoculated in Marine Actinomycete growth (MAG) broth (Starch-1gm, Peptone-0.4gm, Yeast extract-0.2gm, NaCl 1%v/w incubated for 4-5 days and observed for aerial mycelium under 100 x light microscope. The isolates were also studied for their carbohydrate utilization viz. D-glucose, D-Fructose, L-Rhamnose, D-Galactose, Lactose, Sucrose, L-Arabinose, Raffinose, Xylose, Salicin, Cellulose and Inositol (Nonomura, 1974; Buchanan, 1974; Pridham and Gottlieb, 1948). Salt tolerance (NaCl) was determined by growing the organisms on glucose nutrient agar plates supplemented with 0 to 10% (w/v) NaCl (Sujatha et al., 2005; Jensen et al., 1991; Gottlieb, 1973). The isolates were tested for temperature tolerance, oxidase, nitrate reduction, catalase and urease activities. The isolates were also screened for the production of enzymes such as protease, lipase, amylase, chitinase and cellulase activity as per the method of Williams et al. (1983) and Holt (1994). Protease activity was checked by spot inoculating loopful of culture along with the spores, into Nutrient Gelatin Agar (Peptone – 5gm, Beef extract – 3gm, Gelatin – 120 gm pH 6.8) after 24 to 48hrs incubation plates were flooded with gelatine precipitating reagent which consequently liquefy Gelatine to amino acids. A clear zone is the indication for positive result (Williams et al., 1983; Gourdeau et al., 2008). Lipase activity was determined by inoculating loopfull cultures in Tributyrin Agar (Himedia, Mumbai). Clearing zone indicates the positive result. Amylase activity was determined by spot inoculating the cultures in Starch Agar Medium (Peptone- 0.5gm, Beef extract- 0.3gm, Starch - 0.2g, Agar- 2.0g, Sea water- 100ml, pH- 7.2) (Mincer et al., 2002). Degradation of casein was determined following Lindqvist and Storgards (1960). The growth at different temperatures, other physiological and biochemical characteristics were studied using the method described by Williams et al. (1983). All tests were performed at 28°C. For enzymes like chitinase and cellulose, cultures were inoculated in cellulose agar medium and chitinase agar medium for 7 to 15 days. Formation of clear zone was the positive result. The production of melanoïdes pigments was carried out on ISP6 Agar (peptone, 20g; ferric citrate ammoniacal, 0.5g; sodium thiosulfate, 0.08g; yeast extract, 1g; K2HPO4, 1g; Agar 15g; H2O, 1000 ml, pH 7.2) and ISP7 agar (glycerol, 15g; L-tyrosin, 0.5g; L-asparagine, 1g; K2HPO4, 0.5g; MgSO4, 7H2O,0.5g; NaCl, 0.5g; FeSO4, 7H2O, 0.01g; standard saline solution, 1ml; agar, 18g; H20, 1000ml, pH 7.2) (Vijayakumar et al., 2007; Shirling and Gottlieb, 1966).
The susceptibilities of the organisms to various antibiotics were studied on nutrient agar plates containing various antibiotics such as triacylolandomycin, lincomycin and rifampicin. The antibiotics were aseptically mixed with sterile molten agar and the preparations were maintained at 45°C and poured into plates. After inoculation, the plates were incubated at 30°C for 1 week. Growth on the media was compared with growth of a control and was recorded as negative or positive (no growth or growth respectively) (Lechevalier and Lechevalier, 1967).
Thin layer Chromatography was done to determine cell wall composition. To detect the presence of meso Diamino pimelic acid, the sample were pelletized and treated with 6N HCL. Vacuum evaporation was done to evaporate HCL present in the sample and spotted in TLC Plates (Merck). After running for 5-6 hour in solvent system (methanol: distilled water: 6N HCL: Pyridine (80:26:4:10), plates were air dried, sprayed with 0.2% Ninhydrin in acetone. Developed in hot air oven at 100oC for 3 minutes observed for maroon coloured spots (Becker et al., 1965).
RESULTS
Habitat Structure
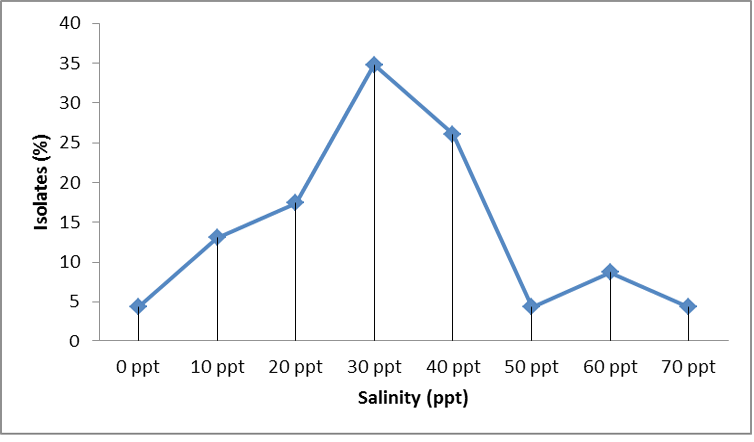
The detailed survey have shown that the entire study area along the coast was dominated mostly by mangrove forests or sandy beaches along with a combination of other habitats like rocky and corals (Table 1). The sediment analysis by standard pipette analysis have shown that most of the stations were loamy followed by sandy, clayey and muddy. The isolates from most of the stations were found to be more salt tolerant especially in the deeper regions. Even though they were showing growth at 0ppt to 70ppt salt concentration, highest percentage of growth was observed in the range 30-40ppt of salt concentration (Figure 2).
Table 1: Physico-chemical parameters of sea bed and water of various stations
|
Station |
S‰ |
S.S.T. °C |
Water pH |
Sediment Properties |
Sea bed Characteristics* |
Isolates (%) |
||
|
pH |
TOC % |
Texture |
||||||
|
South Andaman |
||||||||
|
Burmanallah |
32 |
25 |
8 |
5.4 |
1.23 |
Clay loamy |
R, M |
6.1 |
|
Carbyn’s Beach |
30 |
24 |
8 |
6.3 |
0.5 |
Sandy |
M, R, S |
4.5 |
|
Chattam |
33 |
24 |
8 |
7 |
1 |
Sandy muddy |
S |
3.1 |
|
Chidiyatappu |
35 |
24 |
8 |
6 |
0.86 |
Clay loamy |
M, C |
5.9 |
|
Dignabad |
30 |
25 |
8.3 |
5.4 |
0.43 |
Sandy clay |
S |
2.8 |
|
Junglighat |
32 |
24 |
7.8 |
5.8 |
0.9 |
Muddy sandy |
S |
3.7 |
|
Kodiyaghat |
35 |
25 |
8.1 |
4 |
0.75 |
Sandy loamy |
M, R |
3.6 |
|
Marina Park |
30 |
25 |
8.9 |
6.8 |
0.5 |
Sandy Clay loamy |
S, R |
7.5 |
|
Science Center |
35 |
25 |
8.2 |
6.4 |
0.45 |
Sandy clay loamy |
R, S |
3.7 |
|
Minnie Bay |
34 |
25 |
8.4 |
8 |
0.6 |
Clay loamy |
M |
0.2 |
|
Sippighat |
32 |
25 |
8.2 |
6.8 |
0.8 |
Clay loamy |
M |
2.0 |
|
Collinpur Beach |
35 |
24 |
8.2 |
6.8 |
0.4 |
Sandy loamy |
M, S |
0.9 |
|
Wandoor |
34 |
25 |
7.8 |
7.2 |
0.8 |
Sandy loamy |
M, S |
1.1 |
|
Middle Andaman |
||||||||
|
Billiground |
27 |
24 |
7.3 |
6.6 |
0.56 |
Sandy |
S, with Shells |
3.6 |
|
Betapur |
28 |
24 |
7 |
6.5 |
0.5 |
Sandy loamy |
S |
5.0 |
|
Yeratta |
32 |
23 |
8 |
5.4 |
2.43 |
Clay loamy |
M |
3.9 |
|
Bakultala |
34 |
24 |
8 |
6.8 |
1.23 |
Clay sandy |
M |
3.0 |
|
Rangat |
34 |
24 |
7 |
6.6 |
1.4 |
Clay loamy |
M |
1.7 |
|
Nimbutala |
34 |
25 |
8.2 |
6.4 |
0.9 |
Loamy clay |
M |
2.8 |
|
Kadamtala |
33 |
24 |
8.1 |
6.8 |
0.9 |
clay loamy sandy |
M |
3.1 |
|
Karmatang |
33 |
24 |
8 |
6.9 |
2.45 |
Sandy clay |
M, R |
3.3 |
|
Austin Creek |
34 |
25 |
7.7 |
7 |
0.5 |
Sandy |
M |
3.4 |
|
Mayabunder |
35 |
23 |
8 |
6.8 |
0.55 |
Coarse sandy |
M, R |
2.5 |
|
North Andaman |
||||||||
|
Panchavati |
33 |
25 |
8 |
6.5 |
0.7 |
Gravel sandy |
S, R |
1.7 |
|
Aerial Bay Jetty |
35 |
24 |
7 |
6 |
0.6 |
Muddy sandy |
M, S |
2.0 |
|
Durgapur |
34 |
25 |
8.1 |
6.5 |
0.5 |
Sandy |
S |
1.9 |
|
Kalipur |
35 |
25 |
8 |
7 |
0.4 |
Sandy clay |
S |
5.0 |
|
Shyamkunj |
33 |
24 |
7.8 |
6.8 |
1.64 |
Clay loamy sandy |
S, M |
2.5 |
|
RRO Camp |
34 |
25 |
8 |
6.9 |
0.5 |
Loamy sandy |
S |
1.7 |
|
Culbert Bay |
34 |
24 |
8.2 |
6.8 |
0.372 |
Loamy sandy |
S |
3.7 |
|
Aamkunj |
34 |
25 |
8 |
6.4 |
1.32 |
Loamy sandy |
S |
2.2 |
|
Shivpur |
34 |
24 |
8.1 |
6.8 |
0.3288 |
Coarse sandy |
S |
2.0 |
*S = Sandy; R = Rocky; M = Mangrove; C = Coral
Table 2: Zone wise and Depth wise Distribution of Actinobacteria
|
Zone |
Station |
0-1 m |
5-6 m |
10-11 m |
Total |
% (Zone wise) |
% (Total) |
|
South Andaman |
Burmanallah |
23 |
6 |
10 |
39 |
13.4 |
6.1 |
|
Carbyn’s Beach |
10 |
9 |
10 |
29 |
10.0 |
4.5 |
|
|
Chattam |
4 |
16 |
0 |
20 |
6.9 |
3.1 |
|
|
Chidiyatappu |
14 |
6 |
18 |
38 |
13.1 |
5.9 |
|
|
Dignabad |
9 |
6 |
3 |
18 |
6.2 |
2.8 |
|
|
Junglighat |
11 |
9 |
4 |
24 |
8.3 |
3.7 |
|
|
Kodiyaghat |
9 |
4 |
10 |
23 |
7.9 |
3.6 |
|
|
Marina Park |
22 |
18 |
8 |
48 |
16.6 |
7.5 |
|
|
Science Center |
8 |
12 |
4 |
24 |
8.3 |
3.7 |
|
|
Minni Bay |
1 |
0 |
0 |
1 |
0.3 |
0.2 |
|
|
Sippighat |
4 |
2 |
7 |
13 |
4.5 |
2.0 |
|
|
Collinpur Beach |
3 |
2 |
1 |
6 |
2.1 |
0.9 |
|
|
Wandoor |
5 |
0 |
2 |
7 |
2.4 |
1.1 |
|
|
Total |
123 |
90 |
77 |
290 |
|
45 |
|
|
|
% |
42 |
31 |
27 |
|
|
|
|
Middle Andaman
|
Billiground |
7 |
8 |
8 |
23 |
11.1 |
3.6 |
|
Betapur |
7 |
10 |
15 |
32 |
15.5 |
5.0 |
|
|
Yeratta |
11 |
7 |
7 |
25 |
12.1 |
3.9 |
|
|
Bakultala |
6 |
4 |
9 |
19 |
9.2 |
3.0 |
|
|
Rangat |
6 |
1 |
4 |
11 |
5.3 |
1.7 |
|
|
Nimbutala |
4 |
7 |
7 |
18 |
8.7 |
2.8 |
|
|
Kadamtala |
5 |
4 |
11 |
20 |
9.7 |
3.1 |
|
|
Karmatang |
7 |
10 |
4 |
21 |
10.1 |
3.3 |
|
|
Austin Creek |
11 |
6 |
5 |
22 |
10.6 |
3.4 |
|
|
Mayabunder |
11 |
2 |
3 |
16 |
7.7 |
2.5 |
|
|
Total |
75 |
59 |
73 |
207 |
|
32 |
|
|
% |
36 |
29 |
35 |
|
|
|
|
|
North Andaman |
Panchavati |
5 |
5 |
1 |
11 |
7.5 |
1.7 |
|
Aerial Bay Jetty |
2 |
8 |
3 |
13 |
8.9 |
2.0 |
|
|
Durgapur |
9 |
1 |
2 |
12 |
8.2 |
1.9 |
|
|
Kalipur |
16 |
10 |
6 |
32 |
21.9 |
5.0 |
|
|
Shyamkunj |
9 |
4 |
3 |
16 |
11.0 |
2.5 |
|
|
RRO Camp |
9 |
1 |
1 |
11 |
7.5 |
1.7 |
|
|
Culbert Bay |
9 |
12 |
3 |
24 |
16.4 |
3.7 |
|
|
Hamkunj |
6 |
3 |
5 |
14 |
9.6 |
2.2 |
|
|
Shivpur |
6 |
2 |
5 |
13 |
8.9 |
2.0 |
|
|
Total |
71 |
46 |
29 |
146 |
|
23 |
|
|
% |
49 |
32 |
20 |
|
|
|
|
|
GRAND TOTAL |
269 |
195 |
179 |
643 |
|
|
|
|
|
% |
42 |
30 |
28 |
|
|
|
General Distribution and Abundance
During the present study, 643 isolates were isolated from the sediment samples collected along the coast of Andaman Islands (Table 2). The highest abundance of Actinobacteria population was recorded from the South Andaman Zone in total as well as in all the sampled depths (2.90 x 10 2 cfu/g) followed by North Andaman (2.05 x 102 cfu/g) and Middle Andaman (1.46 x 102 cfu/g). A depth wise analysis have shown that the depth 0-1m inhabits highest number (269 isolates) followed by 5-7m (195) and 10-12m (179).
The most number of Actinobacteria were recorded from Marina Park (7.5%) of South Andaman followed by Burmanallah (6.1%) and Chidiyatappu (5.9%). While only one isolate was isolated from Minnie Bay. In the 0-1m depth, most number of Actinobacteria were recorded from Burmanallah (23 isolates) followed by Marina Park (22), Kalipur (16) and Chidiyatappu (14), etc. In the depth 5-6m, maximum number was observed in Marina Park (18 isolates) followed by Chattam (16), Culbert Bay (12), Science Centre (12) and Betapur (10). In the depth zone 10-11m, most number of Actinomycetes were observed from Chidiyatappu (18 isolates) followed by Betapur (15), Kadamtala (11), Burmanallah (10), Carbyn’s Cove (10) and Kodiyaghat (10). Actinobacteria could not be recorded from the stations Chattam and Minnie Bay.
Distribution and Abundance in South Andaman Zone
Maximum number of isolates (123) was recorded at 0-1m depth followed by 5-6m (90) and the least was recorded from 10-11m (77) (Table 2). Marina Park station was recorded most number of Actinomycetes contributing to 16.6% and the least was in Minnie Bay (0.3%). In 0-1m depth zone, Burmanallah recorded the highest (23 colonies) and in the depth 10-11m, highest number was recorded in Chidiyatappu (18).
Distribution and Abundance in Middle Andaman
In Middle Andaman, out of 207 Actinomycetes, 0-1m showed the maximum (75 colonies) followed by 10-11m (73) and the least was recorded from 5-6m (59). Betapur station recorded the most number of Actinomycetes (32 colonies) contributing to 15.5% from the zone. In 0-1m depth zone, Yeratta, Austin Creek and Mayabunder contributed the highest (11 colonies each). Betapur and Karmatang recorded maximum number of Actinomycetes from the depth 5-6m (10 each) followed by Billiground (8), Yeratta (7), etc. In the depth 10-11m, highest number was recorded in the station Betapur (15).
Distribution and Abundance in North Andaman
In North Andaman, out of 146 Actinobacteria recorded, 0-1m showed the maximum (71 colonies) followed by 5-6m (46) and the least was recorded from 10-11m (29). Kalipur station recorded the most number of Actinobacteria (32 colonies) contributing to 21.9% from the zone. In 0-1m depth zone, Kalipur contributed the highest (16 colonies). Culbert Bay recorded maximum number of Actinobacteria from the depth 5-6m (12). While in the depth 10-11m, highest number was recorded in the station Kalipur (6).
Distribution of various Actinomycete genera
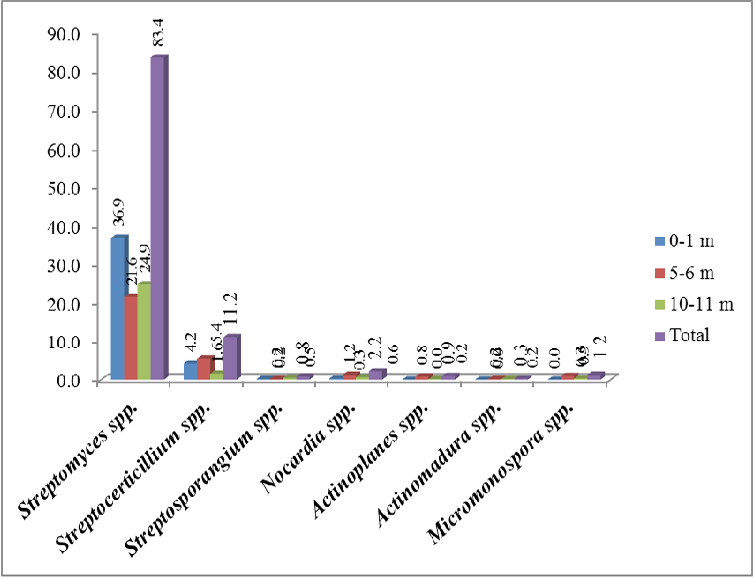
The seven major Actinobacteria genera identified from the morphologically different isolates appeared in the media are Streptomyces spp., Streptoverticillium spp., Nocardia spp., Micromonospora spp., Actinoplanes spp., Streptosporangium spp. and Actinomadura spp. (Table 3). It could be seen from the analysis of the species composition that 83.4% of colonies were Streptomyces spp. (Figure 3). Streptoverticillium spp. was found to be the second most dominant genus (11.2%). Streptomyces spp. showed dominance spatially as well as bathymetrically. Spatially, Streptomyces spp. showed the highest percentage distribution in South Andaman (38.4%) compared to Middle (28%) and North Andaman Islands (17%). The highest percentage was recorded from 0-1m depth (36.9%) followed by 10-11m (24.9%) and 5-6m (21.6%) (Table 4). Streptoverticillium spp. could be recorded from all the depths and zones, and spatially, highest contribution was from South Andaman (4.2%) followed by North Andaman (3.9%) and Middle Andaman (Table 5). Depth-wise, 5-6m has recorded the maximum with 5.4%.
Table 3: Biochemical characterization of Actinobacteria isolated from Andaman coast
|
Characteristics |
Colonies shown positive result |
Possible Species |
|
Aerial mass colour |
||
|
White |
128 |
SM, SV, AM, NO, AP, MM |
|
White cream/yellow |
136 |
SM, SV, AM, NO, SP |
|
White changing to grey |
58 |
SM, SV |
|
White change to red |
42 |
SM, SV |
|
Grey |
145 |
SM, SV |
|
Red/Orange/Pink |
27 |
SM, MM, NO, SP |
|
Substrate Mycelium |
||
|
Substrate Mycelium |
594 |
MM, AP, AM, SP, SM, SV |
|
Fragmented Substrate Mycelium |
14 |
NO |
|
Sporophore morphology |
||
|
Sporarngia Formation |
578 |
AP, AM, SP, SM |
|
Straight |
145 |
SM |
|
Spiral |
230 |
SM, SV |
|
Flexous |
111 |
SM |
|
Retinaculum apertum |
50 |
SM |
|
Conidia Formation |
13 |
AM, AP |
|
Pigment production |
||
|
Melanin |
129 |
SM, SV |
|
Reverse colour |
115 |
SM, SV |
|
Soluble colour |
134 |
SM, SV |
|
Isolates showing pigmentation |
158 |
SM, SV |
|
Carbohydrates utilised by the isolates |
||
|
D-glucose |
640 |
SM, SV, SP, AP, NO, MM, AM |
|
D-Fructose |
632 |
SM, SV, SP, AP, NO, MM, AM |
|
L-Rhamnose |
450 |
SM, SV, SP, AP, AM |
|
D-Galactose |
148 |
SM, SV, SP, MM, AM |
|
Lactose |
219 |
SM |
|
Sucrose |
354 |
SM, SV, SP, MM |
|
L-Arabinose |
630 |
SM, SV, SP, AP, NO, AM |
|
Raffinose |
604 |
SM, SV, SP, MM |
|
Xylose |
630 |
SM, SV, SP, NO, MM |
|
Salicin |
405 |
SM, SP, AP, MM, AM |
|
Cellulose |
309 |
SM, SP, MM |
|
Inositol |
617 |
SM, SV, SP, MM |
|
Oxidase |
386 |
SM, SV, SP |
|
Tyrosine |
14 |
NO |
|
Xanthine |
14 |
NO |
|
Hypoxanthine |
16 |
NO, AP |
|
Enzyme Activity |
||
|
Catalase |
514 |
SM |
|
Urease |
386 |
NO, SM, SV |
|
Amylase |
568 |
SM, MM, NO, AP, AM |
|
Protease |
571 |
SM, MM, SV |
|
Lipase |
549 |
MM, SV, NO, SM |
|
Chitinase |
12 |
SM, MM |
|
Cellulase |
18 |
SM, MM, NO |
|
Lysozyme |
14 |
NO |
|
Caesin |
452 |
SM, SV, MM, AP, AM |
|
Presence of Di Amino Pimelic Acid |
549 |
SM, SV |
SM – Streptomyces; SV – Streptoverticillium spp.; MM – Micromonospora spp.; AP – Actinoplanes spp.; AM – Actinomadhura spp.; NO – Nocardia spp.; SP – Streptosporangium spp.
Nocardia spp. was the third most abundant group with a total of 14 colonies recorded from all the three zones with a maximum representation from South Andamans (1.2%) (Table 6). Micromonospora spp. was recorded maximum from South Andaman (0.6%) and was absent in the 0-1m depth and observed mostlyfrom the 5-6m depth (0.9%). Actinoplanes spp. also found to distribute in all the three zones with highest contribution from North Andamans (0.5%). It was absent in the depth 0-1m and highest concentration was found in 5-6m (0.8%). Streptosporangium spp. could not be recorded from Middle Andaman and only 5 numbers could be recorded during the present study. Whereas, Actinomadura spp. could be recorded only from Middle Andamans and was absent in the 0-1m depth.
Streptomyces spp. was recorded from all the stations. The highest percentage was recorded from Burmanallah (7.3%) followed by Marina Park (7.1), Chidiyatyappu (6.5), Betapur (5.8) and Kalipur (4.9). Streptomyces spp. dominated in the stations Burmanallah (7.3%) followed by Marina Park (7.1), Chidiyatyappu (6.5) in South Andaman. Depth-wise, the highest percentage composition was found in the 0-1m depth zone at Burmanallah (4.3%) and Chattam at the depth 5-6m (4.1%). In the Middle Andaman, Betapur recorded maximum Streptomyces spp. (5.8%) Kalipur station could record maximum number of Strepto> myces spp. (4.9%) in the North Andaman Zone. 0-1m depth at Kalipur station has recorded highest (3%).
Table 4: Depth-wise percentage distribution of Streptomyces spp.
|
Zone |
Station |
0-1 m |
5-6 m |
10-11 m |
Total |
% (Zone Wise) |
% (Total |
|
South Andaman |
Burmanallah |
23 |
6 |
10 |
39 |
15.8 |
7.3 |
|
Carbyn’s Beach |
9 |
5 |
8 |
22 |
8.9 |
4.1 |
|
|
Chattam |
3 |
14 |
0 |
17 |
6.9 |
3.2 |
|
|
Chidiyatappu |
14 |
3 |
18 |
35 |
14.2 |
6.5 |
|
|
Dignabad |
9 |
6 |
3 |
18 |
7.3 |
3.4 |
|
|
Junglighat |
10 |
7 |
4 |
21 |
8.5 |
3.9 |
|
|
Kodiyaghat |
9 |
4 |
7 |
20 |
8.1 |
3.7 |
|
|
Marina Park |
21 |
8 |
9 |
38 |
15.4 |
7.1 |
|
|
Science Center |
8 |
6 |
2 |
16 |
6.5 |
3.0 |
|
|
Minni Bay |
1 |
0 |
0 |
1 |
0.4 |
0.2 |
|
|
Sippighat |
4 |
1 |
5 |
10 |
4.0 |
1.9 |
|
|
Collinpur Beach |
2 |
2 |
1 |
5 |
2.0 |
0.9 |
|
|
Wandoor |
4 |
0 |
1 |
5 |
2.0 |
0.9 |
|
|
Total |
117 |
62 |
68 |
247 |
|
46.1 |
|
|
Middle Andaman |
Billiground |
7 |
6 |
8 |
21 |
11.7 |
3.9 |
|
Betapur |
7 |
9 |
15 |
31 |
17.2 |
5.8 |
|
|
Yeratta |
6 |
7 |
7 |
20 |
11.1 |
3.7 |
|
|
Bakultala |
6 |
3 |
8 |
17 |
9.4 |
3.2 |
|
|
Rangat |
6 |
0 |
4 |
10 |
5.6 |
1.9 |
|
|
Nimbutala |
3 |
3 |
7 |
13 |
7.2 |
2.4 |
|
|
Kadamtala |
5 |
3 |
11 |
19 |
10.6 |
3.5 |
|
|
Karmatang |
7 |
9 |
4 |
20 |
11.1 |
3.7 |
|
|
Austin Creek |
6 |
4 |
5 |
15 |
8.3 |
2.8 |
|
|
Mayabunder |
10 |
2 |
2 |
14 |
7.8 |
2.6 |
|
|
Total |
63 |
46 |
71 |
180 |
|
33.6 |
|
|
North Andaman |
Panchavati |
2 |
4 |
1 |
7 |
6.4 |
1.3 |
|
Aerial Bay Jetty |
2 |
4 |
1 |
7 |
6.4 |
1.3 |
|
|
Durgapur |
3 |
1 |
2 |
6 |
5.5 |
1.1 |
|
|
Kalipur |
16 |
6 |
4 |
26 |
23.9 |
4.9 |
|
|
Shyamkunj |
9 |
3 |
2 |
14 |
12.8 |
2.6 |
|
|
RRO Camp |
9 |
1 |
1 |
11 |
10.1 |
2.1 |
|
|
Culbert Bay |
5 |
8 |
3 |
16 |
14.7 |
3.0 |
|
|
Hamkunj |
5 |
2 |
2 |
9 |
8.3 |
1.7 |
|
|
Shivpur |
6 |
2 |
5 |
13 |
11.9 |
2.4 |
|
|
Total |
57 |
31 |
21 |
109 |
|
20.3 |
|
|
Grand Total |
237 |
139 |
160 |
536 |
|
|
|
Table 5: Depth-wise distribution of Streptoverticillium spp.
|
Zone |
Station |
0-1 m |
5-6 m |
10-11 m |
Total |
% (ZoneWise) |
% (Total |
|
South Andaman |
Carbyn’s Beach |
0 |
3 |
|
3 |
11.1 |
4.2 |
|
Chidiyatappu |
|
3 |
|
3 |
11.1 |
4.2 |
|
|
Kodiyaghat |
|
3 |
3 |
11.1 |
4.2 |
||
|
Marina Park |
|
8 |
|
8 |
29.6 |
11.1 |
|
|
Science Center |
|
6 |
2 |
8 |
29.6 |
11.1 |
|
|
Collinpur Beach |
1 |
|
1 |
3.7 |
1.4 |
||
|
Wandoor |
1 |
|
1 |
3.7 |
1.4 |
||
|
Total |
2 |
20 |
5 |
27 |
|
37.5 |
|
|
Middle Andaman |
Billiground |
|
2 |
|
2 |
10.0 |
2.8 |
|
Yeratta |
5 |
|
5 |
25.0 |
6.9 |
||
|
Bakultala |
|
1 |
1 |
5.0 |
1.4 |
||
|
Rangat |
|
1 |
|
1 |
5.0 |
1.4 |
|
|
Nimbutala |
1 |
3 |
|
4 |
20.0 |
5.6 |
|
|
Austin Creek |
5 |
1 |
|
6 |
30.0 |
8.3 |
|
|
Mayabunder |
1 |
|
1 |
5.0 |
1.4 |
||
|
Total |
12 |
7 |
1 |
20 |
|
27.8 |
|
|
North Andaman |
Panchavati |
2 |
|
|
2 |
8.0 |
2.8 |
|
Aerial Bay Jetty |
|
3 |
1 |
4 |
16.0 |
5.6 |
|
|
Durgapur |
6 |
|
|
6 |
24.0 |
8.3 |
|
|
Kalipur |
|
2 |
|
2 |
8.0 |
2.8 |
|
|
Culbert Bay |
4 |
2 |
|
6 |
24.0 |
8.3 |
|
|
Hamkunj |
1 |
1 |
3 |
5 |
20.0 |
6.9 |
|
|
Total |
13 |
8 |
4 |
25 |
|
34.7 |
|
|
Grand Total |
27 |
35 |
10 |
72 |
|
|
|
Streptoverticillium spp. could be recorded from a total of 20 stations during the present study (Table 3). Highest percentage composition was found from Marina Park and Science Center stations (11.1% each) in South Andaman. Depth wise, maximum contribution was found from Marina Park Station (11.1%) followed by Science Centre (8.3%) at the depth 5-6m. In Middle Andaman, Austin Creek has recorded highest percentage composition of 8.3%. Depth-wise, 0-1m at Yeratta and Austin Creek has recorded the highest (5% each). While in North Andaman, highest recordings were from Durgapur and Culbert Bay (8.3% each) in the 0-1m depth.
Highest concentration of Nocardia spp. was found in Carbyn’s Cove of South Andaman (28.6%) followed by Junglighat (21.4%) and was reported from 7 stations only (Table 4). Micromonospora spp. was recorded from 5 stations viz. Chattam, Sippighat, Kadamtala, Aerial Bay Jetty and Culbert Bay. Only 4 stations recorded Actinoplanes spp. and Kalipur contributed maximum (50%) followed by Bakultala, Betapur (17% each) and Chidiyatappu. Streptosporangium spp. was found equal percentage distribution in Culbert Bay, Chattam, Kodiyaghat, Marina Park and Kalipur (20% each). Actinomadhura spp. was found to be the rarest and could be recorded only from two stations viz. Karmatang and Mayabunder.
DISCUSSION
During the present study it could be noticed that most of the stations were found to be a combination of either mangroves or sandy with other habitats. The domination of Actinobacteria was discernible in the South Andaman when compared to other zones. This zone is mainly dominated by mangrove habitats followed by sandy and rocky habitats. In stations like Wandoor, Burmanallah and Chidiyatappu, the corals are mostly a continuation of the mangrove ecosystem and the inhabitants are found interacting very closely. The human interferences including tourism activities were found to be higher in areas like Junglighat, Minnie Bay, Marina Park, Science Centre, Wandoor and Chidiyatapu. Rocky bottoms were found in the stations viz. Burmanallah, Carbyn’s Cove, Kodiyaghat, Marina Park, and Science Centre. While in other zones, mangroves and other types of habitats co-exist in stations like Yerratta, Kadamthala, Rangat, Karmatang, Panchavati, Nimbuthala, Bakulthala etc. where a lot of anthropogenic activities like fishing, fire wood collection, etc. taking place. Sandy beaches dominated in stations like RRO camp, Culbert Bay, Shivpur, Aamkunj. The rich biodiversity of the Andaman and Nicobar Islands is already been reported by many workers (Qasim and Wafar, 1990; Dagar and Singh, 1999; Roy, 2003; Ramya, 2008; Roy et al., 2009; Ramkumar et al., 2013).
While considering the sediment texture, the present results have shown that the clay loamy and loamy sandy sediments hold maximum number of Actinomycete population. Ravikumar et al. (2010) have recorded highest number of Actinobacteria isolates from the mangrove sediments at Thondi Tamil Nadu, where the sediment was clayey which can hold maximum number of Actinobacteria.
Table 6: Depth-wise distribution of other Actinobacteria genera
|
Zone |
Station |
0-1 m |
5-6 m |
10-11 m |
Total |
% (Zone wise) |
% (Total) |
|
Streptosporangia spp. |
|||||||
|
South Andaman |
Chattam Marina Park Wandoor Total |
1 0 0 1 |
0 0 0 0 |
0 1 1 2 |
1 1 1 3 |
33.3 33.3 33.3
|
20.0 20.0 20.0 60.0 |
|
North Andaman |
Culbert Bay Kalipur Total |
0 0 0 |
1 0 1 |
0 1 1 |
1 1 2 |
50.0 50.0
|
20.0 20.0 40.0 |
|
Grand Total |
1 |
1 |
3 |
5 |
|
||
|
Nocardia spp. |
|||||||
|
South Andaman |
Carbyn’s Beach Junglighat Marina Park Total |
1 0 0 1 |
1 2 1 4 |
2 1 0 3 |
4 3 1 8 |
50.0 37.5 12.5
|
28.6 21.4 7.1 57.1 |
|
Middle Andaman |
Nimbutala Austin Creek Total |
0 0 0 |
1 1 2 |
0 0 0 |
1 1 2 |
50.0 50.0
|
7.1 7.1 14.3 |
|
North Andaman |
Panchavati Shyamkunj Total |
1 0 1 |
1 1 2 |
0 1 1 |
2 2 4 |
50.0 50.0
|
14.3 14.3 28.6 |
|
Grand Total |
2 |
8 |
4 |
14 |
|
|
|
|
Actinoplanes spp. |
|||||||
|
South Andaman |
Sippighat Total |
0 0 |
1 1 |
0 0 |
1 1 |
100
|
16.7 16.7 |
|
Middle Andaman |
Betapur Bakultala Total |
0 0 0 |
1 1 2 |
0 0 0 |
1 1 2 |
50.0 50.0
|
16.7 16.7 33.3 |
|
North Andaman |
Kalipur Total |
0 0 |
2 2 |
1 1 |
3 3 |
100
|
50.0 50.0 |
|
Grand Total |
0 |
5 |
1 |
6 |
|
|
|
|
Actinomadura spp. |
|||||||
|
Middle Andaman |
Karmatang Mayabunder Grand Total |
0 0 0 |
1 0 1 |
0 1 1 |
1 1 2 |
50.0 50.0
|
50.0 50.0
|
|
Micromonospora spp. |
|||||||
|
South Andaman |
Chattam Sippighat Total |
0 0 0 |
2 1 3 |
0 1 1 |
2 2 4 |
50.0 50.0
|
25.0 25.0 50.0 |
|
Middle Andaman |
Kadamtala Total |
0 0 |
1 1 |
0 0 |
1 1 |
100
|
12.5 12.5 |
|
North Andaman |
Aerial Bay Jetty Culbert Bay Total |
0 0 0 |
1 1 2 |
1 0 1 |
2 1 3 |
66.7 33.3
|
25.0 12.5 37.5 |
|
Grand Total |
0 |
6 |
2 |
8 |
|
|
|
The dominance of Streptomyces spp. was already reported from the coast of Andaman Islands along with Micromonospora spp., Nocardia spp., Streptoverticillium spp. and Saccharopolyspora spp. (Sujatha et al., 2005; Sivakumar et al., 2007; Abirami et al., 2013). While Balagurunathan et al. (1989) has reported some common species like Rhodococcos sp., Streptomyces spp., Salinospora sp. and Micromonospora sp. from the mangrove sediments. Similarly, the dominance of Streptomyces spp. was evident in the present study also and the abundance of the other genera was negligible. In the present study the saccarolytic Actinomycetes like Streptomyces spp. load was high and most of them were isolated from depth ranges 0-1m and 5 – 6m depth zones. According to Grein and Meyers (1958), Streptomyces spp. are not autochthonous marine flora, they may be terrestrial forms adapted to salinity of sea water and sediment. Some scientists considered Actinomycetes to be part of indigenous marine micro flora (Zobell, 1940; Okazaki and Okami, 1976; Weyland, 1981) where as others considered them as wash in components that merely survived in marine and littoral sediment as spores (Goodfellow and Haynes, 1984; Takizawa et al., 1993; Jensen et al., 2005). This view is supported by the observation that the numbers of Actinomycetes in marine habitat decreases with increasing distance from land (Weyland, 1969). The present study have shown a general trend that the 0-1m depth have recorded highest concentration of Actinomycetes followed by 5-6m and 10-11m. Mincer et al. (2002) has reported that littoral inshore zone is most favourable for the survival. This indicates that these isolates include true marine forms as well as the washed away forms from terrestrial environment which showed a wide salt tolerance.
Andaman and Nicobar coast is one among the virgin coasts in the world and a hot spot of biodiversity which is not explored systematically and thoroughly. So the present study have shown the importance and potential of this region in terms of actinobacterial diversity and the future prospects of exploration for strains of bacterial flora for novel drugs. This work can be a baseline for the future studies in the field of pharmacological research to be conducted in the country.
ACKNOWLEDGEMENT
The authors thankfully acknowledge the funding received under the scheme “Women Scientist program (WOS-A) Department of Science and Technology, Government of India (DST No: SR/WOS-A/LS-205/2008 Dated 28/08/2009)” and the facilities provided by the Director, CIARI for conducting this work.
REFERENCES